Semi-synthetic terpenoids with differential adjuvant properties as sustainable replacements for shark squalene in vaccine emulsions
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Synthetic biology has allowed for the industrial production of supply-limited sesquiterpenoids such as the antimalarial drug artemisinin and β-farnesene. One of the only unmodified
animal products used in medicine is squalene, a triterpenoid derived from shark liver oil, which when formulated into an emulsion is used as a vaccine adjuvant to enhance immune responses in
licensed vaccines. However, overfishing is depleting deep-sea shark populations, leading to potential supply problems for squalene. We chemically generated over 20 squalene analogues from
fermentation-derived β-farnesene and evaluated adjuvant activity of the emulsified compounds compared to shark squalene emulsion. By employing a desirability function approach that
incorporated multiple immune readouts, we identified analogues with enhanced, equivalent, or decreased adjuvant activity compared to shark squalene emulsion. Availability of a library of
structurally related analogues allowed elucidation of structure-function relationships. Thus, combining industrial synthetic biology with chemistry and immunology enabled generation of
sustainable terpenoid-based vaccine adjuvants comparable to current shark squalene-based adjuvants while illuminating structural properties important for adjuvant activity. SIMILAR CONTENT
BEING VIEWED BY OTHERS SYNTHETIC BIOLOGY-DERIVED TRITERPENES AS EFFICACIOUS IMMUNOMODULATING ADJUVANTS Article Open access 13 October 2020 IMMUNOINFORMATICS APPROACHES IN DEVELOPING A NOVEL
MULTI-EPITOPE CHIMERIC VACCINE PROTECTIVE AGAINST _SAPROLEGNIA PARASITICA_ Article Open access 27 January 2024 CARBOHYDRATE FATTY ACID MONOSULPHATE: OIL-IN-WATER ADJUVANT ENHANCES SARS-COV-2
RBD NANOPARTICLE-INDUCED IMMUNOGENICITY AND PROTECTION IN MICE Article Open access 14 February 2023 INTRODUCTION Squalene
((6_E_,10_E_,14_E_,18_E_)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene) is a triterpene and belongs to the large natural product family of terpenoids of which there are over
55,000 known members1. Many terpenoids are produced at low concentrations in plants, making their industrial production economically infeasible, but squalene is unusual in that it occurs at
high concentrations in shark liver oil from which it has traditionally been isolated2. Shark-derived squalene has been used as a vaccine adjuvant in hundreds of millions of influenza vaccine
doses, demonstrating an excellent safety profile and vaccine dose-sparing capability3. Moreover, multiple late-stage COVID-19 vaccine candidates that include shark squalene-based
formulations are expected to play an important role in the global pandemic response due—in part—to stability profiles that enable easier distribution logistics (i.e., refrigerated, not
frozen) than the currently authorized mRNA vaccines4. Shark squalene is also a key component in vaccine candidates under clinical evaluation for other indications, including tuberculosis,
malaria, schistosomiasis, and leishmaniasis5. Indeed, next to aluminum salts, shark squalene-based emulsions are the most widely employed vaccine adjuvant formulation in licensed inactivated
or protein-based vaccines3. However, shark populations have been subject to overfishing, and the global abundance of oceanic sharks and rays has declined by 71% since 1970, resulting in
calls for prohibitions and precautionary catch limits to avert population collapse6. A replacement for shark squalene with equivalent or better vaccine adjuvant properties when appropriately
formulated, but sourced sustainably, would be desirable to alleviate the population pressure on sharks and secure the long-term future of this class of vaccine adjuvants. Synthetic biology
applied to metabolic engineering has resulted in industrial-scale production volumes of natural products that were hitherto either subject to supply constraints or unavailable in the
quantities and purities required for commercial usage. An early example of the development and use of bioengineered yeast to produce a terpenoid at commercial scale was the semi-synthetic
version of the antimalarial drug artemisinin, whereby yeast (_Saccharomyces cerevisiae_) was engineered to produce the sesquiterpenoid artemisinic acid by fermentation, which was then
converted chemically to artemisinin7,8. Subsequently, _S. cerevisiae_ was engineered to produce the sesquiterpene β-farnesene ((6_E_)-7,11-dimethyl-3-methylidenedodeca-1,6,10-triene) by
fermentation at high yield and productivity9, leading to its commercial manufacture10. A major commercial product derived from β-farnesene by semi-synthesis is high-purity (92–94% with
>99% saturated triterpene content) squalane, which provides a sustainable, renewable source of this emollient for the cosmetic market and displaces shark-derived squalane, manufactured by
hydrogenation of shark-derived squalene11. Despite the importance of shark squalene emulsion adjuvants in contemporary human vaccines, their mechanisms of action are not completely
understood. For instance, it has been shown that shark squalene-based emulsions increase vaccine antigen uptake, enhance recruitment and activation of various immune cells at the injection
site and the draining lymph node, and cause production of danger-associated molecular patterns that result in proinflammatory signaling cascades12; however, it is not clear how the
structural properties of squalene relate to these mechanisms. We leveraged the ready availability of fermentation-derived, isomerically pure β-farnesene to investigate the structure-activity
relationship (SAR) of squalene analogues as vaccine adjuvant components. As the β-farnesene feedstock is derived from sugarcane by yeast fermentation10, the terpene moieties of these
molecules are renewable and sustainably produced. Additional terpenoids were obtained from alternative non-animal sources, including plants or synthetic chemistry. Our initial investigation
started with two kinds of farnesene dimers: dehydroisosqualene (DHIS) and farnesene thermal dimers (Table 1). DHIS is structurally similar to squalene with one extra double bond and some
differences in the location and geometry of the double bonds in the center of the molecule. Similarly, farnesene thermal dimers each feature a farnesyl analogue and a geranyl sidechain (C10)
group in common with squalene with different hydrocarbon groups connecting the two chains. Preliminary evaluation of these two materials formulated in oil-in-water emulsions encouraged
further exploration of the structure-activity relationships of additional compounds similar to squalene. Specifically, we sought to assess the impact of (1) variations in the overall length
of terpene analogues, (2) conformationally restricted versions of squalene to learn about the preferred geometry of the pharmacophore, (3) saturation of the double bonds in the squalene
structure, (4) hydrophobicity (logP) and charge on the activity of a series of analogues, and (5) inserting chain-extending core substituents to increase the overall length of the molecule.
We formulated terpenoids with emulsifiers and other excipients under high-pressure homogenization to generate oil-in-water nanoemulsions. Following assessment of emulsion physicochemical
stability, the biological activity of the emulsions was evaluated by measuring innate immune responses in human whole blood stimulated with the emulsions as well as by characterizing
adaptive immune responses in mice immunized with a split, inactivated influenza vaccine mixed with the emulsions. We incorporated multiple immunological readouts into a desirability function
to provide an overall rank score for each compound compared to shark squalene. This work identifies non-shark-derived triterpenoids as vaccine adjuvant raw materials with promising
stability and biological activity profiles, elucidates structure-function relationships for squalene-like terpenoids, and represents a major step forward from our previous reports of
potential squalene alternatives for pharmaceutical use13,14,15. RESULTS COMPOUND SYNTHESIS AND CHARACTERIZATION The structures of synthesized compounds are shown in Table 1. Synthesis
procedures, characterization results, and International Union of Pure and Applied Chemistry (IUPAC) names for each compound are described in the Supplementary Information (Supplementary
Methods and Supplementary Table 1). In general, purified compounds were characterized by gas chromatography with flame ionization detection (GC-FID), high-performance liquid chromatography
(HPLC), 1H and 13 C nuclear magnetic resonance (NMR), and mass spectrometry (MS). All synthesized compounds achieved a purity of ≥90% as determined by GC-FID or HPLC-MS, with the exception
of two compounds that were obtained at 82–85% purity (Table 1). Plant-derived solanesol was obtained from a commercial vendor at a purity of ≥93% as reported by the manufacturer using HPLC.
EMULSION FORMULATION AND PHYSICOCHEMICAL STABILITY Terpenoid oils were formulated with excipients that comprise the previously reported composition known as stable emulsion (SE), consisting
of a mixture of emulsifiers (dimyristoyl phosphatidylcholine (DMPC) and poloxamer 188), an antioxidant agent (α-tocopherol), a tonicity agent (glycerol), and a buffer system (25 mM ammonium
phosphate, pH 5.8), and processed by high-pressure homogenization to generate 4% v/v oil-in-water emulsions16. A non-terpenoid SE based on a long-chain triglyceride oil (grapeseed) that was
previously shown to have no immunostimulatory properties was included as a negative control15,17. An alternative surfactant composition based on the adjuvant emulsion known as MF59 was
employed for some terpenoids (consisting of polysorbate 80, sorbitan trioleate, and citrate buffer3). Emulsion droplet diameter and polydispersity index among all successfully formulated
oils were highly similar to the shark squalene and long-chain triglyceride emulsion controls for SE and MF59-like preparations (Table 2 and Supplementary Figs. 1–2). For instance, droplet
sizes of all successfully filtered SE formulations were 71–126 nm compared to 91 nm for shark squalene SE. Likewise, droplet sizes of MF59-like compositions were 151–199 nm compared to 144
nm for shark squalene MF59-like emulsion. Some terpenoid oils were not successfully formulated in the SE excipient composition: compounds 1 and ether 6 were too viscous to be formulated, and
acids C and diols B resulted in emulsions with average droplet diameters >200 nm and high polydispersity index values, which prevented terminal filtration through a 0.22-µm
polyethersulfone membrane. Where possible, alternative approaches were devised to circumvent these challenges. Thus, in the case of diols B, acceptable emulsification (droplet diameter
<200 nm) was achieved by replacing the SE excipient composition described above with the MF59-like composition. Similarly, diesters B—which is structurally related to diols B—was
formulated in the MF59-like excipient composition. In the case of ether 6, the oil viscosity was decreased by mixing 1:1 v:v with the long-chain triglyceride oil. Emulsions generally
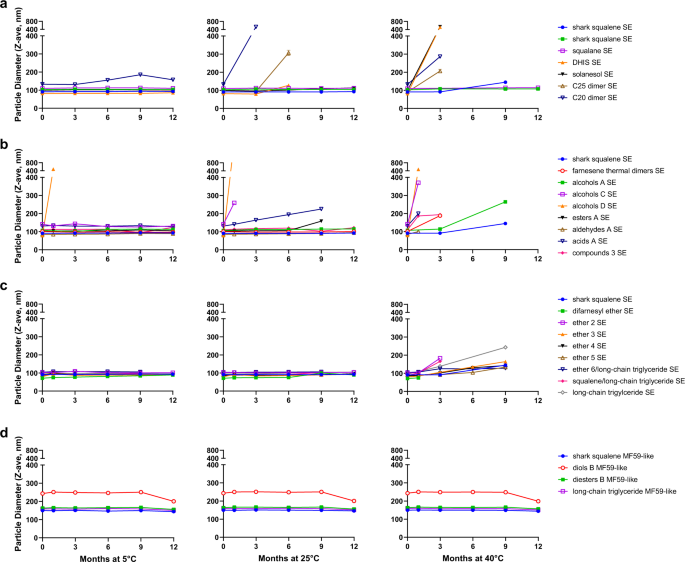
exhibited negative zeta potentials (Table 2). For successfully formulated emulsions, a minimum stability benchmark of <20% droplet diameter growth after storage for 3 months at 5 °C was
established to determine which emulsions should be tested for biological activity. The average droplet diameter and polydispersity were monitored over time on emulsions stored at 5 °C, 25
°C, and 40 °C (Fig. 1 and Supplementary Fig. 2). Many terpenoid oil emulsions demonstrated comparable physicochemical stability as shark-derived squalene emulsion when stored at 5 °C and 25
°C. At 40 °C, squalane SE formulations and all MF59-like compositions demonstrated the most robust physical stability, followed by the shark squalene SE, the ether SEs, and alcohols A SE.
Terpenoid oils that resulted in physically unstable emulsions as evidenced by increasing droplet diameter, polydispersity index, and/or phase separation were removed from further stability
testing. SEs made with C20 dimer, C25 dimer, DHIS, solanesol, acids A, alcohols C, or esters A were less physically stable than the shark squalene SE at elevated temperatures. At 5 °C
storage, alcohols D emulsion was physically unstable within 1 month following manufacture, and compounds 3 emulsion evidenced visible particulates after ~7 months. Finally, a selection of
emulsified oils was monitored for chemical stability by HPLC with charged aerosol detection (Supplementary Fig. 2). In this regard, solanesol demonstrated comparable chemical stability to
shark squalene and squalane at all temperatures. IN VITRO INNATE IMMUNE STIMULATION ACTIVITY Emulsions demonstrating physical stability (<20% growth in diameter) for a minimum of 3 months
when stored at 5 °C were selected for evaluation of innate immunomodulatory activity on whole blood from human subjects. Three experimental sets of emulsions were incubated with heparinized
blood for 18–24 h at 37 °C, and supernatants were subsequently assessed for production of interleukin (IL)-8, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1β
(MIP-1β), and IL-6 cytokines. These cytokines were selected as potential indicators of innate stimulation rather than a comprehensive profile of all cytokine responses. Various terpenoid oil
emulsions demonstrated comparable or enhanced dose-dependent innate immune stimulation compared to the shark squalene emulsion, whereas other terpenoid oil emulsions resulted in little or
no innate immune stimulation (Fig. 2). In some cases (e.g., C20 dimer SE, compounds 3 SE, and diols B MF59-like), emulsions elicited high cytokine levels at low doses but little or no
cytokine induction at high doses. To assess cytolytic behavior, selected emulsions were evaluated for their effect on viability of human peripheral blood mononuclear cells (PBMCs). Several
emulsions along with shark squalene SE had a minimal impact on PBMC viability, whereas some emulsions (C20 dimer SE, compounds 3 SE, diols B MF59-like, and acids A SE) substantially
decreased cell viability (Supplementary Fig. 3). IN VIVO ADJUVANTED INFLUENZA VACCINE IMMUNOGENICITY In preparation for vaccination studies in the mouse model, selected emulsions were mixed
with split, inactivated H5N1 vaccine antigen and monitored for physicochemical compatibility for up to 24 h after mixing, with emulsion droplet diameter measured by dynamic light scattering
and antigen conformation assessed by single radial immunodiffusion (SRID). Adjuvant particle size and antigen conformation demonstrated only minor changes 24 h after mixing (Supplementary
Fig. 4). In order to determine an optimal antigen dose for revealing the impact of an adjuvant, female C57BL/6 mice (5/group) were immunized intramuscularly with 1, 0.1, or 0.01 µg of
hemagglutinin (HA) equivalent in the split, inactivated H5N1 vaccine, with or without the shark squalene emulsion. The ability of the shark squalene emulsion to significantly enhance
antigen-specific serum antibody titers, functional hemagglutination inhibition (HAI) titers, and long-lived antibody-secreting plasma cells was most evident at the 0.01-µg HA dose
(Supplementary Fig. 5). Based on their stability performance and in vitro bioactivity, 17 emulsions with appropriate controls were selected for in vivo evaluation with split, inactivated
H5N1 antigen. Using 0.01 µg as the antigen dose based on the previous study, C57BL/6 mice (4 male and 4 female mice per group) were immunized intramuscularly at Day 0 and Day 21 with
influenza vaccine antigen alone or the vaccine antigen mixed with the indicated oil-in-water emulsions at 2% v/v oil in three separate experiments, each involving a different experimental
set of oils (Supplementary Fig. 6 and Supplementary Table 2). Three weeks following the first immunization, blood was collected to assess antigen-specific serum antibody titers and
functional serum HAI titers. Three weeks following the second immunization, blood, bone marrow, and spleen samples were acquired to assess antigen-specific serum antibody titers and
functional serum HAI titers, long-lived antibody-secreting plasma cells, and cytokine production from antigen-stimulated splenocytes, respectively. After two immunizations, various terpenoid
emulsions elicited significantly enhanced antigen-specific serum immunoglobulin (Ig)G, HAI, and/or long-lived antibody-secreting plasma cells compared to vaccine antigen alone, and
comparable (or enhanced in the case of acids A SE) to the effects of shark squalene emulsion (Fig. 3). Although significant augmentation of antigen-specific serum IgG isotypes (IgG1 and/or
IgG2c) was evident for many emulsions, there was no statistically significant shift in the IgG2c/IgG1 titer ratio (Supplementary Fig. 7), and cytokine levels from stimulated splenocytes were
generally low, confirming that there was no definitive shift in TH bias of the immune response (Supplementary Fig. 8). Immune responses measured 3 weeks after a single immunization
exhibited trends consistent with the results obtained following the second immunization (Supplementary Fig. 9). To generate a meaningful overall ranking of the emulsions tested, involving
multiple in vivo immunological readouts and three separate in vivo experiments, we employed a desirability function approach adapted from previous reports18,19. Briefly, data from each
readout were log-transformed, the average values from each experimental group were ratioed to the average shark squalene emulsion value from the same experiment, and the resulting values
were normalized on a unitless scale of 0–1. A weighted composite desirability score was then calculated using the equation \(D = \root {p} \of {{d_1^{w_1} \times d_2^{w_2} \times \ldots
\times d_n^{w_n}}}\) where _d__i_ = partial desirability attributed to the _i_th immunological response (_i_ = 1; 2;…; _n_), _w__i_ = weighting attributed to the _i_th response, and _p_ =
\(\mathop {\sum}\nolimits_1^n {w_i}\)20. The weighting system was designed to rank the various immunological readouts, with 5 representing the greatest importance and 1 representing the
least importance (Table 3). For example, HAI titers are considered a correlate of protection for influenza vaccines21 and were thus given the highest weight. Nevertheless, the weighting
approach is subjective and may be tailored based on the desired target immune profile. Thus, an editable desirability function spreadsheet is located in the Supplementary Data, allowing user
modification of the weighting factors to determine the impact on the overall desirability ranking of the terpenoid formulations tested here. The partial and composite desirability scores
representing all formulations tested in vivo are shown in Fig. 4, employing the weighting scheme from Table 3. The desirability scores for the shark squalene emulsion groups from each
experiment are by definition the same. The composite desirability ranking can be used to broadly categorize emulsions that elicited enhanced, equivalent, or reduced immune responses compared
to shark squalene emulsion. Thus, emulsions made with acids A, farnesene thermal dimers, solanesol, or alcohols A performed substantially or somewhat better than shark squalene emulsion
(>10% improvement in composite desirability score); emulsions made with aldehydes A, alcohols C, diols B, DHIS, ether 4, difarnesyl ether, ether 5, C25 dimer, esters A, and ether 3
performed similar to shark squalene emulsion (≤10% change in composite desirability score); and emulsions made with long-chain triglyceride, ether 2, C20 dimer, shark squalane, squalane, or
without any emulsion (antigen alone) performed poorly compared to shark squalene emulsion (>10% decrease in composite desirability score). Moreover, partial desirability scores
representing specific immunological readouts indicate that rapid antibody immune responses were elicited by acids A and farnesene thermal dimers emulsions, whereas solanesol emulsion may
favor response durability and aldehydes A the highest IgG2c/IgG1 ratio. CORRELATIONS Several structural features correlating with changes in adjuvant activity in vivo were identified. First,
for unsaturated linear terpenoid emulsions, adjuvant activity was strongly associated with chain length (represented by cLogP, molecular weight, or number of carbons, see Fig. 5a and
Supplementary Fig. 10a, b). Thus, C20 and C25 analogues of DHIS showed lower composite desirability scores than squalene or DHIS, whereas the C45 solanesol substantially enhanced responses,
with a ranking of C20 dimer < C25 dimer < C30 squalene < C30 DHIS < C45 solanesol. Second, we considered that conformationally restricted analogues such as acids A, alcohols A,
aldehydes A, alcohols C, and esters A may impact bioactivity since they are more similar to the extended conformation of squalene at C11-C12 rather than a folded conformation at C11-C12
(squalene can exist in both conformations, see Supplementary Fig. 11). Clearly, this set of compounds has members with good to excellent activity (Fig. 4), indicating that the C11-C12 folded
conformation is not required for activity. Thus, these results support the C11-C12 extended form as the active conformation of squalene. Third, dramatically reduced adjuvant activity was
associated with chain saturation as evidenced by comparing the composite desirability scores of unsaturated shark squalene emulsion with emulsions made from saturated squalane (shark-derived
or semi-synthetic, see Table 1 and Fig. 4). Nevertheless, alterations in the double bond configuration did not appear to have detrimental impacts to bioactivity, as evidenced by the highly
similar composite desirability scores of shark squalene and DHIS emulsions. Fourth, the influence of charge was dramatic, with acids A SE achieving the highest composite desirability score
(Fig. 4). Unfortunately, the other charged compounds did not generate stable emulsions and were thus not evaluated for bioactivity. Fifth, a range of different connecting chain lengths in a
series of a mono ether and diethers of farnesyl chloride made by alkylation of alcohols and phenols was tolerated, with some emulsified compounds demonstrating similar activity to shark
squalene emulsion (Supplementary Table 3). Interestingly, the geometry of main chain linkage to the central ring structure had a substantial impact on composite desirability score, with
ether 5 (1,2-linkage) outperforming ether 2 (1,3-linkage). Moreover, adjuvant activity was at least maintained and possibly enhanced with the introduction of a central ring structure: for
instance, the cyclohexene-containing farnesene thermal dimers exhibited a higher composite desirability score than shark squalene or DHIS, and the benzene-containing ether 5 performed
similarly to ether 4 or shark squalene (Fig. 4). However, for uncharged cyclohexene-containing terpenoids, the molecular weight of the side chain conjugated to the cyclohexene was inversely
correlated with the composite desirability score (Fig. 5b), indicating a steric effect could be causing activity reduction. Although several terpenoid emulsions stimulated innate responses
from human cells in vitro and adaptive responses from immunized mice in vivo comparable to shark squalene emulsion, there was not a strong correlation between these readouts covering all
emulsions tested. For instance, the top performer in the mouse immunogenicity studies was the acids A emulsion, but little or no cytokine stimulation was evident when this emulsion was
incubated with human whole blood. On the other hand, the C20 dimer emulsion was extremely potent in vitro at low doses but was a poor performer in vivo. Nevertheless, when the analysis is
limited to linear unsaturated terpenoids, a correlation between the in vivo composite desirability score and the in vitro MCP-1 cytokine stimulation was identified (Fig. 5c). Moreover, for
all SE formulations, we identified an inverse correlation between in vitro cytokine production and emulsion particle size (Fig. 5d and Supplementary Fig. 10c). Thus, the ability to broadly
predict adaptive in vivo immune response from the innate in vitro cytokine stimulation profile is limited, in part because in vitro results may be confounded by the effect of emulsion
particle size. DISCUSSION Evaluation of adjuvant activity of squalene analogues other than squalane has only recently been reported22 despite the importance of squalene as an adjuvant
component in commercial vaccines. For instance, the shark squalene emulsion MF59 is approved for use in a commercial influenza vaccine and boosts responses in elderly and pediatric
populations23. The lack of tested analogues may be due in part to the structure of squalene, which is a highly symmetrical molecule with six nearly equivalent trisubstituted double bonds,
meaning that attempting to achieve a highly selective industrially acceptable functionalization is not likely to succeed. However, the procedures described in the present report using
yeast-produced β-farnesene as a starting material enabled the synthesis of a set of squalene-like triterpenoid analogues to explore structure-activity relationships. Maintenance of
biological activity—despite terpenoid structural changes—is consistent with a previous report evaluating two novel triterpenoids22, and the reduced biological activity of squalane compared
to squalene is consistent with a previous report that correlated biological activity with ability to induce reactive oxygen species24 but contradicts an earlier report describing squalene
and squalane as equally effective vaccine adjuvant emulsion components25. In any case, the present study describes systematic structural changes other than chain saturation that resulted in
substantial loss or enhancement of biological activity, thus informing a new structure-activity based understanding of squalene-like molecules. Additional work will be needed to refine the
structure-activity map of squalene-like molecules. In particular, since the negatively charged acids A compound was the top performing molecule in terms of the mouse immunogenicity composite
desirability score, additional study is warranted regarding the effect of charge on terpenoid adjuvant activity. A possible explanation for this striking bioactivity enhancement is that the
salt of the acid may cause reordering of emulsion droplet morphology such that the anion of acids A is localized at the oil-water interface with increased bioavailability. Alternatively, it
could be considered that acids A is substantially less lipophilic than the other molecules tested. All of the other molecules tested in vivo were highly lipophilic. The only other molecules
with substantial hydrophilic character (acids C, compounds 1, and compounds 3) were not evaluated in vivo due to emulsion instability, although compounds 3 emulsion showed interesting in
vitro bioactivity. While acids A SE was the most potent formulation in the in vivo studies, it was not as physically stable as other leading candidates. Indeed, it is important to note that
the phospholipid/surfactant emulsification systems employed here were previously optimized for shark squalene14,26,27,28,29. Thus, it is highly possible that development of optimized
emulsifier compositions could generate emulsions with more suitable stability profiles for the leading candidates identified here, and additional work is merited in this regard.
Nevertheless, most candidates were stable when stored at 5 °C, and long-term stability at elevated temperatures was demonstrated for several candidates (Fig. 1). Another potential benefit of
formulation optimization would be to reduce cell viability loss evident with some candidates in the in vitro assays. While some cell necrosis and/or death may represent important mechanisms
of action for squalene emulsions and other adjuvants30,31,32, formulation development may optimize both adjuvant activity and biocompatibility. The best example of this type of scenario is
the saponin adjuvant QS-21, which is highly hemolytic unless formulated in cholesterol-containing platforms such as liposomes33. We considered the possibility that the bioactivity of
aldehydes A might be enhanced if the aldehyde could provide a costimulatory signal by forming an imine bond with a T cell surface receptor amino group similar to a proposed mechanism for the
saponin QS-2134,35, but no benefit was apparent in this regard (Fig. 4). Indeed, cellular responses overall were modest in our studies, and it is possible that inclusion of additional
adjuvant components (such as TLR ligands) might be required for more potent T cell-based immunity. Despite generating and evaluating the immunogenicity of multiple compounds, we recognize
that additional testing would be necessary in future to advance such compounds as alternatives to shark squalene in vaccine products. For instance, protective efficacy testing in larger
animal models of selected lead compounds would be necessary to establish that they are suitable replacements of shark squalene. Moreover, elucidation of specific mechanisms of action would
be desirable in future for comparison to the various reported mechanisms of shark squalene emulsions. However, such efforts were outside the scope of the present report. Another limitation
of the current work is that a semi-synthetic process for generating squalene itself was not reported as work is ongoing in this regard. Semi-synthetic approaches to the manufacture of
pharmaceuticals have been practiced for decades. Possibly the best-known examples are the semi-synthetic β-lactam antibiotics whose synthesis commences with a natural product (penicillin G)
from _Penicillium chrysogenum_36,37. The approach we have taken is that the fermentation-derived precursor, β-farnesene, is the product of metabolic engineering and development of its
industrial fermentative production from engineered yeast. The ready availability of the β-farnesene precursor enabled us to synthesize a range of squalene analogues that would have been
inaccessible had the tools of synthetic biology not been applied to engineering and industrializing yeast for its production. The syntheses employed are industrially scalable, and—given the
ready availability of the precursor (β-farnesene)—these compounds could be produced at commercial scale. Although synthesis of solanesol was not attempted here, we note that this molecule
showed promising emulsion stability and adjuvant activity, is commercially available from solanaceous plants, and is an intermediate for the synthesis of coenzyme Q10 and vitamin K2. Shark
populations are under severe ecological pressure, and an alternative source of a terpenoid adjuvant to replace shark-derived squalene would be environmentally beneficial. In summary, we
designed and synthesized >20 distinct terpenoids using a sustainable semi-synthetic approach and compared vaccine adjuvant activity of the emulsified terpenoids to shark squalene
emulsion. Several of the compounds (e.g., alcohols A, farnesene thermal dimers, diols B, ether 4, and ether 5) possess similar or improved adjuvant activity properties compared to shark
squalene as well as acceptable physicochemical stability, which could make them promising alternatives for shark squalene in vaccine adjuvant formulations for both human and veterinary
usage. Furthermore, changes in squalene analogue structure were correlated with biological activity. Thus, these results demonstrate a path for obtaining squalene-like molecules from
sustainable non-animal sources and for systematically mapping their structure-activity relationship in vaccine adjuvant emulsions. METHODS EXPERIMENTAL DESIGN The objectives of the study
were to design and generate multiple terpenoid compounds using sustainable approaches and evaluate them for emulsion stability and vaccine adjuvant bioactivity compared to shark-derived
squalene emulsion. Pre-specified criteria included a target of ≥90% compound purity as determined by GC-FID or HPLC-MS and a physical emulsion stability requirement of <20% size growth
after 3 months storage at 5 °C. COMPOUND SYNTHESIS AND CHARACTERIZATION Detailed synthesis, characterization information, and IUPAC names for each compound are located in the Supplementary
Information. EMULSION FORMULATION Plant-derived solanesol was obtained from TCI America. Shark-derived squalene, squalane, and sorbitan trioleate were obtained from Sigma-Aldrich. Napa
Valley Naturals (Stonewall Kitchen) grapeseed oil was purchased from a local grocery store. DMPC was obtained from Lipoid. Poloxamer 188, α-tocopherol, and glycerol were purchased from
Spectrum Chemical. Polysorbate 80 was obtained from NOF. Buffer components were obtained from J.T.Baker and Fluka. Split, inactivated H5N1 (A/Vietnam/1194/2004) was obtained from the
National Institute for Biological Standards and Control (NIBSC). For the SE compositions, terpenoid oils were formulated with a mixture of emulsifiers (DMPC and poloxamer 188), an
antioxidant agent (α-tocopherol), a tonicity agent (glycerol), and a buffer system (25 mM ammonium phosphate, pH 5.8) and processed by high-shear mixing and high-pressure homogenization to
generate 4% v/v oil-in-water nanoemulsions16. For the MF59-like compositions, terpenoid oils were formulated with a mixture of non-ionic surfactants (polysorbate 80 and sorbitan trioleate)
and a buffer system (10 mM citrate, pH 6.0), and processed by high-shear mixing and high-pressure homogenization to generate 4% v/v oil-in-water nanoemulsions27. IN VITRO CYTOKINE
STIMULATION ASSAY Fresh heparinized whole blood from 6–12 human volunteers (equal numbers of male and female) was obtained from Bloodworks Northwest. Biological sample collections were
approved by WCG IRB. All participants reviewed and signed informed consent forms. Emulsions at the indicated % v/v concentrations (diluted with saline) were incubated with the blood for
18–24 h at 37 °C and 5% CO213,15. After incubation, supernatants were aspirated and assessed by enzyme-linked immunosorbent assay (ELISA) kits for production of IL-6, IL-8, MCP-1 (Life
Technologies #88-7066-77, 88-8086-77, 88-7399-77, respectively), and MIP-1β (R&D Systems #DY271) cytokines according to manufacturer’s instructions. Cytokine levels that were below the
lowest concentration of the standard curve were assigned half the value elicited by the lowest standard curve concentration. MICE, IMMUNIZATIONS, AND SAMPLE COLLECTION C57BL/6 (B6) mice were
purchased from The Jackson Laboratory. Experimental groups consisted of equal numbers of 6–8-week-old male and female mice. Mice were immunized by intramuscular injection of 100 µL total
volume (50 µL in each hind leg) of vaccine containing split, inactivated H5N1 A/Vietnam/1194/2004 (NIBSC) at the indicated dose and 2% v/v oil-in-water emulsion at Study Day 0 and Study Day
21. All animal experiments were performed in accordance with national and institutional guidelines for animal care of laboratory animals and approved by the Infectious Disease Research
Institute Institutional Animal Care and Use Committee. BLOOD AND TISSUE COLLECTION AND PROCESSING Peripheral blood was collected at Study Day 21 by the retro-orbital route (prior to the
boost immunization) under light sedation using isoflurane and at Study Day 42 via cardiac puncture after euthanasia. Blood was collected in Sarstedt Microvette capillary blood collection
tubes and centrifuged to separate the serum. Serum was stored at −20 °C until analysis. On Study Day 42, mice were euthanized by controlled administration of carbon dioxide inhalation,
followed by cervical dislocation, and spleens and bone marrow were removed aseptically. Lymphocytes were isolated from the spleen using manual disruption. Red blood cells contained in
spleens were lysed using Red Blood Cell Lysis Buffer (eBioscience). Lymphocytes were used for cytokine secretion ELISA (interferon (IFN)-γ and IL-5) and cytokine ELISpot (IFN-γ and IL-5)
assays as described below. Bone marrow was exposed by snipping ends of harvested femurs followed by rinse and centrifugation cycles in complete Roswell Park Memorial Institute (RPMI) medium
(consisting of RPMI medium supplemented with 10% v/v fetal bovine serum (FBS) and penicillin-streptomycin). The red blood cells were removed with Red Blood Cell Lysis Buffer (eBioscience).
Bone marrow-derived cells were used for B-cell ELISpot assay as described below. SERUM AND CYTOKINE ELISA Antigen-specific total IgG (IgGT), IgG1, and IgG2c were measured in the serum
samples from the immunized animals using antibodies purchased from Southern Biotech (#1031-05, 1070-05, and 1079-05, respectively) that were diluted 1:5000, 1:3000, and 1:2000, respectively.
Briefly, 384-well plates were coated with 1 µg/mL recombinant H5 A/Vietnam/1194/2004 antigen (Sino Biological) overnight. The next day, plates were washed and blocked with
phosphate-buffered saline (PBS) with 0.1% polysorbate 20 and 1% bovine serum albumin (BSA). After washing, plates were incubated with the serum followed by incubation with horseradish
peroxidase (HRP)-conjugated antibodies and 3,3',5,5'-tetramethylbenzidine (TMB) substrate. The reaction was stopped using 1 N H2SO4 and read within 30 min using a Victor _X_4
(PerkinElmer) plate reader. Endpoint titers were interpolated using a sigmoidal curve fit and an arbitrary cutoff value of 0.5 or 0.75 depending on background signal. Endpoint titers below
the standard curve range were assigned the value corresponding to 0.5_x_ of the lowest standard curve dilution. Splenocytes from immunized animals were stimulated with 10 µg/mL recombinant
H5 A/Vietnam/1194/2004 antigen (Sino Biological) for 72 h. IFN-γ and IL-5 were quantified in the supernatant by ELISA, according to the manufacturer’s instructions (Invitrogen #88-7314-88
and #88-7054-88, respectively). HAI ASSAY Serum samples were treated with receptor destroying enzyme (RDE) at a ratio of 1:3 v:v (sample:RDE) in a 96-well V-bottom plate. Following overnight
incubation at 37 °C, samples were centrifuged at 400 x g for 1 min, and 6 parts of PBS were added for a final serum dilution of 1:10. Horse red blood cell (HRBC) suspension (10% HRBC) was
prepared by washing with PBS three times, resuspending to a final concentration of 1% HRBC, and storing at 4 °C for same-day use. Treated serum samples or control sera were added to 96-well
V-bottom plates in duplicate, after which 25 µL of split, inactivated H5N1 A/Vietnam/1194/2004 (NIBSC) (67 µg/mL stock diluted 1:200 in PBS) were added to each well. Plates were then
incubated at ambient temperature for 15 min, after which 50 µL of 1% HRBC suspension were added to each well. Plates were then further incubated at ambient temperature undisturbed for 30–60
min. The HAI titer is the reciprocal of the greatest dilution that completely inhibits the agglutination of the HRBCs as indicated by the absence of a tear-shaped streaming of erythrocytes
upon tilting of the plate. BONE MARROW PLASMA CELL ELISPOT ELISpot plates (Millipore) were coated with 2 µg/mL recombinant H5 A/Vietnam/1194/2004 antigen (Sino Biological) and incubated
overnight at 4 °C. Plates were washed with PBS with 0.1% v/v polysorbate 20 (PBST), blocked with complete RPMI medium for 2 h at ambient temperature, and then washed again. The first and
third experimental set of oils demonstrated high viability cell preparations (>66%), and single-cell suspensions were seeded at 1.0 × 106 cells per well with 3-fold serial dilutions. The
second set of experimental oils demonstrated low viability cell preparations (mean value: 21%), so data from single-cell suspensions were only included if the seeded cell count before
dilution was above 1.0 × 104 cells per well, with a multiplication factor employed to normalize to a 1.0 × 106 cell count. The plates were incubated at 37 °C with 5% CO2 for 3 h, washed with
PBST, then incubated overnight at 4 °C with HRP-conjugated anti-mouse IgG (H + L) (Southern Biotech #1031-05) diluted 1:1000. After incubation, the plates were washed with PBS and developed
with 3-amino-9-ethylcarbazole (AEC) substrate kits (Vector Laboratories) according to the manufacturer’s protocol. The reaction was stopped by washing the plates with deionized water,
plates were dried in the dark, and spots were counted using an automated ELISpot reader (CTL Analyzer, Cellular Technology Limited). Data were analyzed using ImmunoSpot version 7
professional software (Cellular Technology Limited). SPLENOCYTE CYTOKINE ELISPOT ELISpot plates were coated with IFN-γ (BD Biosciences #551881 or eBioscience #88-7384) and IL-5 (BD
Biosciences #551880 or eBioscience #88-7825) capture antibodies, diluted 1:200, overnight at 4 °C. Plates were washed with PBST, blocked with complete RPMI medium for 2 h at ambient
temperature, and then washed again. Splenocytes were plated at 2.0 × 105 cells per well and were stimulated with 10 µg/mL recombinant H5 A/Vietnam/1194/2004 antigen (Sino Biological) at 37
°C with 5% CO2 for 48 h. The plates were washed with PBST, detection antibodies (BD Biosciences #551881 and #551880 or eBioscience #88-7384 and #88-7825), diluted 1:250, were added, and the
plates were then incubated overnight at 4 °C. After incubation, plates were washed with PBST, and Avidin D (Av)-HRP (Invitrogen #50-112-3249), diluted 1:250, was added for a 45-min
incubation at ambient temperature followed by a PBS wash. The plates were developed with AEC substrate kits according to the manufacturer’s protocol. The reaction was stopped by washing the
plates with deionized water, plates were dried in the dark, and spots were counted using an automated ELISpot reader (CTL Analyzer). Data were analyzed using ImmunoSpot version 7
professional software (Cellular Technology Ltd). STATISTICAL ANALYSIS Adaptive immunity responses measured in vaccinated animals were log-transformed as indicated and then assessed for
distribution normality by the D’Agostino-Pearson test (for group sizes of _n_ = 8) or the Shapiro-Wilk test (for group sizes _n_ < 8). Normally distributed data were compared by one-way
ANOVA with Sidak’s correction for multiple comparisons, whereas non-normally distributed data were evaluated by the non-parametric Kruskal–Wallis test with Dunn’s correction for multiple
comparisons as indicated in figure legends. Pearson’s correlation coefficient was computed on normally distributed data (tested by D’Agostino-Pearson or Shapiro-Wilk tests as described
above). Statistical analysis was performed using GraphPad Prism version 9.3.1. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary
linked to this article. DATA AVAILABILITY The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Materials
were transferred between institutions under a Material Transfer Agreement (MTA), and MTAs would be required for any sample requests. REFERENCES * Ajikumar, P. K. et al. Terpenoids:
Opportunities for biosynthesis of natural product drugs using engineered microorganisms. _Mol. Pharm._ 5, 167–190 (2008). Article CAS PubMed Google Scholar * Kim, S. K. & Karadeniz,
F. Biological importance and applications of squalene and squalane. _Adv. Food Nutr. Res._ 65, 223–233 (2012). Article PubMed Google Scholar * Fox, C. B. & Haensler, J. An update on
safety and immunogenicity of vaccines containing emulsion-based adjuvants. _Expert Rev. Vaccines_ 12, 747–758 (2013). Article CAS PubMed Google Scholar * O’Hagan, D. T., van der Most,
R., Lodaya, R. N., Coccia, M. & Lofano, G. “World in motion”—emulsion adjuvants rising to meet the pandemic challenges. _NPJ Vaccines_ 6, 158 (2021). Article PubMed PubMed Central
Google Scholar * Fox, C.B., Carter, D., Kramer, R.M., Beckmann, A.M. & Reed, S.G. Current status of TLR4 ligand vaccine adjuvants. in _Immunopotentiators in Modern Vaccines_ Edn. 2nd
(eds. O’Hagan D.T. & Schijns V.) 105–127 (Academic Press, Cambridge, 2017). * Pacoureau, N. et al. Half a century of global decline in oceanic sharks and rays. _Nature_ 589, 567–571
(2021). Article CAS PubMed Google Scholar * Paddon, C. J. & Keasling, J. D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. _Nat.
Rev. Microbiol._ 12, 355–367 (2014). Article CAS PubMed Google Scholar * Paddon, C. J. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. _Nature_ 496,
528–532 (2013). Article CAS PubMed Google Scholar * Meadows, A. L. et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. _Nature_ 537, 694–697 (2016).
Article CAS PubMed Google Scholar * Benjamin, K. R., Silva, I. R., Cherubim, J. O. P., McPhee, D. & Paddon, C. J. Developing commercial production of semi-synthetic artemisinin, and
of ²-Farnesene, an isoprenoid produced by fermentation of Brazilian sugar. _J. Braz. Chem. Soc_. 27, 1339–1345 (2016). CAS Google Scholar * McPhee, D., Pin, A., Kizer, L. & Perelman,
L. Deriving renewable squalane from sugarcane. _Cosmet. Toiletries_ 129, 20–26 (Allured Business Media, 2014). * Orr, M. T. & Fox, C. B. AS03 stresses out macrophages: commentary on
‘Activation of the endoplasmic reticulum stress sensor IRE1α by the vaccine adjuvant AS03 contributes to its immunostimulatory properties’. _npj Vaccines_ 3, 27 (2018). Article PubMed
PubMed Central Google Scholar * Adlington, K. et al. Molecular design of squalene/squalane countertypes via the controlled oligomerization of Isoprene and evaluation of vaccine adjuvant
applications. _Biomacromolecules_ 17, 165–172 (2016). Article CAS PubMed Google Scholar * Fox, C. B. et al. Monitoring the effects of component structure and source and formulation
stability and adjuvant activity of oil-in-water emulsions. _Coll. Surf. B: Biointerfaces_ 65, 98–105 (2008). Article CAS Google Scholar * Fox, C. B. et al. Vaccine adjuvant activity of
emulsified oils from species of the Pinaceae family. _Phytomedicine_ 64, 152927 (2019). Article CAS PubMed PubMed Central Google Scholar * Misquith, A. et al. In vitro evaluation of
TLR4 agonist activity: formulation effects. _Coll. Surf. B: Biointerfaces_ 113, 312–319 (2014). Article CAS Google Scholar * Fox, C. B., Baldwin, S. L., Duthie, M. S., Reed, S. G. &
Vedvick, T. S. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. _Vaccine_ 29, 9563–9572 (2011). Article CAS PubMed PubMed Central Google Scholar *
Abhyankar, M. M. et al. Optimizing a multi-component intranasal _Entamoeba histolytica_ vaccine formulation using a design of experiments strategy. _Front. Immunol._ 12, 683157 (2021).
Article CAS PubMed PubMed Central Google Scholar * Poncet, D. et al. Preclinical optimization of an enterotoxigenic _Escherichia coli_ adjuvanted subunit vaccine using response surface
design of experiments. _npj Vaccines_ 5, 83 (2020). Article CAS PubMed PubMed Central Google Scholar * Derringer, G. & Suich, R. Simultaneous optimization of several response
variables. _J. Qual. Technol._ 12, 214–219 (1980). Article Google Scholar * Cowling, B. J. et al. Influenza hemagglutination-inhibition antibody titer as a mediator of vaccine-induced
protection for Influenza B. _Clin. Infect. Dis._ 68, 1713–1717 (2019). Article CAS PubMed Google Scholar * Tateno, M. et al. Synthetic biology-derived triterpenes as efficacious
immunomodulating adjuvants. _Sci. Rep._ 10, 17090 (2020). Article CAS PubMed PubMed Central Google Scholar * Black, S. Safety and effectiveness of MF-59 adjuvanted influenza vaccines in
children and adults. _Vaccine_ 33, B3–B5 (2015). Article CAS PubMed Google Scholar * Huang, C.-H., Huang, C.-Y. & Huang, M.-H. Unsaturated squalene content in emulsion vaccine
adjuvants plays a crucial role in ROS-mediated antigen uptake and cellular immunity. _Mol. Pharm._ 15, 420–429 (2018). Article CAS PubMed Google Scholar * Allison, A. C. Squalene and
squalane emulsions as adjuvants. _Methods_ 19, 87–93 (1999). Article CAS PubMed Google Scholar * Fox, C. B., Baldwin, S. L., Duthie, M. S., Reed, S. G. & Vedvick, T. S.
Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions. _AAPS PharmSciTech_ 13, 498–506 (2012). Article CAS PubMed PubMed Central Google Scholar
* Fox, C. B. et al. Adjuvanted pandemic influenza vaccine: variation of emulsion components affects stability, antigen structure, and vaccine efficacy. _Influenza Other Respi Viruses_ 7,
815–826 (2013). Article CAS Google Scholar * Fox, C. B. et al. Effects of emulsifier concentration, composition, and order of addition in squalene-phosphatidylcholine oil-in-water
emulsions. _Pharm. Dev. Technol._ 16, 511–519 (2011). Article CAS PubMed Google Scholar * O’Hagan, D. T., Ott, G. S., Van Nest, G., Rappuoli, R. & Giudice, G. D. The history of MF59
adjuvant: a phoenix that arose from the ashes. _Exp. Rev. Vaccines_ 12, 13–30 (2013). Article Google Scholar * Wang, Y. J., Wu, C. A. & Morrow, W. J. Cell death induced by vaccine
adjuvants containing surfactants. _Vaccine_ 22, 1524–1536 (2004). Article Google Scholar * Yang, Y. W. & Shen, S. S. Enchanced antigen delivery via cell death induced by the vaccine
adjuvants. _Vaccine_ 25, 7763–7772 (2007). Article CAS PubMed Google Scholar * Kim, E. H. et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody
responses, through a RIPK3-dependent pathway. _eLife_ 9, e52687 (2020). Article CAS PubMed PubMed Central Google Scholar * Brunner, L., Barnier-Quer, C. & Collin, N. QS-21 adjuvant:
laboratory-scale purification method and formulation into liposomes in _Vaccine Adjuvants: Methods and Protocols_. (ed. C.B. Fox) 73–86 (Springer New York, NY, 2017). * Soltysik, S. et al.
Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. _Vaccine_ 13, 1403–1410 (1995). Article CAS PubMed Google
Scholar * Lacaille-Dubois, M. A. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: a review. _Phytomedicine_ 60, 152905 (2019). Article CAS
PubMed PubMed Central Google Scholar * Nayler, J. H. C. Semi-synthetic approaches to novel penicillins. _Trends Biochem. Sci._ 16, 234–237 (1991). Article CAS PubMed Google Scholar *
Lynn, B. The semi-synthetic penicillins. _Antibiotica et. Chemotherapia. Fortschr. Adv. Prog._ 13, 125–226 (1965). CAS Google Scholar * Shinagawa, F. B., de Santana, F. C., Araujo, E.,
Purgatto, E. & Mancini-Filho, J. Chemical composition of cold pressed Brazilian grape seed oil. _Food Sci. Technol._ 38, 164–171 (2018). Article Google Scholar Download references
ACKNOWLEDGEMENTS Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under grant
R01AI135673. 100% of the total project costs at $4.1 million was financed with Federal money. The content is solely the responsibility of the authors and does not necessarily represent the
official views of NIH. The authors are grateful to Dr. Valerie Soza for editorial assistance. AUTHOR INFORMATION Author notes * Tony Phan Present address: Neoleukin, Seattle, WA, USA * Hong
Liang & Mark T. Orr Present address: Bristol-Myers Squibb, Seattle, WA, USA * Darrick Carter Present address: HDT Bio Corp., Seattle, WA, USA * Darrick Carter Present address: PAI Life
Sciences Inc., Seattle, WA, USA AUTHORS AND AFFILIATIONS * Amyris, Inc., Emeryville, CA, USA Karl J. Fisher & Christopher J. Paddon * Access to Advanced Health Institute, formerly
Infectious Disease Research Institute, Seattle, WA, USA Robert Kinsey, Raodoh Mohamath, William R. Lykins, Jeffrey A. Guderian, Julie Bakken, David Argilla, Gabi Ramer-Denisoff, Elise
Larson, Yizhi Qi, Sandra Sivananthan & Christopher B. Fox * Infectious Disease Research Institute, Seattle, WA, USA Tony Phan, Hong Liang, Mark T. Orr, Karina Smolyar & Darrick
Carter * Department of Global Health, University of Washington, Seattle, WA, USA Christopher B. Fox Authors * Karl J. Fisher View author publications You can also search for this author
inPubMed Google Scholar * Robert Kinsey View author publications You can also search for this author inPubMed Google Scholar * Raodoh Mohamath View author publications You can also search
for this author inPubMed Google Scholar * Tony Phan View author publications You can also search for this author inPubMed Google Scholar * Hong Liang View author publications You can also
search for this author inPubMed Google Scholar * Mark T. Orr View author publications You can also search for this author inPubMed Google Scholar * William R. Lykins View author publications
You can also search for this author inPubMed Google Scholar * Jeffrey A. Guderian View author publications You can also search for this author inPubMed Google Scholar * Julie Bakken View
author publications You can also search for this author inPubMed Google Scholar * David Argilla View author publications You can also search for this author inPubMed Google Scholar * Gabi
Ramer-Denisoff View author publications You can also search for this author inPubMed Google Scholar * Elise Larson View author publications You can also search for this author inPubMed
Google Scholar * Yizhi Qi View author publications You can also search for this author inPubMed Google Scholar * Sandra Sivananthan View author publications You can also search for this
author inPubMed Google Scholar * Karina Smolyar View author publications You can also search for this author inPubMed Google Scholar * Darrick Carter View author publications You can also
search for this author inPubMed Google Scholar * Christopher J. Paddon View author publications You can also search for this author inPubMed Google Scholar * Christopher B. Fox View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.B.F., C.J.P., D.C., K.J.F., and M.T.O. conceived the study. C.B.F., C.J.P., and M.T.O. supervised the
research. C.B.F., C.J.P., E.L., and S.S. administered the project. C.J.P. provided resources. C.B.F. and C.J.P. acquired funding. D.A., E.L., G.R.-D., H.L., J.A.G., J.B., K.J.F., K.S.,
R.K., R.M., T.P., and W.R.L. contributed to the investigation. C.B.F., C.J.P., E.L., H.L., J.A.G., K.J.F., M.T.O., R.K., T.P., and Y.Q. contributed to methodology. S.S. curated the data.
C.B.F., H.L., J.A.G., K.J.F., M.T.O., R.M., W.R.L., G.R.-D., and T.P. collected and analyzed the data. C.B.F., C.J.P., K.J.F., and R.M. wrote the manuscript. CORRESPONDING AUTHORS
Correspondence to Christopher J. Paddon or Christopher B. Fox. ETHICS DECLARATIONS COMPETING INTERESTS C.B.F., C.J.P., and K.J.F. declare no Competing Non-Financial Interests but the
following Competing Financial Interests. C.J.P. owns shares and possesses stock options in Amyris, Inc. C.B.F. is an inventor on patents and/or patent applications involving terpenoid
emulsion adjuvant formulations. K.J.F. and C.J.P. are inventors on a patent application covering novel compounds for use in adjuvant formulations. All other authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY DATA SUPPLEMENTARY INFORMATION REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fisher, K.J., Kinsey, R., Mohamath, R. _et al._ Semi-synthetic terpenoids with
differential adjuvant properties as sustainable replacements for shark squalene in vaccine emulsions. _npj Vaccines_ 8, 14 (2023). https://doi.org/10.1038/s41541-023-00608-y Download
citation * Received: 28 July 2022 * Accepted: 24 January 2023 * Published: 16 February 2023 * DOI: https://doi.org/10.1038/s41541-023-00608-y SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
