Rapid magnetic isolation of extracellular vesicles via lipid-based nanoprobes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

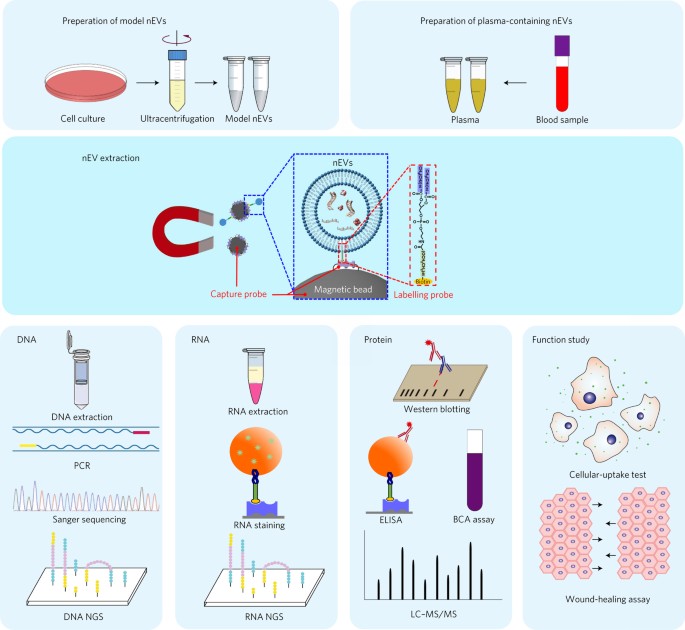
ABSTRACT Extracellular vesicles (EVs) can mediate intercellular communication by transferring cargo proteins and nucleic acids between cells. The pathophysiological roles and clinical value
of EVs are under intense investigation, yet most studies are limited by technical challenges in the isolation of nanoscale EVs (nEVs). Here, we report a lipid-nanoprobe system that enables
spontaneous labelling of nEVs for subsequent magnetic enrichment in 15 minutes, with isolation efficiency and cargo composition similar to what can be achieved by the much slower and bulkier
method of ultracentrifugation. We also show that this approach allows for downstream analyses of nucleic acids and proteins, enabling the identification of _EGFR_ and _KRAS_ mutations
following nEV isolation from the blood plasma of non-small-cell lung-cancer patients. The efficiency and versatility of the lipid-nanoprobe approach opens up opportunities in point-of-care
cancer diagnostics. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12
digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS RAPID PURIFICATION AND MULTIPARAMETRIC CHARACTERIZATION OF CIRCULATING SMALL EXTRACELLULAR VESICLES UTILIZING A LABEL-FREE LAB-ON-A-CHIP DEVICE Article
Open access 25 October 2023 MODELING AND OPTIMIZATION OF PARALLELIZED IMMUNOMAGNETIC NANOPORE SORTING FOR SURFACE MARKER SPECIFIC ISOLATION OF EXTRACELLULAR VESICLES FROM COMPLEX MEDIA
Article Open access 16 August 2023 APPLICATION OF PEPTIDES WITH AN AFFINITY FOR PHOSPHOLIPID MEMBRANES DURING THE AUTOMATED PURIFICATION OF EXTRACELLULAR VESICLES Article Open access 30
October 2020 REFERENCES * Colombo, M., Raposo, G. & Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. _Annu. Rev. Cell. Dev.
Biol._ 30, 255–289 (2014). Article CAS Google Scholar * Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. _J. Cell Biol._ 200, 373–383 (2013).
Article CAS Google Scholar * Cocucci, E. & Meldolesi, J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. _Trends Cell Biol_. 25, 364–372 (2015). Article
CAS Google Scholar * Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. _J. Extracell. Vesicles_ 4, 27066 (2015). Article Google
Scholar * Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. _Nat. Rev. Immunol._ 2, 569–579 (2002). Article CAS Google Scholar * Li, Y., Shen,
Z. & Yu, X.-Y. Transport of microRNAs via exosomes. _Nat. Rev. Cardiol_. 12, 198–198 (2015). Article Google Scholar * Alderton, G. K. Diagnosis: fishing for exosomes. _Nat. Rev.
Cancer_ 15, 453–453 (2015). Article CAS Google Scholar * Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. _Nature_ 523, 177–182 (2015).
Article CAS Google Scholar * Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. _Cell Res._ 24, 766–769 (2014). Article CAS Google Scholar *
Kahlert, C. et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated _KRAS_ and p53 DNA in the serum exosomes of patients with pancreatic cancer. _J. Biol.
Chem._ 289, 3869–3875 (2014). Article CAS Google Scholar * Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. _Nat. Cell Biol._ 17,
816–826 (2015). Article CAS Google Scholar * Melo, S. A. et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. _Cancer Cell_ 26, 707–721 (2014).
Article CAS Google Scholar * Yeo, R. W. Y. et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. _Adv. Drug Deliv. Rev._ 65, 336–341 (2013). Article CAS
Google Scholar * Lai, R. C., Yeo, R. W. Y., Tan, K. H. & Lim, S. K. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. _Biotechnol. Adv._ 31, 543–551
(2013). Article CAS Google Scholar * Azmi, A. S., Bao, B. & Sarkar, F. H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. _Cancer Metast.
Rev_. 32, 623–642 (2013). Article CAS Google Scholar * Ohno, S.-i. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. _Mol. Ther._
21, 185–191 (2013). Article CAS Google Scholar * Liga, A., Vliegenthart, A. D. B., Oosthuyzen, W., Dear, J. W. & Kersaudy-Kerhoas, M. Exosome isolation: a microfluidic road-map. _Lab
Chip_ 15, 2388–2394 (2015). Article CAS Google Scholar * Christianson, H. C., Svensson, K. J., van Kuppevelt, T. H., Li, J.-P. & Belting, M. Cancer cell exosomes depend on
cell-surface heparan sulfate proteoglycans for their internalization and functional activity. _Proc. Natl Acad. Sci. USA_ 110, 17380–17385 (2013). Article CAS Google Scholar * Bechstein,
D. J. B. et al. High performance wash-free magnetic bioassays through microfluidically enhanced particle specificity. _Sci. Rep_. 5, 11693 (2015). Article Google Scholar * Caballero, J.
N., Frenette, G., Belleannée, C. & Sullivan, R. CD9-positive microvesicles mediate the transfer of molecules to bovine spermatozoa during epididymal maturation. _PLoS ONE_ 8, e65364
(2013). Article CAS Google Scholar * Tauro, B. J. et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. _Mol. Cell. Proteomics_ 12,
587–598 (2013). Article CAS Google Scholar * Thery, C. et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. _Nat. Immunol._ 3, 1156–1162 (2002). Article
CAS Google Scholar * Lambertz, U. et al. Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both old and new world Leishmania providing evidence for conserved
exosomal RNA packaging. _BMC Genomics_ 16, 1–26 (2015). Article CAS Google Scholar * Gormally, E., Caboux, E., Vineis, P. & Hainaut, P. Circulating free DNA in plasma or serum as
biomarker of carcinogenesis: practical aspects and biological significance. _Mutat. Res._ 635, 105–117 (2007). Article CAS Google Scholar * Wei, Z., Batagov, A. O., Carter, D. R. &
Krichevsky, A. M. Fetal bovine serum RNA interferes with the cell culture derived extracellular RNA. _Sci. Rep_. 6, 31175 (2016). Article CAS Google Scholar * Kowal, J. et al. Proteomic
comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. _Proc. Natl Acad. Sci. USA_ 113, E968–E977 (2016). Article CAS Google Scholar
* Asano, H. et al. Detection of _EGFR_ gene mutation in lung cancer by mutant-enriched polymerase chain reaction assay. _Clin. Cancer Res._ 12, 43–48 (2006). Article CAS Google Scholar
* Vlassov, A. V., Magdaleno, S., Setterquist, R. & Conrad, R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. _Biochim.
Biophys. Acta_ 1820, 940–948 (2012). Article CAS Google Scholar * Klymchenko, A. S. & Kreder, R. Fluorescent probes for lipid rafts: from model membranes to living cells. _Chem.
Biol._ 21, 97–113 (2014). Article CAS Google Scholar * Wijesinghe, D., Arachchige, M. C. M., Lu, A., Reshetnyak, Y. K. & Andreev, O. A. pH dependent transfer of nano-pores into
membrane of cancer cells to induce apoptosis. _Sci. Rep_. 3, 3560 (2013). Article Google Scholar * Lobb, R. J. et al. Optimized exosome isolation protocol for cell culture supernatant and
human plasma. _J. Extracell. Vesicles_ 4, 27031 (2015). Article Google Scholar * Jeong, S. et al. Integrated magneto–electrochemical sensor for exosome analysis. _ACS Nano_ 10, 1802–1809
(2016). Article CAS Google Scholar * Im, H. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. _Nat. Biotechnol._ 32, 490–495 (2014). Article
CAS Google Scholar * Zhao, Z., Yang, Y., Zeng, Y. & He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. _Lab Chip_ 16,
489–496 (2016). Article CAS Google Scholar * Weber, R. J., Liang, S. I., Selden, N. S., Desai, T. A. & Gartner, Z. J. Efficient targeting of fatty-acid modified oligonucleotides to
live cell membranes through stepwise assembly. _Biomacromolecules_ 15, 4621–4626 (2014). Article CAS Google Scholar * Charbonneau, D. M. & Tajmir-Riahi, H.-A. Study on the interaction
of cationic lipids with bovine serum albumin. _J. Phys. Chem. B_ 114, 1148–1155 (2010). Article CAS Google Scholar * Bobrie, A., Colombo, M., Krumeich, S., Raposo, G. & Théry, C.
Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. _J. Extracell. Vesicles_
1, 18397 (2012). Article CAS Google Scholar * Van Niel, G. et al. Intestinal epithelial cells secrete exosome-like vesicles. _Gastroenterology_ 121, 337–349 (2001). Article CAS Google
Scholar * Colombo, M. et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. _J. Cell Sci._ 126,
5553–5565 (2013). Article CAS Google Scholar * Rupp, A. K. et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. _Gynecol. Oncol._ 122,
437–446 (2011). Article CAS Google Scholar * Boch, C. et al. The frequency of _EGFR_ and _KRAS_ mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe
from a cohort study. _BMJ Open_ 3, e002560 (2013). Article Google Scholar * Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies _. Sci.
Transl. Med._ 6, 224ra224 (2014). Article Google Scholar * Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. _Nat. Med_. 20,
548–554 (2014). Article CAS Google Scholar * Cheng, G. et al. The GO/rGO-Fe3O4 composites with good water-dispersibility and fast magnetic response for effective immobilization and
enrichment of biomolecules. _J. Mater. Chem._ 22, 21998–22004 (2012). Article CAS Google Scholar * Cheng, G., Zhang, J.-L., Liu, Y.-L., Sun, D.-H. & Ni, J.-Z. Synthesis of novel
Fe3O4@SiO2@CeO2 microspheres with mesoporous shell for phosphopeptide capturing and labeling. _Chem. Comm_. 47, 5732–5734 (2011). Article CAS Google Scholar * Deng, Y., Qi, D., Deng, C.,
Zhang, X. & Zhao, D. Superparamagnetic high-magnetization microspheres with an Fe3O4@ SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. _J. Am.
Chem. Soc._ 130, 28–29 (2008). Article CAS Google Scholar * Morel, A.-L. et al. Sonochemical approach to the synthesis of Fe3O4@SiO2 core−shell nanoparticles with tunable properties. _ACS
Nano_ 2, 847–856 (2008). Article CAS Google Scholar * Ding, H. et al. Fe3O4@SiO2 core/shell nanoparticles: the silica coating regulations with a single core for different core sizes and
shell thicknesses. _Chem. Mater._ 24, 4572–4580 (2012). Article CAS Google Scholar * Wan, Y. et al. Surface-immobilized aptamers for cancer cell isolation and microscopic cytology.
_Cancer Res._ 70, 9371–9380 (2010). Article CAS Google Scholar * Wang, S., Wan, Y. & Liu, Y. Effects of nanopillar array diameter and spacing on cancer cell capture and cell
behaviors. _Nanoscale_ 6, 12482–12489 (2014). Article CAS Google Scholar * Xue, P. et al. Isolation and elution of Hep3B circulating tumor cells using a dual-functional herringbone chip.
_Microfluid. Nanofluid._ 16, 605–612 (2014). Article CAS Google Scholar * Lu, N.-N . et al. Biotin-triggered decomposable immunomagnetic beads for capture and release of circulating tumor
cells. _ACS Appl. Mater. Interfaces_ 7, 8817–8826 (2015). Article CAS Google Scholar * Wan, Y. et al. Nanotextured substrates with immobilized aptamers for cancer cell isolation and
cytology. _Cancer_ 118, 1145–1154 (2012). Article CAS Google Scholar * Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of
non–small-cell lung cancer to gefitinib. _N. Engl. J. Med._ 350, 2129–2139 (2004). Article CAS Google Scholar * Li, H & Durbin, R. Fast and accurate short read alignment with
Burrows-Wheeler transform. _Bioinformatics_. 25, 1754–17320 (2009). Article CAS Google Scholar * Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing
genomic features. _Bioinformatics_ 26, 841–842 (2010). Article CAS Google Scholar * Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. _EMBnet.journal_
17, 10–12 (2011). Article Google Scholar * Dobin, A . et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS Google Scholar * Karolchik, D.,
Hinrichs, A. S. & Kent, W. J. The UCSC Genome Browser. _Curr. Protoc. Bioinform._ Ch. 1, Unit 1 4 (2007). * Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with
high-throughput sequencing data. _Bioinformatics_ 31, 166–169 (2015). * Alam, S. et al. The eleventh and twelfth data releases of the Sloan Digital Sky Survey: final data from SDSS-III.
_Astrophys. J. Suppl. Ser._ 219, 12 (2015). Article Google Scholar * Anders, S. & Huber, W. Differential expression analysis for sequence count data. _Genome Biol._ 11, R016 (2010).
Article Google Scholar * Wickham, H. _ggplot2: Elegant Graphics for Data Analysis_ (Springer, 2009). * Wan, Y. et al. Dataset for rapid magnetic isolation of extracellular vesicles via
lipid-based nanoprobes. _figshare_http://dx.doi.org/10.6084/m9.figshare.4728856 (2017). Download references ACKNOWLEDGEMENTS S.-Y.Z. thanks the Penn State Materials Research Institute, the
Huck Institute of Life Sciences, the Penn State Hershey Cancer Institute, the Penn State proteomic and mass spectrometry facilities at Hershey and University Park, the Penn State Microscopy
and Cytometry Facility, and the Penn State Genomics Facility for their support. This work was partially supported by the Pennsylvania State University start-up fund and the National Cancer
Institute of the National Institutes of Health under Award Number DP2CA174508. We thank the Applied Bioinformatics Center (BFX) at the New York University (NYU) School of Medicine for
providing bioinformatics support and for helping with the analysis and interpretation of the data. This work used computing resources at the High Performance Computing Facility (HPCF) of the
Center for Health Informatics and Bioinformatics at the NYU Langone Medical Center. We also thank the Genome Technology Center (GTC) for library preparation and sequencing. This shared
resource is partially supported by the Cancer Center Support Grant, P30CA016087, at the Laura and Isaac Perlmutter Cancer Center. We would like to thank S. Hafenstein at Penn State Hershey
for discussions on cryo-SEM and C. Zhang at the Dana-Farber Cancer Institute for his advice on genomic analysis. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biomedical
Engineering, Micro and Nano Integrated Biosystem (MINIBio) Laboratory, The Pennsylvania State University, University Park, 16802, Pennsylvania, USA. Yuan Wan, Gong Cheng, Si-Jie Hao, Merisa
Nisic, Chuan-Dong Zhu, Yi-Qiu Xia, Wen-Qing Li, Zhi-Gang Wang, Wen-Long Zhang & Si-Yang Zheng * Penn State Materials Research Institute, The Pennsylvania State University, University
Park, 16802, Pennsylvania, USA. Yuan Wan, Gong Cheng, Si-Jie Hao, Yi-Qiu Xia, Wen-Qing Li, Zhi-Gang Wang, Wen-Long Zhang & Si-Yang Zheng * Penn State Milton S. Hershey Medical Center,
The Pennsylvania State University, Hershey, 17033, Pennsylvania, USA. Xin Liu, Shawn J. Rice & Chandra P. Belani * Penn State Hershey Cancer Institute, The Pennsylvania State University,
500 University Drive, Hershey, 17033, Pennsylvania, USA. Xin Liu, Shawn J. Rice & Chandra P. Belani * The Huck Institutes of the Life Sciences, The Pennsylvania State University,
University Park, 16802, Pennsylvania, USA. Merisa Nisic, Istvan Albert & Si-Yang Zheng * The Second Hospital of Nanjing, Affiliated to Medical School of Southeast University, Nanjing,
210003, China. Chuan-Dong Zhu * Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, 16802, Pennsylvania, USA. Aswathy Sebastian & Istvan
Albert * Department of Electrical Engineering, The Pennsylvania State University, University Park, 16802, Pennsylvania, USA. Si-Yang Zheng Authors * Yuan Wan View author publications You
can also search for this author inPubMed Google Scholar * Gong Cheng View author publications You can also search for this author inPubMed Google Scholar * Xin Liu View author publications
You can also search for this author inPubMed Google Scholar * Si-Jie Hao View author publications You can also search for this author inPubMed Google Scholar * Merisa Nisic View author
publications You can also search for this author inPubMed Google Scholar * Chuan-Dong Zhu View author publications You can also search for this author inPubMed Google Scholar * Yi-Qiu Xia
View author publications You can also search for this author inPubMed Google Scholar * Wen-Qing Li View author publications You can also search for this author inPubMed Google Scholar *
Zhi-Gang Wang View author publications You can also search for this author inPubMed Google Scholar * Wen-Long Zhang View author publications You can also search for this author inPubMed
Google Scholar * Shawn J. Rice View author publications You can also search for this author inPubMed Google Scholar * Aswathy Sebastian View author publications You can also search for this
author inPubMed Google Scholar * Istvan Albert View author publications You can also search for this author inPubMed Google Scholar * Chandra P. Belani View author publications You can also
search for this author inPubMed Google Scholar * Si-Yang Zheng View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W. and S.-Y.Z. designed
the research. Y.W. conducted experiments and analysed data. G.C. prepared the MMPs, assisted with peptide-sample preparation and performed proteomic analyses. S.-J.H. assisted with the
preparation of NGS samples, the analysis of RNA NGS data, and the fluorescence imaging. M.N. prepared blood plasma. C.-D.Z. and W.-Q.L. assisted with the cell culture, nEV collection and gel
electrophoresis. Y.-Q.X. performed the wound-healing assay. Z.-G.W. performed the electron microscopy. W.-L.Z. assisted with the image processing. A.S and I.A analysed NGS DNA data. X.L.,
S.J.R. and C.P.B. recruited patients and provided blood samples, tissue NGS data and clinical support. Y.W. and S.-Y.Z. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Si-Yang
Zheng. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary tables, figures and
references (PDF 7817 kb) SUPPLEMENTARY DATASET 1 Top-1,000 expressed mRNAs (XLSX 47 kb) SUPPLEMENTARY DATASET 2 Top-1,000 expressed miRNAs (XLSX 43 kb) SUPPLEMENTARY DATASET 3 Cargo proteins
in the nanoscale extracellular vesicles (XLSX 384 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wan, Y., Cheng, G., Liu, X. _et al._ Rapid
magnetic isolation of extracellular vesicles via lipid-based nanoprobes. _Nat Biomed Eng_ 1, 0058 (2017). https://doi.org/10.1038/s41551-017-0058 Download citation * Received: 30 April 2016
* Accepted: 07 March 2017 * Published: 10 April 2017 * DOI: https://doi.org/10.1038/s41551-017-0058 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
