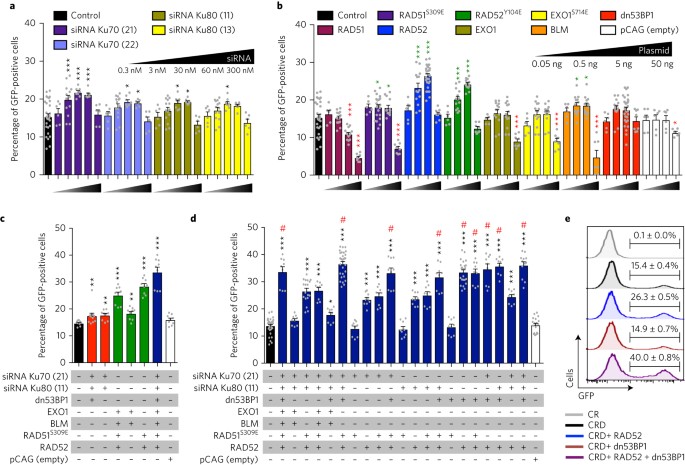
Ectopic expression of rad52 and dn53bp1 improves homology-directed repair during crispr–cas9 genome editing
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Gene disruption by clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9) is highly efficient and relies on the error-prone
non-homologous end-joining pathway. Conversely, precise gene editing requires homology-directed repair (HDR), which occurs at a lower frequency than non-homologous end-joining in mammalian
cells. Here, by testing whether manipulation of DNA repair factors improves HDR efficacy, we show that transient ectopic co-expression of RAD52 and a dominant-negative form of tumour protein
p53-binding protein 1 (dn53BP1) synergize to enable efficient HDR using a single-stranded oligonucleotide DNA donor template at multiple loci in human cells, including patient-derived
induced pluripotent stem cells. Co-expression of RAD52 and dn53BP1 improves multiplexed HDR-mediated editing, whereas expression of RAD52 alone enhances HDR with Cas9 nickase. Our data show
that the frequency of non-homologous end-joining-mediated double-strand break repair in the presence of these two factors is not suppressed and suggest that dn53BP1 competitively antagonizes
53BP1 to augment HDR in combination with RAD52. Importantly, co-expression of RAD52 and dn53BP1 does not alter Cas9 off-target activity. These findings support the use of RAD52 and dn53BP1
co-expression to overcome bottlenecks that limit HDR in precision genome editing. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel
any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MULTI-PATHWAY DNA-REPAIR REPORTERS REVEAL COMPETITION BETWEEN END-JOINING,
SINGLE-STRAND ANNEALING AND HOMOLOGOUS RECOMBINATION AT CAS9-INDUCED DNA DOUBLE-STRAND BREAKS Article Open access 08 September 2022 PREVALENT INTEGRATION OF GENOMIC REPETITIVE AND REGULATORY
ELEMENTS AND DONOR SEQUENCES AT CRISPR-CAS9-INDUCED BREAKS Article Open access 20 January 2025 TARGETING DOUBLE-STRAND BREAK INDEL BYPRODUCTS WITH SECONDARY GUIDE RNAS IMPROVES CAS9
HDR-MEDIATED GENOME EDITING EFFICIENCIES Article Open access 09 May 2022 REFERENCES * Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity.
_Science_ 337, 816–821 (2012). Article CAS PubMed Google Scholar * Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. _Science_ 339, 819–823 (2013). Article CAS
PubMed PubMed Central Google Scholar * Jinek, M. et al. RNA-programmed genome editing in human cells. _eLife_ 2, e00471 (2013). Article PubMed PubMed Central Google Scholar * Mali, P.
et al. RNA-guided human genome engineering via Cas9. _Science_ 339, 823–826 (2013). Article CAS PubMed PubMed Central Google Scholar * Hsu, P. D., Lander, E. S. & Zhang, F.
Development and applications of CRISPR–Cas9 for genome engineering. _Cell_ 157, 1262–1278 (2014). Article CAS PubMed PubMed Central Google Scholar * Ding, Q. et al. Enhanced efficiency
of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. _Cell Stem Cell_ 12, 393–394 (2013). Article CAS PubMed PubMed Central Google Scholar * Hruscha, A.
et al. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. _Development_ 140, 4982–4987 (2013). Article CAS PubMed Google Scholar * Li, D. et al. Heritable
gene targeting in the mouse and rat using a CRISPR–Cas system. _Nat. Biotechnol._ 31, 681–683 (2013). Article CAS PubMed Google Scholar * Mandal, P. K. et al. Efficient ablation of genes
in human hematopoietic stem and effector cells using CRISPR/Cas9. _Cell Stem Cell_ 15, 643–652 (2014). Article CAS PubMed PubMed Central Google Scholar * Ran, F. A. et al. In vivo
genome editing using _Staphylococcus aureus_ Cas9. _Nature_ 520, 186–191 (2015). Article CAS PubMed PubMed Central Google Scholar * Hendel, A. et al. Chemically modified guide RNAs
enhance CRISPR–Cas genome editing in human primary cells. _Nat. Biotechnol._ 33, 985–989 (2015). Article CAS PubMed PubMed Central Google Scholar * Ceccaldi, R., Rondinelli, B. &
D’Andrea, A. D. Repair pathway choices and consequences at the double-strand break. _Trends Cell Biol._ 26, 52–64 (2016). Article CAS PubMed Google Scholar * Campbell, C. R., Keown, W.,
Lowe, L., Kirschling, D. & Kucherlapati, R. Homologous recombination involving small single-stranded oligonucleotides in human cells. _New Biol._ 1, 223–227 (1989). CAS PubMed Google
Scholar * Igoucheva, O., Alexeev, V. & Yoon, K. Targeted gene correction by small single-stranded oligonucleotides in mammalian cells. _Gene Ther._ 8, 391–399 (2001). Article CAS
PubMed Google Scholar * Te Riele, H., Maandag, E. R. & Berns, A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs.
_Proc. Natl Acad. Sci. USA_ 89, 5128–5132 (1992). Article CAS PubMed PubMed Central Google Scholar * Mao, Z., Bozzella, M., Seluanov, A. & Gorbunova, V. Comparison of nonhomologous
end joining and homologous recombination in human cells. _DNA Repair (Amst.)_ 7, 1765–1771 (2008). Article CAS Google Scholar * Jensen, N. M. et al. An update on targeted gene repair in
mammalian cells: methods and mechanisms. _J. Biomed. Sci._ 18, 10 (2011). Article CAS PubMed PubMed Central Google Scholar * Karanam, K., Kafri, R., Loewer, A. & Lahav, G.
Quantitative live cell imaging reveals a gradual shift between DNA repair mechanisms and a maximal use of HR in mid S phase. _Mol. Cell_ 47, 320–329 (2012). Article CAS PubMed PubMed
Central Google Scholar * Branzei, D. & Foiani, M. Regulation of DNA repair throughout the cell cycle. _Nat. Rev. Mol. Cell Biol._ 9, 297–308 (2008). Article CAS PubMed Google
Scholar * Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. _Nature_ 510, 235–240 (2014). Article CAS PubMed PubMed Central Google Scholar *
Dever, D. P. et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. _Nature_ 539, 384–389 (2016). Article CAS PubMed Google Scholar * Chu, V. T. et al. Increasing
the efficiency of homology-directed repair for CRISPR–Cas9-induced precise gene editing in mammalian cells. _Nat. Biotechnol._ 33, 543–548 (2015). Article CAS PubMed Google Scholar *
Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. _eLife_ 3, e04766 (2014). PubMed
PubMed Central Google Scholar * Maruyama, T. et al. Increasing the efficiency of precise genome editing with CRISPR–Cas9 by inhibition of nonhomologous end joining. _Nat. Biotechnol._ 33,
538–542 (2015). Article CAS PubMed PubMed Central Google Scholar * Robert, F., Barbeau, M., Ethier, S., Dostie, J. & Pelletier, J. Pharmacological inhibition of DNA-PK stimulates
Cas9-mediated genome editing. _Genome Med._ 7, 93 (2015). Article PubMed PubMed Central Google Scholar * Suzuki, K. et al. In vivo genome editing via CRISPR/Cas9 mediated
homology-independent targeted integration. _Nature_ 540, 144–149 (2016). Article CAS PubMed PubMed Central Google Scholar * Yu, C. et al. Small molecules enhance CRISPR genome editing
in pluripotent stem cells. _Cell Stem Cell_ 16, 142–147 (2015). Article CAS PubMed PubMed Central Google Scholar * Gu, Y. et al. Growth retardation and leaky SCID phenotype of
Ku70-deficient mice. _Immunity_ 7, 653–665 (1997). Article CAS PubMed Google Scholar * Frank, K. M. et al. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA
ligase IV. _Nature_ 396, 173–177 (1998). Article CAS PubMed Google Scholar * O’Driscoll, M. et al. DNA ligase IV mutations identified in patients exhibiting developmental delay and
immunodeficiency. _Mol. Cell_ 8, 1175–1185 (2001). Article PubMed Google Scholar * Beerman, I., Seita, J., Inlay, M. A., Weissman, I. L. & Rossi, D. J. Quiescent hematopoietic stem
cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. _Cell Stem Cell_ 15, 37–50 (2014). Article CAS PubMed PubMed Central Google Scholar * Xie, A. et
al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. _Mol. Cell_ 28, 1045–1057 (2007). Article CAS PubMed PubMed Central Google
Scholar * Frock, R. L. et al. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. _Nat. Biotechnol._ 33, 179–186 (2014). Article CAS PubMed Google
Scholar * Renaud, J. B. et al. Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR–Cas9 nucleases. _Cell Rep._ 14, 2263–2272 (2016).
Article CAS PubMed Google Scholar * Richardson, C. D., Ray, G. J., DeWitt, M. A., Curie, G. L. & Corn, J. E. Enhancing homology-directed genome editing by catalytically active and
inactive CRISPR–Cas9 using asymmetric donor DNA. _Nat. Biotechnol._ 34, 339–344 (2016). Article CAS PubMed Google Scholar * Pierce, A. J., Hu, P., Han, M., Ellis, N. & Jasin, M. Ku
DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. _Genes Dev._ 15, 3237–3242 (2001). Article CAS PubMed PubMed Central Google Scholar *
Delacote, F., Han, M., Stamato, T. D., Jasin, M. & Lopez, B. S. An xrcc4 defect or Wortmannin stimulates homologous recombination specifically induced by double-strand breaks in
mammalian cells. _Nucleic Acids Res._ 30, 3454–3463 (2002). Article CAS PubMed PubMed Central Google Scholar * Kurosawa, A. et al. DNA ligase IV and artemis act cooperatively to
suppress homologous recombination in human cells: implications for DNA double-strand break repair. _PLoS ONE_ 8, e72253 (2013). Article CAS PubMed PubMed Central Google Scholar *
Sorensen, C. S. et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. _Nat. Cell Biol._ 7, 195–201 (2005). Article CAS PubMed Google
Scholar * Honda, M., Okuno, Y., Yoo, J., Ha, T. & Spies, M. Tyrosine phosphorylation enhances RAD52-mediated annealing by modulating its DNA binding. _EMBO J._ 30, 3368–3382 (2011).
Article CAS PubMed PubMed Central Google Scholar * Bolderson, E. et al. Phosphorylation of Exo1 modulates homologous recombination repair of DNA double-strand breaks. _Nucleic Acids
Res._ 38, 1821–1831 (2010). Article CAS PubMed Google Scholar * Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. _Cell_ 154, 1380–1389
(2013). Article CAS PubMed PubMed Central Google Scholar * Xie, K., Minkenberg, B. & Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing
system. _Proc. Natl Acad. Sci. USA_ 112, 3570–3575 (2015). Article CAS PubMed PubMed Central Google Scholar * Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human
cells. _Science_ 343, 84–87 (2014). Article CAS PubMed Google Scholar * Yin, L. et al. Multiplex conditional mutagenesis using transgenic expression of Cas9 and sgRNAs. _Genetics_ 200,
431–441 (2015). Article CAS PubMed PubMed Central Google Scholar * Kabadi, A. M., Ousterout, D. G., Hilton, I. B. & Gersbach, C. A. Multiplex CRISPR/Cas9-based genome engineering
from a single lentiviral vector. _Nucleic Acids Res._ 42, e147 (2014). Article PubMed PubMed Central Google Scholar * Lee, J. et al. mRNA-mediated glycoengineering ameliorates deficient
homing of human stem cell-derived hematopoietic progenitors. _J. Clin. Invest._ 127, 2433–2437 (2017). Article PubMed PubMed Central Google Scholar * Mitchell, J. R., Wood, E. &
Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. _Nature_ 402, 551–555 (1999). Article CAS PubMed Google Scholar * Hengel, S. R. et al.
Small-molecule inhibitors identify the RAD52–ssDNA interaction as critical for recovery from replication stress and for survival of BRCA2 deficient cells. _eLife_ 5, e14740 (2016). Article
PubMed PubMed Central Google Scholar * Hou, P. et al. Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection. _Sci. Rep._ 5, 15577 (2015). Article CAS PubMed
PubMed Central Google Scholar * Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. _Clin. Cancer Res._ 23, 2255–2266 (2017). Article
CAS PubMed Google Scholar * Su, S. et al. CRISPR–Cas9 mediated efficient PD-1 disruption on human primary T cells from cancer patients. _Sci. Rep._ 6, 20070 (2016). Article CAS PubMed
PubMed Central Google Scholar * Symington, L. S. & Gautier, J. Double-strand break end resection and repair pathway choice. _Annu. Rev. Genet._ 45, 247–271 (2011). Article CAS PubMed
Google Scholar * Bothmer, A. et al. Regulation of DNA end joining, resection, and immunoglobulin class switch recombination by 53BP1. _Mol. Cell_ 42, 319–329 (2011). Article CAS PubMed
PubMed Central Google Scholar * Bothmer, A. et al. 53BP1 regulates DNA resection and the choice between classical and alternative end joining during class switch recombination. _J. Exp.
Med._ 207, 855–865 (2010). Article CAS PubMed PubMed Central Google Scholar * Ochs, F. et al. 53BP1 fosters fidelity of homology-directed DNA repair. _Nat. Struct. Mol. Biol._ 23,
714–721 (2016). Article CAS PubMed Google Scholar * Bhargava, R., Onyango, D. O. & Stark, J. M. Regulation of single-strand annealing and its role in genome maintenance._Trends
Genet._ 32, 566–575 (2016). Article CAS PubMed PubMed Central Google Scholar * Storici, F., Snipe, J. R., Chan, G. K., Gordenin, D. A. & Resnick, M. A. Conservative repair of a
chromosomal double-strand break by single-strand DNA through two steps of annealing. _Mol. Cell Biol._ 26, 7645–7657 (2006). Article CAS PubMed PubMed Central Google Scholar * Rijkers,
T. et al. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. _Mol. Cell Biol._ 18, 6423–6429 (1998). Article CAS PubMed PubMed
Central Google Scholar * Yamaguchi-Iwai, Y. et al. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. _Mol. Cell Biol._ 18, 6430–6435 (1998).
Article CAS PubMed PubMed Central Google Scholar * Symington, L. S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. _Microbiol. Mol.
Biol. Rev._ 66, 630–670 (2002). Article CAS PubMed PubMed Central Google Scholar * Hanamshet, K., Mazina, O.M. & Mazin, A.V. Reappearance from obscurity: mammalian Rad52 in
homologous recombination. _Genes (Basel)_ 7, E63 (2016). Article Google Scholar * Feng, Z. et al. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. _Proc. Natl Acad. Sci.
USA_ 108, 686–691 (2011). Article CAS PubMed Google Scholar * Lok, B. H., Carley, A. C., Tchang, B. & Powell, S. N. RAD52 inactivation is synthetically lethal with deficiencies in
BRCA1 and PALB2 in addition to BRCA2 through RAD51-mediated homologous recombination. _Oncogene_ 32, 3552–3558 (2013). Article CAS PubMed Google Scholar * Daley, J. M. & Sung, P.
53BP1, BRCA1, and the choice between recombination and end joining at DNA double-strand breaks. _Mol. Cell Biol._ 34, 1380–1388 (2014). Article PubMed PubMed Central Google Scholar *
Kakarougkas, A. et al. Opposing roles for 53BP1 during homologous recombination. _Nucleic Acids Res._ 41, 9719–9731 (2013). Article CAS PubMed PubMed Central Google Scholar * Panier, S.
& Boulton, S. J. Double-strand break repair: 53BP1 comes into focus. _Nat. Rev. Mol. Cell Biol._ 15, 7–18 (2014). Article CAS PubMed Google Scholar * Bunting, S. F. et al. 53BP1
inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. _Cell_ 141, 243–254 (2010). Article CAS PubMed PubMed Central Google Scholar *
Finney-Manchester, S. P. & Maheshri, N. Harnessing mutagenic homologous recombination for targeted mutagenesis in vivo by TaGTEAM. _Nucleic Acids Res._ 41, e99 (2013). Article CAS
PubMed PubMed Central Google Scholar * Daley, J. M. & Wilson, T. E. Rejoining of DNA double-strand breaks as a function of overhang length. _Mol. Cell Biol._ 25, 896–906 (2005).
Article CAS PubMed PubMed Central Google Scholar * Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified
mRNA. _Cell Stem Cell_ 7, 618–630 (2010). Article CAS PubMed PubMed Central Google Scholar * Park, I. H., Lerou, P. H., Zhao, R., Huo, H. & Daley, G. Q. Generation of human-induced
pluripotent stem cells. _Nat. Protoc._ 3, 1180–1186 (2008). Article CAS PubMed Google Scholar * Moon, D. H. et al. Poly(A)-specific ribonuclease (PARN) mediates 3′-end maturation of the
telomerase RNA component. _Nat. Genet._ 47, 1482–1488 (2015). Article CAS PubMed PubMed Central Google Scholar * Hu, J. et al. Detecting DNA double-stranded breaks in mammalian genomes
by linear amplification-mediated high-throughput genome-wide translocation sequencing. _Nat. Protoc._ 11, 853–871 (2016). Article CAS PubMed PubMed Central Google Scholar * Aguet, F.,
Antonescu, C. N., Mettlen, M., Schmid, S. L. & Danuser, G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. _Dev.
Cell_ 26, 279–291 (2013). Article CAS PubMed PubMed Central Google Scholar * Reshef, D. N. et al. Detecting novel associations in large data sets. _Science_ 334, 1518–1524 (2011).
Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in part by National Institutes of Health grants R01AI020047 and R01AI077595
(to F.W.A.) and RO1HL107630, HL107440, UC4DK104218 and U19HL129903 (to D.J.R.), the Translational Research Program (Boston Children’s Hospital), Pedals for Pediatrics (Dana-Farber Cancer
Institute) awards (to S.A. and B.B.), The Leona M. and Harry B. Helmsley Charitable Trust (to D.J.R.) and the New York Stem Cell Foundation (to D.J.R.). The HEK293 broken-GFP reporter cell
line was kindly provided by G. Church. The gRNA constructs targeting B2M were provided by C. Cowan. AUTHOR INFORMATION Author notes * Bruna S. Paulsen and Pankaj K. Mandal contributed
equally to this work. AUTHORS AND AFFILIATIONS * Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, MA, 02138, USA Bruna S. Paulsen, Pankaj K. Mandal, Paula
Gutierrez-Martinez, Wataru Ebina & Derrick J. Rossi * Program in Cellular and Molecular Medicine at Boston Children’s Hospital, Boston, MA, 02115, USA Bruna S. Paulsen, Pankaj K. Mandal,
Richard L. Frock, Srigokul Upadhyayula, Paula Gutierrez-Martinez, Wataru Ebina, Tomas Kirchhausen, Frederick W. Alt & Derrick J. Rossi * Department of Pediatrics, Harvard Medical
School, Boston, MA, 02115, USA Pankaj K. Mandal, Srigokul Upadhyayula, Tomas Kirchhausen, Suneet Agarwal & Derrick J. Rossi * Department of Genetics, Harvard Medical School, Boston, MA,
02115, USA Richard L. Frock & Frederick W. Alt * Division of Hematology/Oncology, Boston Children’s Hospital, Boston, MA, 02115, USA Baris Boyraz & Suneet Agarwal * Department of
Basic Oncology, Hacettepe University Cancer Institute, Ankara, Turkey Baris Boyraz * Molecular Neurogenetics Unit, Psychiatric and Neurodevelopmental Genetics Unit, Center for Genomic
Medicine, Massachusetts General Hospital, Boston, MA, 02114, USA Rachita Yadav & Michael E. Talkowski * Broad Institute, Cambridge, MA, 02142, USA Rachita Yadav & Michael E.
Talkowski * Department of Neurology, Harvard Medical School, Boston, MA, 02115, USA Rachita Yadav & Michael E. Talkowski * Department of Cell Biology, Harvard Medical School, Boston, MA,
02115, USA Srigokul Upadhyayula & Tomas Kirchhausen * Department of Pediatrics, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden Anders Fasth * Stem Cell Program,
Boston Children’s Hospital, Boston, MA, 02115, USA Suneet Agarwal * Harvard Stem Cell Institute, Cambridge, MA, 02138, USA Suneet Agarwal & Derrick J. Rossi * The Howard Hughes Medical
Institute, Boston Children’s Hospital, Boston, MA, 02115, USA Frederick W. Alt Authors * Bruna S. Paulsen View author publications You can also search for this author inPubMed Google Scholar
* Pankaj K. Mandal View author publications You can also search for this author inPubMed Google Scholar * Richard L. Frock View author publications You can also search for this author
inPubMed Google Scholar * Baris Boyraz View author publications You can also search for this author inPubMed Google Scholar * Rachita Yadav View author publications You can also search for
this author inPubMed Google Scholar * Srigokul Upadhyayula View author publications You can also search for this author inPubMed Google Scholar * Paula Gutierrez-Martinez View author
publications You can also search for this author inPubMed Google Scholar * Wataru Ebina View author publications You can also search for this author inPubMed Google Scholar * Anders Fasth
View author publications You can also search for this author inPubMed Google Scholar * Tomas Kirchhausen View author publications You can also search for this author inPubMed Google Scholar
* Michael E. Talkowski View author publications You can also search for this author inPubMed Google Scholar * Suneet Agarwal View author publications You can also search for this author
inPubMed Google Scholar * Frederick W. Alt View author publications You can also search for this author inPubMed Google Scholar * Derrick J. Rossi View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS B.S.P., P.K.M. and D.J.R. designed the experiments. B.S.P. and P.K.M. performed the experiments. P.K.M., R.L.F. and F.W.A.
designed and performed the HTGTS experiments. B.S.P., B.B., A.F. and S.A. designed and performed the human DKC1 iPS cell line experiments. B.S.P., P.G.-M. and W.E. designed and performed the
experiments for the selection of the candidate factors. P.K.M., R.Y. and M.E.T. designed and performed the capture deep sequencing experiments. S.U. and T.K. performed the image analyses.
All authors were involved in data analysis. B.S.P., P.K.M and D.J.R. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Derrick J. Rossi. ETHICS DECLARATIONS COMPETING INTERESTS
D.J.R. is an academic co-founder of Intellia Therapeutics (Cambridge, MA), a biotechnology company focused on developing CRISPR–Cas9 therapies. ADDITIONAL INFORMATION PUBLISHER’S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION
Supplementary figures and methods LIFE SCIENCES REPORTING SUMMARY SUPPLEMENTARY TABLES Supplementary tables RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Paulsen, B.S., Mandal, P.K., Frock, R.L. _et al._ Ectopic expression of RAD52 and dn53BP1 improves homology-directed repair during CRISPR–Cas9 genome editing. _Nat Biomed Eng_ 1,
878–888 (2017). https://doi.org/10.1038/s41551-017-0145-2 Download citation * Received: 08 December 2016 * Accepted: 13 September 2017 * Published: 09 October 2017 * Issue Date: November
2017 * DOI: https://doi.org/10.1038/s41551-017-0145-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
