Single-cell chromatin accessibility maps reveal regulatory programs driving early mouse organogenesis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

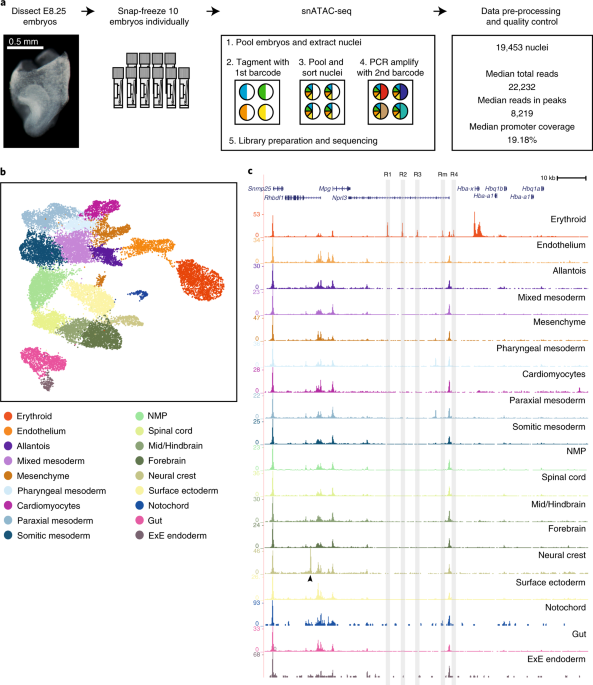
ABSTRACT During mouse embryonic development, pluripotent cells rapidly divide and diversify, yet the regulatory programs that define the cell repertoire for each organ remain ill-defined. To
delineate comprehensive chromatin landscapes during early organogenesis, we mapped chromatin accessibility in 19,453 single nuclei from mouse embryos at 8.25 days post-fertilization.
Identification of cell-type-specific regions of open chromatin pinpointed two TAL1-bound endothelial enhancers, which we validated using transgenic mouse assays. Integrated gene expression
and transcription factor motif enrichment analyses highlighted cell-type-specific transcriptional regulators. Subsequent in vivo experiments in zebrafish revealed a role for the ETS factor
FEV in endothelial identity downstream of ETV2 (Etsrp in zebrafish). Concerted in vivo validation experiments in mouse and zebrafish thus illustrate how single-cell open chromatin maps,
representative of a mammalian embryo, provide access to the regulatory blueprint for mammalian organogenesis. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A SINGLE-CELL ATLAS OF CHROMATIN ACCESSIBILITY IN MOUSE
ORGANOGENESIS Article 08 July 2024 SINGLE-NUCLEUS CHROMATIN LANDSCAPES DURING ZEBRAFISH EARLY EMBRYOGENESIS Article Open access 19 July 2023 MAPPING THE CHROMATIN ACCESSIBILITY LANDSCAPE OF
ZEBRAFISH EMBRYOGENESIS AT SINGLE-CELL RESOLUTION BY SPATAC-SEQ Article 08 July 2024 DATA AVAILABILITY Raw sequencing data and processed data are available at GEO with accession number
GSE133244. Previously published sequencing data that were re-analysed here are available under accession codes GSM1436367 and GSM1436368 (ETV2 ChIP-seq) and GSM1692843, GSM1692848 and
GSM1692858 (TAL1 ChIP-seq). Processed TAL1 ChIP-seq data used in this publication is also available at http://codex.stemcells.cam.ac.uk/. Data are available in processed form for download
and interactive browsing at https://gottgens-lab.stemcells.cam.ac.uk/snATACseq_E825. Cell type tracks can be explored at https://tinyurl.com/snATACseq-GSE133244-UCSC. All other data
supporting the findings of this study are available from the corresponding author on reasonable request. CODE AVAILABILITY All code is available upon request and at
https://github.com/BPijuanSala/MouseOrganogenesis_snATACseq_2020. REFERENCES * Cao, J. et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells.
_Science_ 361, 1380–1385 (2018). Article CAS PubMed PubMed Central Google Scholar * Cusanovich, D. A. et al. Multiplex single-cell profiling of chromatin accessibility by combinatorial
cellular indexing. _Science_ 348, 910–914 (2015). Article CAS PubMed PubMed Central Google Scholar * Pijuan-Sala, B., Guibentif, C. & Göttgens, B. Single-cell transcriptional
profiling: a window into embryonic cell-type specification. _Nat. Rev. Mol. Cell Biol._ 19, 399–412 (2018). Article CAS PubMed Google Scholar * Preissl, S. et al. Single-nucleus analysis
of accessible chromatin in developing mouse forebrain reveals cell-type-specific transcriptional regulation. _Nat. Neurosci._ 21, 432–439 (2018). Article CAS PubMed PubMed Central
Google Scholar * Cusanovich, D. A. et al. The _cis_-regulatory dynamics of embryonic development at single-cell resolution. _Nature_ 555, 538–542 (2018). Article CAS PubMed PubMed
Central Google Scholar * Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. _Nature_ 566, 496 (2019). Article CAS PubMed PubMed Central Google Scholar
* Ibarra-Soria, X. et al. Defining murine organogenesis at single-cell resolution reveals a role for the leukotriene pathway in regulating blood progenitor formation. _Nat. Cell Biol._ 20,
127–134 (2018). Article CAS PubMed PubMed Central Google Scholar * Pijuan-Sala, B. et al. A single-cell molecular map of mouse gastrulation and early organogenesis. _Nature_ 566, 490
(2019). Article CAS PubMed PubMed Central Google Scholar * González-Blas, C. B. et al. cisTopic: cis-regulatory topic modeling on single-cell ATAC-seq data. _Nat. Methods_ 16, 397–400
(2019). Article CAS PubMed Central Google Scholar * Bellomo, D., Lander, A., Harragan, I. & Brown, N. A. Cell proliferation in mammalian gastrulation: the ventral node and notochord
are relatively quiescent. _Dev. Dyn._ 205, 471–485 (1996). Article CAS PubMed Google Scholar * Ilgren, E. B. Polyploidization of extraembryonic tissues during mouse embryogenesis.
_Development_ 59, 103–111 (1980). CAS Google Scholar * Anguita, E. et al. Deletion of the mouse α-globin regulatory element (HS−26) has an unexpectedly mild phenotype. _Blood_ 100,
3450–3456 (2002). Article CAS PubMed Google Scholar * Hay, D. et al. Genetic dissection of the α-globin super-enhancer in vivo. _Nat. Genet._ 48, 895–903 (2016). Article CAS PubMed
PubMed Central Google Scholar * Hughes, J. R. et al. Annotation of cis-regulatory elements by identification, subclassification, and functional assessment of multispecies conserved
sequences. _Proc. Natl Acad. Sci. USA_ 102, 9830–9835 (2005). Article CAS PubMed PubMed Central Google Scholar * Craig, M. L. & Russell, E. S. A developmental change in hemoglobins
correlated with an embryonic red cell population in the mouse. _Dev. Biol._ 10, 191–201 (1964). Article CAS PubMed Google Scholar * Hanssen, L. L. P. et al. Tissue-specific
CTCF–cohesin-mediated chromatin architecture delimits enhancer interactions and function in vivo. _Nat. Cell Biol._ 19, 952–961 (2017). Article CAS PubMed PubMed Central Google Scholar
* Tzouanacou, E., Wegener, A., Wymeersch, F. J., Wilson, V. & Nicolas, J.-F. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. _Dev.
Cell_ 17, 365–376 (2009). Article CAS PubMed Google Scholar * Tremblay, M., Sanchez-Ferras, O. & Bouchard, M. GATA transcription factors in development and disease. _Development_
145, dev164384 (2018). Article CAS PubMed Google Scholar * Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated
accessibility from single-cell epigenomic data. _Nat. Methods_ 14, 975–978 (2017). Article CAS PubMed PubMed Central Google Scholar * Moon, K. R. et al. Visualizing structure and
transitions in high-dimensional biological data. _Nat. Biotechnol._ 37, 1482–1492 (2019). Article CAS PubMed PubMed Central Google Scholar * Ralston, A. et al. Gata3 regulates
trophoblast development downstream of Tead4 and in parallel to Cdx2. _Development_ 137, 395–403 (2010). Article CAS PubMed Google Scholar * Nuez, B., Michalovich, D., Bygrave, A.,
Ploemacher, R. & Grosveld, F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. _Nature_ 375, 316 (1995). Article CAS PubMed Google Scholar *
Parkins, A. C., Sharpe, A. H. & Orkin, S. H. Lethal β-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. _Nature_ 375, 318 (1995). Article Google Scholar *
Desjardins, C. A. & Naya, F. J. The function of the MEF2 family of transcription factors in cardiac development, cardiogenomics, and direct reprogramming. _J. Cardiovasc. Dev. Dis._ 3,
26 (2016). Article CAS PubMed PubMed Central Google Scholar * Kallianpur, A. R., Jordan, J. E. & Brandt, S. J. The SCL/TAL-1 gene is expressed in progenitors of both the
hematopoietic and vascular systems during embryogenesis. _Blood_ 83, 1200–1208 (1994). Article CAS PubMed Google Scholar * Shivdasani, R. A., Mayer, E. L. & Orkin, S. H. Absence of
blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. _Nature_ 373, 432–434 (1995). Article CAS PubMed Google Scholar * Silver, L. & Palis, J. Initiation of
murine embryonic erythropoiesis: a spatial analysis. _Blood_ 89, 1154–1164 (1997). Article CAS PubMed Google Scholar * Palis, J. Hematopoietic stem cell‐independent hematopoiesis:
emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo. _FEBS Lett._ 590, 3965–3974 (2016). Article CAS PubMed Google Scholar * Downs, K. M., Gifford, S.,
Blahnik, M. & Gardner, R. L. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. _Dev. Camb. Engl._ 125, 4507–4520 (1998). CAS Google
Scholar * Ng, C. E. L. et al. A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. _Stem Cells_ 28, 1869–1881 (2010). Article CAS PubMed Google
Scholar * Nottingham, W. T. et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. _Blood_ 110, 4188–4197 (2007). Article CAS PubMed
PubMed Central Google Scholar * Goode, D. K. et al. Dynamic gene regulatory networks drive hematopoietic specification and differentiation. _Dev. Cell_ 36, 572–587 (2016). Article CAS
PubMed PubMed Central Google Scholar * Beck, D. et al. Genome-wide analysis of transcriptional regulators in human HSPCs reveals a densely interconnected network of coding and noncoding
genes. _Blood_ 122, e12–e22 (2013). Article CAS PubMed Google Scholar * Wilson, N. K. et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of
ten major transcriptional regulators. _Cell Stem Cell_ 7, 532–544 (2010). Article CAS PubMed Google Scholar * Pinto do O, P., Kolterud, A. & Carlsson, L. Expression of the
LIM-homeobox gene LH2 generates immortalized steel factor-dependent multipotent hematopoietic precursors. _EMBO J._ 17, 5744–5756 (1998). Article PubMed PubMed Central Google Scholar *
Butko, E., Pouget, C. & Traver, D. Complex regulation of HSC emergence by the Notch signaling pathway. _Dev. Biol._ 409, 129–138 (2016). Article CAS PubMed Google Scholar * Kothary,
R. et al. A transgene containing lacZ inserted into the dystonia locus is expressed in neural tube. _Nature_ 335, 435 (1988). Article CAS PubMed Google Scholar * Pennacchio, L. A. et al.
In vivo enhancer analysis of human conserved non-coding sequences. _Nature_ 444, 499 (2006). Article CAS PubMed Google Scholar * Craig, M. P. & Sumanas, S. ETS transcription factors
in embryonic vascular development. _Angiogenesis_ 19, 275–285 (2016). Article PubMed PubMed Central Google Scholar * Koyano-Nakagawa, N. et al. Etv2 is expressed in the yolk sac
hematopoietic and endothelial progenitors and regulates Lmo2 gene expression. _Stem Cells_ 30, 1611–1623 (2012). Article CAS PubMed PubMed Central Google Scholar * Liu, F. et al.
Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. _EMBO Rep._ 16, 654–669 (2015). Article CAS PubMed PubMed Central Google
Scholar * Wang, L. et al. Fev regulates hematopoietic stem cell development via ERK signaling. _Blood_ 122, 367–375 (2013). Article CAS PubMed Google Scholar * Pham, V. N. et al.
Combinatorial function of ETS transcription factors in the developing vasculature. _Dev. Biol._ 303, 772–783 (2007). Article CAS PubMed Google Scholar * Landry, J.-R. et al. Expression
of the leukemia oncogene Lmo2 is controlled by an array of tissue-specific elements dispersed over 100 kb and bound by Tal1/Lmo2, Ets, and Gata factors. _Blood_ 113, 5783–5792 (2009).
Article CAS PubMed Google Scholar * Göttgens, B. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets
and GATA factors. _EMBO J._ 21, 3039–3050 (2002). Article PubMed PubMed Central Google Scholar * Göttgens, B. et al. The _scl_ +18/19 stem cell enhancer is not required for
hematopoiesis: identification of a 5′ bifunctional hematopoietic-endothelial enhancer bound by Fli-1 and Elf-1. _Mol. Cell. Biol._ 24, 1870–1883 (2004). Article CAS PubMed PubMed Central
Google Scholar * Fang, R.et al. Fast and accurate clustering of single cell epigenomes reveals cis-regulatory elements in rare cell types. Preprint available at
https://www.biorxiv.org/content/10.1101/615179v2 (2019). * Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. _Nat. Methods_
14, 959–962 (2017). Article CAS PubMed PubMed Central Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). CAS
PubMed PubMed Central Google Scholar * Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). _Genome Biol._ 9, R137 (2008). Article CAS PubMed PubMed Central Google Scholar *
The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. _Nature_ 489, 57–74 (2012). Article CAS PubMed Central Google Scholar * Wolock, S. L.,
Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Preprint at https://www.biorxiv.org/content/10.1101/357368v1 (2018).
* Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589
(2010). Article CAS PubMed PubMed Central Google Scholar * Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the
zebrafish. _Dev. Dyn._ 203, 253–310 (1995). Article CAS PubMed Google Scholar * Sumanas, S. & Lin, S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. _PLoS
Biol._ 4, e10 (2006). Article CAS PubMed Google Scholar * Xue, Y. et al. A 3D atlas of hematopoietic stem and progenitor cell expansion by multi-dimensional RNA-seq analysis. _Cell Rep._
27, 1567–1578.e5 (2019). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank B. Ren and K. Zhang for making this collaboration between the University of
California San Diego and the University of Cambridge possible; I. Imaz-Rosshandler for statistical advice; T. L. Hamilton and Central Biomedical Services for technical support in embryo
collection; and R. Fang for kindly providing us with the list of constitutive promoters. We also thank S. Kuan for sequencing and B. Li for bioinformatics support. We would like to extend
our gratitude to the QB3 Macrolab at UC Berkeley for purification of the Tn5 transposase. B.P.-S. is funded by the Wellcome Trust 4-Year PhD Programme in Stem Cell Biology and Medicine and
the University of Cambridge, UK. B.P.-S was awarded a Travelling Fellowship from The Company of Biologists (DEV–180505) to perform this study. Research in the authors’ laboratories is
supported by the Wellcome, MRC, Bloodwise, CRUK and NIH-NIDDK; as well as core support grants from the Wellcome to the Wellcome-MRC Cambridge Stem Cell Institute. This work was funded as
part of a Wellcome Strategic Award (105031/Z/14/Z) awarded to W. Reik, B.G., J. Marioni, J. Nichols, L. Vallier, S. Srinivas, B. Simons, S. Teichmann and T. Voet. Work at the Center for
Epigenomics was supported in part by the UC San Diego School of Medicine. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Haematology, University of Cambridge, Cambridge, UK
Blanca Pijuan-Sala, Nicola K. Wilson, Rebecca L. Hannah, Sarah Kinston, Fernando J. Calero-Nieto & Berthold Göttgens * Wellcome-Medical Research Council Cambridge Stem Cell Institute,
University of Cambridge, Cambridge, UK Blanca Pijuan-Sala, Nicola K. Wilson, Rebecca L. Hannah, Sarah Kinston, Fernando J. Calero-Nieto & Berthold Göttgens * State Key Laboratory of
Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing, China Jun Xia & Feng Liu * Center for Epigenomics, Department of Cellular and Molecular Medicine, University
of California, San Diego, School of Medicine, La Jolla, CA, USA Xiaomeng Hou, Olivier Poirion & Sebastian Preissl Authors * Blanca Pijuan-Sala View author publications You can also
search for this author inPubMed Google Scholar * Nicola K. Wilson View author publications You can also search for this author inPubMed Google Scholar * Jun Xia View author publications You
can also search for this author inPubMed Google Scholar * Xiaomeng Hou View author publications You can also search for this author inPubMed Google Scholar * Rebecca L. Hannah View author
publications You can also search for this author inPubMed Google Scholar * Sarah Kinston View author publications You can also search for this author inPubMed Google Scholar * Fernando J.
Calero-Nieto View author publications You can also search for this author inPubMed Google Scholar * Olivier Poirion View author publications You can also search for this author inPubMed
Google Scholar * Sebastian Preissl View author publications You can also search for this author inPubMed Google Scholar * Feng Liu View author publications You can also search for this
author inPubMed Google Scholar * Berthold Göttgens View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.P.-S performed embryo dissections,
bioinformatic analysis (both data pre-processing and biological analysis), created the website and coordinated the study. N.K.W., S.K. and F.J.C.-N. performed enhancer validation
experiments. J.X. performed experiments in zebrafish. X.H. performed snATAC-seq and was assisted by B.P.-S. R.L.H. processed the ETV2 ChIP-seq dataset. O.P. performed data demultiplexing and
barcode extraction. S.P. supervised the snATAC-seq experiment, sequencing and initial data pre-processing. F.L. supervised experiments in zebrafish. B.G. supervised the study. B.P.-S. and
B.G. wrote the manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Berthold Göttgens. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
EXTENDED DATA EXTENDED DATA FIG. 1 SNATAC-SEQ EXPERIMENT. A, E8.25 embryos used for snATAC-seq. This panel includes the embryo in Fig. 1a (top right in this panel). Scale bars: 0.5 mm.
Experiment was performed with 10 embryos. B, Representative FACS gating strategy. The gate used to sort the nuclei regardless of DNA ploidy can be found in the bottom left panel. Gates for
nuclei with 2 (2n) and 4 copies (4n) of DNA can be found in the bottom right panel. EXTENDED DATA FIG. 2 DATA QUALITY CONTROL AND CELL TYPE ANNOTATION. A, Quality control (QC) thresholds.
Top: _X-Y_ plot showing the number of reads in peaks and promoter coverage for each barcode. Promoter coverage is defined as the number of reads in constitutive promoters divided by the
total number of constitutive promoters. Values have been log-transformed. Red square box delimits the nuclei that passed QC for these parameters. Middle: Histogram showing the doublet scores
for the nuclei that passed the first QC. Red line delimits the threshold; those below the line passed QC. _y_ axis has been log-transformed. Bottom: Histogram showing the ratio of reads in
peaks for those nuclei that passed QC in the panels above. Red line delimits the threshold; nuclei above this line passed QC. B, Heatmap illustrating the row-normalised frequency (from dark
blue/low to yellow/high) of nuclei for each cell type with open chromatin in the transcription start site (TSS) of genes that are expressed specifically in them. Marker gene list has been
curated by using a previously reported transcriptomic atlas, containing this stage8. C, Frequency of nuclei based on their DNA content per cell type. For this plot, we only considered the
nuclei sorted with the “4n” and “2n” gates from Extended Data Fig. 1b. Source data EXTENDED DATA FIG. 3 TRANSCRIPTION FACTOR MOTIF ENRICHMENT ANALYSES. A, Heatmap showing the motif
enrichment scores (NES) for transcription factor (TF) motifs enriched in OCRs uniquely contributing to topics 38, 51 and/or 100. Values are represented by a colour gradient from dark blue
(0) to dark red (8.9). Sequence logos are shown on the left. B, UMAP visualisation showing the motif enrichment scores for GATA1–6 using chromVAR on the 19,453 cells. Values are represented
by a colour gradient from dark blue (low, below 0) to red (high, above 0). Cells with values of 0 are depicted in grey. Sequence logos for each member can be found at the bottom right corner
of each plot. C, Histogram showing the number of regions containing GATA binding sites per topic. Source data EXTENDED DATA FIG. 4 MOTIF ENRICHMENT SCORES AND SHARING BETWEEN GUT AND
SURFACE ECTODERM. A, Complete heatmap of transcription factor motif enrichment _Z_-scores (from blue/low/−1 to red/high/+1) showing all transcription factor (TF) names (extended from Fig.
3b). B, Barplot showing the number of cell-type-specific OCRs that are shared in a defined number of cell types, highlighted on the _x_ axis. C, GO terms for genes associated with regions
specific for surface ectoderm that are not shared with gut (n=1,018). D, GO terms for genes associated with regions specific for gut that are not shared with surface ectoderm (n=1,058). E,
GO terms for genes associated with regions specific for gut and surface ectoderm that are shared between these lineages (n=227). Values obtained from one-sided hyperGTest and BH-corrected.
Source data EXTENDED DATA FIG. 5 ALLANTOIC-HAEMATO-ENDOTHELIAL DEVELOPMENT. A, Simplified diagram of allantoic-haemato-endothelial development. Early mesoderm generates different lineages
including allantoic cells, erythrocytes and endothelial cells (ECs). The precursors of the first wave of primitive erythrocytes express genes commonly associated with ECs, thus making the
distinction between erythroid and endothelial precursors (Haemato-endothelial precursors) difficult by their transcriptomes. Yolk sac (YS) ECs give rise to the definitive blood wave by
generating erythro-myeloid progenitors (EMPs). The allantois also contributes to the EC pool by generating allantoic ECs. B, UMAP visualisation (left) and PAGA representation as in Fig. 7a
(right) of the allantoic-haemato-endothelial landscape (n=3,284 cells) showing the chromatin accessibility at the _Runx1 +23_ _kb_ enhancer. Black dots in UMAP on the right correspond to
nuclei where the region is accessible. Accessibility in PAGA is represented by the ratio of nuclei per cluster (from grey to dark blue) that have _Runx1 +23_ _kb_ accessible. C, PAGA
representation as in Fig. 7a showing the mean enrichment scores per cluster (from grey=0 to dark blue=1) for TAL1 ChIP-seq peaks from haemangioblasts, haemogenic endothelium and
haematopoietic progenitors. Subclusters for PAGA have been defined in Fig. 7a. D, Venn diagram showing the number of endothelial-specific regions from the snATAC-seq dataset, the number of
TAL1-bound regions obtained by ChIP-seq in haemogenic endothelial cells from32, and their overlap. ChIP-seq peaks were taken from http://codex.stemcells.cam.ac.uk/. Source data EXTENDED DATA
FIG. 6 _ERG +85_ _KB_ AND _FLI1 −15 KB_ ENHANCERS. A,B, Genome browser tracks showing the _Erg_ (A) and the _Fli1_ (B) loci. Black arrowheads indicate the _Erg +85_ _kb_ (top) and the _Fli1
−15 kb_ (bottom) enhancers. Tracks correspond to the snATAC-seq profiles of the erythroid, endothelium and allantois cell types after cell pooling, TAL1 ChIP-seq for haemogenic endothelial
cells (“HE TAL1 ChIP-seq”, grey), H3K27ac ChIP-seq for haemogenic endothelial cells (“HE H3K27ac”, gold), TAL1 ChIP-seq for haemangioblasts (“Haem. TAL1 ChIP-seq”, grey), H3K27ac ChIP-seq
for haemangioblasts (“Haem. H3K27ac”, gold), TAL1 ChIP-seq for haematopoietic progenitors (“HP TAL1 ChIP-seq”, grey), H3K27ac ChIP-seq for haematopoietic progenitors (“HP H3K27ac”, gold)
from32, TAL1 ChIP-seq for HPC-7 cells (“HPC-7 TAL1 ChIP-seq”, grey) and DNase-seq for HPC-7 cells (blue). Publicly available tracks were obtained from http://codex.stemcells.cam.ac.uk/. C,
UMAP visualisation of the allantoic-haemato-endothelial landscape (n=3,284 cells) showing the enrichment score (from grey/low to dark blue/high) for HPC-7 TAL1 ChIP-seq peaks. Source data
EXTENDED DATA FIG. 7 _FLT1 +67_ _KB_ AND _MAML3 +360_ _KB_ ENHANCERS. A,B, Genome browser tracks showing the _Flt1_ (A) and the _Maml3_ (B) loci. Black arrowheads indicate the _Flt1 +67_
_kb_ (top) and the _Maml3 +360_ _kb_ (bottom) enhancers. Tracks correspond to the snATAC-seq profiles of the erythroid, endothelium and allantois cell types after cell pooling, TAL1 ChIP-seq
for haemogenic endothelial cells (“HE TAL1 ChIP-seq”, grey), H3K27ac ChIP-seq for haemogenic endothelial cells (“HE H3K27ac”, gold), TAL1 ChIP-seq for haemangioblasts (“Haem. TAL1
ChIP-seq”, grey), H3K27ac ChIP-seq for haemangioblasts (“Haem. H3K27ac”, gold), TAL1 ChIP-seq for haematopoietic progenitors (“HP TAL1 ChIP-seq”, grey), H3K27ac ChIP-seq for haematopoietic
progenitors (“HP H3K27ac”, gold) from32, TAL1 ChIP-seq for HPC-7 cells (“HPC-7 TAL1 ChIP-seq”, grey) and DNase-seq for HPC-7 cells (blue). Publicly available tracks were obtained from
http://codex.stemcells.cam.ac.uk/. EXTENDED DATA FIG. 8 EVOLUTIONARY CONSERVATION OF _FLT1 +67_ _KB_ AND _MAML3 +360_ _KB_. Alignment of _Flt1 +67_ _kb_ (A) and _Maml3 +360_ _kb_ (B) across
species. Transcription factor (TF) binding motifs are boxed: red: Ets sites; yellow: Gata sites; blue: E-box sites; purple box: Runx site. EXTENDED DATA FIG. 9 ENDOTHELIAL DEVELOPMENT FROM
ALLANTOIC CELLS. A, UMAP visualisation (n=3,284 cells) showing the pseudotime trajectory from allantois to endothelium as a gradient from grey to blue. Cells scored as 0 in the plot (grey)
were not part of the trajectory. B, Heatmap showing the -log(_P_ value) obtained from a TF motif enrichment analysis on the accessibility patterns found in Fig. 7b using HOMER. -log(_P_
value) ranges from 0 (dark blue) to 311 (dark red). C, UMAP visualisation of the allantoic-haemato-endothelial landscape (n=3,284 cells) showing the enrichment score (from grey=0 to dark
blue=1) for ETV2 ChIP-seq peaks from41. D, Force-directed graph showing cells from the “Mixed mesoderm”, “Allantois”, “Haemato-endothelial progenitors” and “Endothelium” that have been
profiled with single-cell RNA-seq in8 (n=7,631 cells). Cell colours show the different subclusters found when re-analysing this dataset. E, Expression dynamics of highly variable ETS factors
(variance > 0.15) along the trajectory from mixed mesoderm to endothelium (top) and from allantois to endothelium (bottom). _Cdh5_ and _Pecam1_ have been added as positive controls for
mature endothelium. Dots below plots represent the ordered cells coloured by the subclusters in panel (D). Source data EXTENDED DATA FIG. 10 _FEV_ PLAYS A ROLE IN HAEMATOPOIETIC AND
ENDOTHELIAL DEVELOPMENT. A, WISH showing the expression of _lmo2_, _tal1_, _flk1_ and _gata1a_ in _fev_ mutants at 10 s. Dorsal view, anterior to the top. Red arrowheads indicate increased
expression in _fev__+/+_. B, WISH showing that the expression of _fev_ at 12 s was increased _hsp70-fev_-GFP transgenic embryos after heat-shock at 3 s. C, Western blot showing the protein
level of Fev increased in _hsp70-fev_-GFP transgenic embryos compared to control at 12 s after heat-shock at 3 s. D, WISH of _lmo2_, _tal1_, _gata1a_, _flk1_, _myod_ and _runx1_ in control
embryos (top) and embryos injected with _hsp70-fev_-GFP and _tol2_ mRNA and heat-shocked at 3 s (bottom). Black arrowheads indicate the expression (top) and expanded expression (bottom) in
the PLPM. White arrowheads indicate expression (top) and expanded expression (bottom) in the trunk vessels. Embryos are shown on the dorsal view at 12 s stage, and the lateral view at 28
hpf. E, Genome browser tracks showing the Fev locus. Black arrowhead indicates the _Fev +0.7_ _kb_ region accessible in endothelium and bound by ETV2 _in vitro_. Tracks correspond to the
snATAC-seq profiles of the erythroid, endothelium and allantois cell types after cell pooling, the ETV2 ChIP-seq from41 and evolutionary conservation tracks from UCSC. F, UMAP visualisation
(left) and PAGA representation (right) of the allantoic-haemato-endothelial landscape (n=3,284 cells) showing the chromatin accessibility at the _Fev +0.7_ _kb_ region. Sub-clusters are as
in Fig. 7a. Black dots in UMAP on the right correspond to nuclei where the region is accessible. Accessibility in PAGA is represented by the ratio of nuclei per cluster (from grey to dark
blue) that have _Fev +0.7_ _kb_ accessible. G, WISH analyses showing the expression of _fev_ in PLPM from _etsrp_y11-/- mutants, and _etsrp_y11-/- mutants with _hsp70-fev_-GFP and _tol2_
mRNA under heat-shock treatment at 3 s. Red arrowheads highlight the area where _fev_ is reduced in _etsrp_y11-/- mutants and where it is ectopically expressed after heat-shock treatment. H,
Western blot analysis showing the protein level of Fev in sibling and _etsrp_y11-/- mutants. I, WISH of _lmo2_, _tal1_, _gata1a_, _flk1_, _myod_ and _runx1_ in sibling embryos (top),
_etsrp_y11-/- embryos (middle), and _etsrp_y11-/- embryos co-injected with _hsp70-fev_-GFP and _tol2_ mRNA and heat-shocked at 3 s (bottom). Black arrowheads indicate the expression (top),
reduction of expression (middle) and expanded expression (bottom) in the PLPM. White arrowheads indicate these patterns in the trunk vessels. Embryos are shown on the dorsal view at 12 s
stage, and the lateral view at 28 hpf. Fractions in the panels with zebrafish images depict the number of embryos that showed similar results out of the total number of embryos analysed.
Full, unmodified Western blots corresponding to panels (C) and (H) can be found in the Source Data file corresponding to this figure. Scale bars: 200 µm. Source data SUPPLEMENTARY
INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLES 1–10. SUPPLEMENTARY TABLES 1. NUCLEAR BARCODES. Barcode sequences used to label nuclei in single-nucleus ATAC-seq. File tabs:
I1_index_Tn5_i7: tagmentation barcodes used in the first two plates; I2_E85_embryo_all, I2_E85_embryo_smallnuclei_2n, E85_embryo_largenuclei_4n: barcodes added to nuclei in the PCR step for
the sample sorted indiscriminately, the sample sorted in the “2n” gate and the sample sorted in the “4n” gate of Extended Data Fig. 1b, respectively. SUPPLEMENTARY TABLE 2. NUMBER OF READS
PER SEQUENCING RUN. Table indicating the number of reads sequenced in each run. Each read pair from the paired-end sequencing is counted as 2 reads. SUPPLEMENTARY TABLE 3. RETAINED READS
THROUGHOUT THE PRE-PROCESSING PIPELINE. Table specifying the number of reads in the different categories highlighted in the row names. SUPPLEMENTARY TABLE 4. CONSTITUTIVE PROMOTERS. List of
mm10 constitutive promoters, containing the coordinates of 5,006 promoters (TSS / TSS – 2 kb) that are accessible in the majority of datasets based on ENCODE DNase Hypersensitive Sites and
ATAC-seq data. This list was originally generated for ref. 4. SUPPLEMENTARY TABLE 5. METADATA FOR EACH NUCLEUS. File containing information for each nucleus analysed in this study that
passed quality control (19,453 nuclei). For each nucleus, we have provided the barcode (“barcode” column), gating based on DNA content from Extended Data Fig. 1b (“nuclei_type” column),
number of reads (“num_of_reads” column), promoter coverage (“promoter_coverage” column), number of reads in promoters (“read_in_promoter” column), doublet scores (“doublet_scores” column),
number of reads in peaks (“read_in_peak” column), ratio of reads in peaks (“ratio_peaks” column), UMAP coordinates (“umap_X” and “umap_Y” columns), final clusters (“final_clusters” column),
cell type annotation (“ann” column) and sub-clusters for the allantoic-haemato-endothelial landscape (“al_haem_endo_clusters” column). Values for each topic are also included. SUPPLEMENTARY
TABLE 6. METADATA FOR EACH GENOMIC REGION. File containing information for each genomic region analysed in this study. For each genomic region, we have provided the peak ID; peak coordinates
(chromosome, start and end); their general annotation (TSS (−1kb to +100 bp), TTS (−100 bp to +1 kb), intron, exon, intergenic); their distance from the TSS that have been annotated to if
the region is intergenic; and the gene name, ensemble ID and strand of the genes they has been annotated to (if multiple genes have been annotated to the peak, the peak entry will be
repeated). If the region is cell-type-specific, the cell type(s) where it is specific can be found in the “celltype_specificity” column. If the region contributes to a particular topic, you
can find what topic(s) it contributes to in the “topic” column. “topic_stringent” gives the topic information if the regions only contribute to one topic. This table also gives information
on the UMAP coordinates for visualisation in Fig. 4a, and the number of nuclei with each genomic region accessible in linear (“accessibility”) and log10 form (“accessibility_log”). If the
region is part of a dynamic pattern during endothelial establishment, you will find the pattern number in the “Pattern_endothelium” column. Please note that some peak entries may be repeated
due to them being annotated to multiple genes. Therefore, if one wants to plot unique regions independently of metadata regarding gene annotation, we advise to make metadata unique by using
the peakID column. SUPPLEMENTARY TABLE 7. ENDOTHELIAL-SPECIFIC TAL1-BOUND OPEN CHROMATIN REGIONS. File containing the coordinates of endothelial-specific open chromatin regions that
intersect with TAL1 ChIP-seq peaks from haemogenic endothelium. SUPPLEMENTARY TABLE 8. ENDOTHELIAL-SPECIFIC HEPTAD-BOUND OPEN CHROMATIN REGIONS. File containing the coordinates of
endothelial-specific open chromatin regions, already intersected with TAL1 ChIP-seq peaks from haemogenic endothelium, that intersect with HPC-7 ChIP-seq peaks reported as heptad peaks in
ref. 34. SUPPLEMENTARY TABLE 9. MOUSE TRANSGENIC ASSAYS IN NUMBERS. Number of E11.5 mouse transgenic embryos with LacZ staining in different regions (column names). FL: Fetal Liver. YS: Yolk
Sac. SUPPLEMENTARY TABLE 10. METADATA FOR SINGLE-CELL RNA-SEQ SAMPLES. File containing information for each cell from ref. 8 analysed in this study. For each cell (row), we have provided
the cell name, cell barcode, sample stage, sequencing batch and cell type as in ref. 8. Additionally, we provide the force-directed graph coordinates computed for Fig. 7d-f and Extended Data
Fig. 9e (“FA_X”, “FA_Y”), the subcluster identity (“Louvain subclust”), the pseudotime values for the allantoic-to-endothelium trajectory (“DPT_al”) and for the mesoderm-to-endothelium
trajectory (“DPT_meso”). SOURCE DATA SOURCE DATA FIG. 1 Statistical source data to reproduce figure SOURCE DATA FIG. 2 Statistical source data to reproduce figure SOURCE DATA FIG. 3
Statistical source data to reproduce figure SOURCE DATA FIG. 4 Statistical source data to reproduce figure SOURCE DATA FIG. 5 Statistical source data to reproduce figure SOURCE DATA FIG. 7
Statistical source data to reproduce figure SOURCE DATA EXTENDED DATA FIG. 2 Statistical source data to reproduce figure SOURCE DATA EXTENDED DATA FIG. 3 Statistical source data to reproduce
figure SOURCE DATA EXTENDED DATA FIG. 4 Statistical source data to reproduce figure SOURCE DATA EXTENDED DATA FIG. 5 Statistical source data to reproduce figure SOURCE DATA EXTENDED DATA
FIG. 6 Statistical source data to reproduce figure SOURCE DATA EXTENDED DATA FIG. 9 Statistical source data to reproduce figure SOURCE DATA EXTENDED DATA FIG. 10 Statistical source data to
reproduce figure SOURCE DATA EXTENDED DATA FIG. 10 Unprocessed western blots RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pijuan-Sala, B., Wilson,
N.K., Xia, J. _et al._ Single-cell chromatin accessibility maps reveal regulatory programs driving early mouse organogenesis. _Nat Cell Biol_ 22, 487–497 (2020).
https://doi.org/10.1038/s41556-020-0489-9 Download citation * Received: 25 July 2019 * Accepted: 20 February 2020 * Published: 30 March 2020 * Issue Date: April 2020 * DOI:
https://doi.org/10.1038/s41556-020-0489-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
