Nucleus-exported clock acetylates prps to promote de novo nucleotide synthesis and liver tumour growth
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Impairment of the circadian clock is linked to cancer development. However, whether the circadian clock is modulated by oncogenic receptor tyrosine kinases remains unclear. Here we
demonstrated that receptor tyrosine kinase activation promotes CK2-mediated CLOCK S106 phosphorylation and subsequent disassembly of the CLOCK–BMAL1 dimer and suppression of the downstream
gene expression in hepatocellular carcinoma (HCC) cells. In addition, CLOCK S106 phosphorylation exposes its nuclear export signal to bind Exportin1 for nuclear exportation. Cytosolic CLOCK
acetylates PRPS1/2 K29 and blocks HSC70-mediated and lysosome-dependent PRPS1/2 degradation. Stabilized PRPS1/2 promote de novo nucleotide synthesis and HCC cell proliferation and liver
tumour growth. Furthermore, CLOCK S106 phosphorylation and PRPS1/2 K29 acetylation are positively correlated in human HCC specimens and with HCC poor prognosis. These findings delineate a
critical mechanism by which oncogenic signalling inhibits canonical CLOCK transcriptional activity and simultaneously confers CLOCK with instrumental moonlighting functions to promote
nucleotide synthesis and tumour growth. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this
journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now
Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS DNA POLYMERASE BETA CONNECTS TUMORIGENICITY WITH THE CIRCADIAN CLOCK IN LIVER CANCER THROUGH THE EPIGENETIC DEMETHYLATION OF _PER1_ Article
Open access 20 January 2024 CHROMATIN-ASSOCIATED OGT PROMOTES THE MALIGNANT PROGRESSION OF HEPATOCELLULAR CARCINOMA BY ACTIVATING ZNF263 Article 23 June 2023 ANTI-ONCOGENE PTPN13
INACTIVATION BY HEPATITIS B VIRUS X PROTEIN COUNTERACTS IGF2BP1 TO PROMOTE HEPATOCELLULAR CARCINOMA PROGRESSION Article Open access 13 October 2020 DATA AVAILABILITY Mass spectrometry data
have been deposited in ProteomeXchange with the accession code PXD037738. UniProt protein database (EMBL-EBI) was used for protein identification. All other data supporting the findings of
this study are available from the corresponding author on reasonable request. Source data are provided with this paper. REFERENCES * Kinouchi, K. & Sassone-Corsi, P. Metabolic rivalry:
circadian homeostasis and tumorigenesis. _Nat. Rev. Cancer_ 20, 645–661 (2020). Article CAS PubMed Google Scholar * Bass, J. & Takahashi, J. S. Circadian integration of metabolism
and energetics. _Science_ 330, 1349–1354 (2010). Article CAS PubMed PubMed Central Google Scholar * Sahar, S. & Sassone-Corsi, P. Metabolism and cancer: the circadian clock
connection. _Nat. Rev. Cancer_ 9, 886–896 (2009). Article CAS PubMed Google Scholar * Reinke, H. & Asher, G. Crosstalk between metabolism and circadian clocks. _Nat. Rev. Mol. Cell
Biol._ 20, 227–241 (2019). Article CAS PubMed Google Scholar * Patke, A., Young, M. W. & Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. _Nat.
Rev. Mol. Cell Biol._ 21, 67–84 (2020). Article CAS PubMed Google Scholar * Ye, Y. et al. The genomic landscape and pharmacogenomic interactions of clock genes in cancer chronotherapy.
_Cell Syst._ 6, 314–328 e312 (2018). Article CAS PubMed PubMed Central Google Scholar * Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. _Cell
Metab._ 24, 324–331 (2016). Article CAS PubMed PubMed Central Google Scholar * Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. The circadian gene Period2 plays an important role
in tumor suppression and DNA damage response in vivo. _Cell_ 111, 41–50 (2002). Article CAS PubMed Google Scholar * Lee, S., Donehower, L. A., Herron, A. J., Moore, D. D. & Fu, L.
Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. _PLoS One_ 5, e10995 (2010). Article PubMed PubMed Central Google Scholar * Ruan, W., Yuan,
X. & Eltzschig, H. K. Circadian rhythm as a therapeutic target. _Nat. Rev. Drug Discov._ 20, 287–307 (2021). Article CAS PubMed PubMed Central Google Scholar * Dong, Z. et al.
Targeting glioblastoma stem cells through disruption of the circadian clock. _Cancer Discov._ 9, 1556–1573 (2019). Article CAS PubMed PubMed Central Google Scholar * Breuhahn, K. &
Schirmacher, P. Reactivation of the insulin-like growth factor-II signaling pathway in human hepatocellular carcinoma. _World J. Gastroenterol._ 14, 1690–1698 (2008). Article CAS PubMed
PubMed Central Google Scholar * Xu, D. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. _Nature_ 580, 530–535 (2020). Article CAS PubMed Google Scholar *
Ji, H. et al. EGF-induced ERK activation promotes CK2-mediated disassociation of α-Catenin from β-Catenin and transactivation of β-Catenin. _Mol. Cell._ 36, 547–559 (2009). Article CAS
PubMed PubMed Central Google Scholar * Paci, G., Caria, J. & Lemke, E. A. Cargo transport through the nuclear pore complex at a glance. _J. Cell Sci._
https://doi.org/10.1242/jcs.247874 (2021). * Elfgang, C. et al. Evidence for specific nucleocytoplasmic transport pathways used by leucine-rich nuclear export signals. _Proc. Natl Acad. Sci.
USA_ 96, 6229–6234 (1999). Article CAS PubMed PubMed Central Google Scholar * Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine
synthesis by growth signaling through mTOR and S6K1. _Science_ 339, 1323–1328 (2013). Article CAS PubMed PubMed Central Google Scholar * Doi, M., Hirayama, J. & Sassone-Corsi, P.
Circadian regulator CLOCK is a histone acetyltransferase. _Cell_ 125, 497–508 (2006). Article CAS PubMed Google Scholar * Hirayama, J. et al. CLOCK-mediated acetylation of BMAL1 controls
circadian function. _Nature_ 450, 1086–1090 (2007). Article CAS PubMed Google Scholar * Lin, R. et al. CLOCK acetylates ASS1 to drive circadian rhythm of ureagenesis. _Mol. Cell_ 68,
198–209 e196 (2017). Article CAS PubMed Google Scholar * Liu, R. et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. _Mol. Cell_ 81, 2722–2735
e2729 (2021). Article CAS PubMed Google Scholar * Cuervo, A. M. & Wong, E. Chaperone-mediated autophagy: roles in disease and aging. _Cell Res_ 24, 92–104 (2014). Article CAS
PubMed Google Scholar * Kaushik, S. & Cuervo, A. M. The coming of age of chaperone-mediated autophagy. _Nat. Rev. Mol. Cell Biol._ 19, 365–381 (2018). Article CAS PubMed PubMed
Central Google Scholar * Wong, K. M., King, G. G. & Harris, W. P. The treatment landscape of advanced hepatocellular carcinoma. _Curr. Oncol. Rep._ 24, 917–927 (2022). Article CAS
PubMed Google Scholar * Chaudhari, A., Gupta, R., Patel, S., Velingkaar, N. & Kondratov, R. Cryptochromes regulate IGF-1 production and signaling through control of JAK2-dependent
STAT5B phosphorylation. _Mol. Biol. Cell_ 28, 834–842 (2017). Article CAS PubMed PubMed Central Google Scholar * Crosby, P. et al. Insulin/IGF-1 drives period synthesis to entrain
circadian rhythms with feeding time. _Cell_ 177, 896–909 e820 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhang, J., Lv, H., Ji, M., Wang, Z. & Wu, W. Low circadian
clock genes expression in cancers: a meta-analysis of its association with clinicopathological features and prognosis. _PLoS ONE_ 15, e0233508 (2020). Article CAS PubMed PubMed Central
Google Scholar * Li, X. et al. Nucleus-translocated ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. _Mol. Cell_ 66, 684–697 e689 (2017). Article CAS PubMed
PubMed Central Google Scholar * Lee, J. H. et al. EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. _Mol. Cell_ 70, 197–210 e197 (2018). Article
PubMed PubMed Central Google Scholar * Lee, J. H. et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. _Nat. Commun._ 8, 949 (2017). Article
PubMed PubMed Central Google Scholar * Li, X. et al. A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. _Nat. Cell Biol._ 18, 561–571
(2016). Article CAS PubMed PubMed Central Google Scholar * Qian, X. et al. Conversion of PRPS hexamer to monomer by AMPK-mediated phosphorylation inhibits nucleotide synthesis in
response to energy stress. _Cancer Discov._ 8, 94–107 (2018). Article CAS PubMed Google Scholar * Xu, D. Q. et al. PAQR3 controls autophagy by integrating AMPK signaling to enhance
ATG14L-associated PI3K activity. _EMBO J._ 35, 496–514 (2016). Article CAS PubMed PubMed Central Google Scholar * Qian, X. et al. KDM3A senses oxygen availability to regulate
PGC-1α-mediated mitochondrial biogenesis. _Mol. Cell_ 76, 885–895 e887 (2019). Article CAS PubMed Google Scholar * Qian, X. et al. PTEN suppresses glycolysis by dephosphorylating and
inhibiting autophosphorylated PGK1. _Mol. Cell_ 76, 516–527 e517 (2019). Article CAS PubMed Google Scholar * Xu, D. et al. The protein kinase activity of fructokinase A specifies the
antioxidant responses of tumor cells by phosphorylating p62. _Sci. Adv._ 5, eaav4570 (2019). Article CAS PubMed PubMed Central Google Scholar * Lin, S. Y. et al. GSK3-TIP60-ULK1
signaling pathway links growth factor deprivation to autophagy. _Science_ 336, 477–481 (2012). Article CAS PubMed Google Scholar * Du, L. et al. β-Catenin induces transcriptional
expression of PD-L1 to promote glioblastoma immune evasion. _J. Exp. Med._ https://doi.org/10.1084/jem.20191115 (2020). * Xu, D. et al. PAQR3 modulates cholesterol homeostasis by anchoring
Scap/SREBP complex to the Golgi apparatus. _Nat. Commun._ 6, 8100 (2015). Article CAS PubMed Google Scholar * Yu, R., Craik, D. J. & Kaas, Q. Blockade of neuronal α7-nAChR by
α-conotoxin ImI explained by computational scanning and energy calculations. _PLoS Comput. Biol._ 7, e1002011 (2011). Article CAS PubMed PubMed Central Google Scholar * Yu, R. et al.
Molecular determinants conferring the stoichiometric-dependent activity of α-conotoxins at the human α9α10 nicotinic acetylcholine receptor subtype. _J. Med. Chem._ 61, 4628–4634 (2018).
Article CAS PubMed Google Scholar * Yang, W. et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. _Nature_ 480, 118–122 (2011). Article CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China
(2020YFA0803300, Z.L.; 2021YFA0805600, D.X.), the National Natural Science Foundation of China (92157113 and 82072630, D.X.; 82173114, Z.W.; 82072903 and 82272872, T.L.; 82002811, M. Yan;
82188102 and 82030074, Z.L.), the Zhejiang Natural Science Foundation Key Project (LD22H160002, D.X.; LD21H160003, Z.L.), Zhejiang Natural Science Foundation Discovery Project (LQ22H160023,
Z.W.), and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01001, Z.L.). Z.L. is the Kuancheng Wang Distinguished Chair. The authors received no specific
funding for this work. We express our great gratitude to Dr. Hong Wang and his team from the Hangzhou Cosmos Wisdom Mass Spectrometry Center of Zhejiang University Medical School for their
technical support in sample analysis utilizing an integrated nanoLC–ESI–MS/MS and data processing platform. AUTHOR INFORMATION Author notes * These authors contributed equally: Tong Liu,
Zheng Wang, Leiguang Ye, Yuran Duan. AUTHORS AND AFFILIATIONS * Zhejiang Provincial Key Laboratory of Pancreatic Disease, The First Affiliated Hospital, Institute of Translational Medicine,
Zhejiang University School of Medicine, Zhejiang University, Hangzhou, China Tong Liu, Zheng Wang, Yuran Duan, Haiyan He, Liwei Xiao, Qingang Wu, Ke Wu, Guimei Ji, Yuli Shen, Lei Wang, Lin
Li, Peixiang Zheng, Bofei Dong, Zhimin Lu & Daqian Xu * Cancer Center, Zhejiang University, Hangzhou, China Tong Liu, Zheng Wang, Haiyan He, Liwei Xiao, Qingang Wu, Zhimin Lu &
Daqian Xu * Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, China Tong Liu * NHC Key Laboratory of Cell Transplantation, The First Affiliated Hospital of
Harbin Medical University, Harbin, China Tong Liu & Zhiren Zhang * Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China Leiguang Ye * Harbin, China
Leiguang Ye * The Affiliated Hospital of Qingdao University and Qingdao Cancer Institute, Qingdao, China Hongfei Jiang & Fei Shao * Department of Cancer Biology, Department of
Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA Yan Xia * Key Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy,
Ocean University of China, Qingdao, China Mengke Yang & Rilei Yu * Department of Pathology, Harbin Medical University, Harbin, China Meisi Yan * Department of Clinical Laboratory, Cancer
Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China Xu Qian
Authors * Tong Liu View author publications You can also search for this author inPubMed Google Scholar * Zheng Wang View author publications You can also search for this author inPubMed
Google Scholar * Leiguang Ye View author publications You can also search for this author inPubMed Google Scholar * Yuran Duan View author publications You can also search for this author
inPubMed Google Scholar * Hongfei Jiang View author publications You can also search for this author inPubMed Google Scholar * Haiyan He View author publications You can also search for this
author inPubMed Google Scholar * Liwei Xiao View author publications You can also search for this author inPubMed Google Scholar * Qingang Wu View author publications You can also search
for this author inPubMed Google Scholar * Yan Xia View author publications You can also search for this author inPubMed Google Scholar * Mengke Yang View author publications You can also
search for this author inPubMed Google Scholar * Ke Wu View author publications You can also search for this author inPubMed Google Scholar * Meisi Yan View author publications You can also
search for this author inPubMed Google Scholar * Guimei Ji View author publications You can also search for this author inPubMed Google Scholar * Yuli Shen View author publications You can
also search for this author inPubMed Google Scholar * Lei Wang View author publications You can also search for this author inPubMed Google Scholar * Lin Li View author publications You can
also search for this author inPubMed Google Scholar * Peixiang Zheng View author publications You can also search for this author inPubMed Google Scholar * Bofei Dong View author
publications You can also search for this author inPubMed Google Scholar * Fei Shao View author publications You can also search for this author inPubMed Google Scholar * Xu Qian View author
publications You can also search for this author inPubMed Google Scholar * Rilei Yu View author publications You can also search for this author inPubMed Google Scholar * Zhiren Zhang View
author publications You can also search for this author inPubMed Google Scholar * Zhimin Lu View author publications You can also search for this author inPubMed Google Scholar * Daqian Xu
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.X. and Z.L. conceived and designed the study and wrote the manuscript. T.L., Z.W., L.Y.,
Y.D., H.H., K.W., M. Yan, G.J., Y.S., L.W., L.L., P.Z., B.D., F.S. and Z.Z. performed the experiments; H.J., M. Yang and R.Y. were involved in MD simulation analyses. Y.X., L.X., Q.W. and
X.Q. reviewed and edited the manuscript. CORRESPONDING AUTHORS Correspondence to Zhimin Lu or Daqian Xu. ETHICS DECLARATIONS COMPETING INTERESTS Z.L. owns shares in Signalway Biotechnology
(Pearland, TX), which supplied rabbit antibodies that recognize CLOCK pS106 and PRPS1/2 K29ac. Z.L.’s interest in this company had no bearing on its being chosen to supply these reagents.
The remaining authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Cell Biology_ thanks Lars Zender, Tsuyoshi Hirota and the other, anonymous, reviewer(s) for
their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
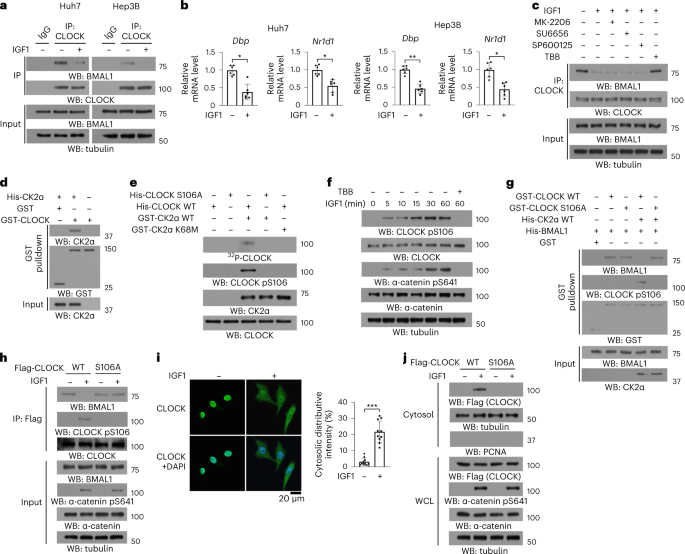
institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 CLOCK S106 PHOSPHORYLATION DISASSEMBLES THE CLOCK–BMAL1 COMPLEX. (B-E, H, J, K, M, N) Immunoprecipitation and immunoblotting
with the indicated antibodies was performed. All experiments were repeated at least twice independently. Data are the mean ± SD. (A) The luciferase activity of the indicated cells expressing
a Per1-driven luciferase reporter was measured after IGF1 treatment (12 h) (n = 6). *_P_ < 0.01; **_P_ < 0.001 by One-way ANOVA post hoc test. (B, C, M, N) The indicated cells were
pretreated with or without the indicated inhibitors for 30 min before IGF1 treatment for 30 min (B, C, M) or transfected with the indicated plasmids before IGF1 treatment for 1 h (N). Total
cell lysates and cytosolic fractions were prepared. (D) Flag-CK2α immunoprecipitated from Huh7 cells treated with or without IGF1 for 30 min was incubated with or without CIP for 30 min. (E)
Purified GST-CK2α was mixed with or without active His-ERK2 for _in vitro_ kinase assay, followed by incubation with purified His-CLOCK. Proteins precipitated by GST pulldown assay were
incubated with or without CIP for 30 min. (F) _In vitro_ kinase assays were performed by mixing purified His–CLOCK with or without purified GST-CK2α in the presence of ATP.
Mass-spectrometric analysis was performed. (G) Alignment of CLOCK to the consensus CK2α-phosphorylated substrate motif (SXXD/E). (H, I) Huh7 cells expressing Flag-CLOCK were treated with or
without IGF1 for 1 h (H). Immunoblotting (H) or IHC analyses of human HCC samples (I) were performed with the indicated antibodies and a CLOCK pS106-blocking peptide. (J-L) The indicated
cells expressing the indicated plasmids (J) were treated with or without IGF1 for 1 h (J-L). Total cell lysates and cytosolic and nuclear fractions were prepared (K). The relative CLOCK
abundance was quantified (n = 10) (L). *_P_ < 0.05; ***_P_ < 0.0001 by two-tailed Student’s t test. Source data EXTENDED DATA FIG. 2 CLOCK S106 PHOSPHORYLATION IS REQUIRED FOR ITS
NUCLEAR EXPORTATION. (E-I) Immunoprecipitation and immunoblotting analyses were performed with the indicated antibodies. All experiments were repeated three times independently with similar
results. (A) Alignment of protein sequences spanning CLOCK S106 and the adjacent noncanonical NES from different species. (B, D) MD simulation of CLOCK bound with BMAL1 WT (B) or BMAL1 4Mut
(D144A/D231A/D299A/E303A) (D). Evolution of the backbone root-mean-square-deviation (RMSD) of the CLOCK-BMAL1 complex from the initial frame in 250 ns MD simulations. RMSD of the CLOCK-BMAL1
complex with and without CLOCK S106 phosphorylation are shown in red and black, respectively. (C) The conformation of the CLOCK-BMAL1 complex of the last frame extracted from 250 ns MD
simulation. CLOCK and BMAL1 structures are shown in green and blue, respectively. The red arrow indicates CLOCK S106. D144, D232, D299, and E303 of BMAL1, which are in a close proximity with
CLOCK S106, are shown. (E) Bacterially purified WT GST-CLOCK or GST-CLOCK S106A was incubated with or without His-CK2α in the presence of ATP for an _in vitro_ kinase assay, followed by
incubation with the indicated His-BMAL1 proteins. (F) Huh7 cells stably transfected with the indicated plasmids were treated with or without IGF1 for 1 h. Total cell lysates and cytosolic
fractions were prepared. (G) Huh7 cells were pretreated with or without TBB for 30 min before treatment with or without IGF1 for 1 h. (H) The indicated cells expressing CLOCK shRNA with
reconstituted expression of the indicated CLOCK proteins were harvested. (I) Hep3B cells expressing CLOCK shRNA with reconstituted expression of Flag-rCLOCK proteins were treated with or
without IGF1 for 1 h. Source data EXTENDED DATA FIG. 3 CLOCK ACETYLATES PRPS1/2 K29. (D, J, M) Immunoprecipitation and immunoblotting with the indicated antibodies was performed. All
experiments were repeated at least twice independently. (A) Huh7 cells with or without expression of Flag–CLOCK were treated with or without IGF1 for 1 h. The immunoprecipitated Flag–CLOCK
was eluted with Flag peptide and stained with Coomassie Brilliant Blue after SDS-PAGE. Mass spectrometry-identified protein peptide hits are shown. (B) Purified GST–CLOCK was incubated with
or without His-CK2α and TBB for an _in vitro_ kinase assay, followed by incubation with or without the indicated His-PRPS1/2 proteins in the presence of acetyl-CoA. Mass-spectrometric
analysis was performed. (C) Alignment of protein sequences spanning PRPS1/2 K29 from different species. (D, E) Huh7 cells expressing Flag-PRPS1 were treated with or without IGF1 for 1 h (D).
Immunoblotting analyses (D) or IHC analyses of human HCC samples (E) were performed with the indicated antibodies and a PRPS1/2 K29 acetylation-blocking peptide. (F-J) Genomic DNA was
extracted from two individual clones of the indicated cells with knock-in expression of PRPS1/2 K29R. PCR products amplified from the indicated DNA fragments were shown (F, G) and sequenced
(H, I). The red line indicates the sgRNA-targeting sequence. The black line indicates the PAM. Blue arrows indicate mutated nucleotides. A mutated amino acid and its WT counterpart are
indicated by the solid red box (H, I). These cells were transfected with or without constitutively active IGF1R-CA (J). (K) Purified GST–CLOCK were incubated with or without His-CK2α for an
_in vitro_ kinase assay, followed by incubation with or without the indicated His-PRPS1/2 proteins in the presence of acetyl-CoA. (L, M) Huh7 (L, M) and Hep3B (L) cells expressing CLOCK
shRNA with reconstituted expression of the indicated CLOCK proteins were harvested or (L) treated with or without IGF1 for 1 h (M). Source data EXTENDED DATA FIG. 4 CYTOSOLIC CLOCK PROMOTES
PRPS1/2 STABILIZATION. (A, D-H, J, K, L, O) Immunoprecipitation and immunoblotting with the indicated antibodies was performed. All experiments were repeated three times independently. (A)
The indicated cells were stably transfected with active IGF1R-CA. (B, C) Huh7 cells stimulated with IGF1 for the indicated time (B) or expressing IGF1R-CA (C) were harvested. The mRNA
expression levels of the PRPS1/2 genes were measured using qPCR (n = 6). Data are the mean ± SD. N.S., not significant by One-way ANOVA post hoc test (B) or two-tailed Student’s t test (C).
(D-G) The indicated cells expressing CLOCK shRNA and active IGF1R-CA with reconstituted expression of the indicated CLOCK protein were treated with CHX for the indicated time (D, F). The
quantification of PRPS1/2 levels is shown. Data are the mean ± SD, n = 6, **_P_ < 0.001; ***_P_ < 0.0001 by One-way ANOVA post hoc test (E, G). (H) The indicated cells were treated
with DMSO, MG132 (10 μM), PS341 (10 μM), CQ (25 μM) or bafilomycin A1 (BFA) (10 nM) for 8 h. (I) Alignment of protein sequences spanning PRPS1/2 K29 and the adjacent HSC70 recognized motif
from different species. (J) LAMP2A shRNA was expressed in the indicated cells. (K-N) Huh7 cells expressing PRPS1/2 shRNA with reconstituted expression of the indicated PRPS1/2 proteins were
harvested for immunoblotting (k, l) or qPCR (n = 6) to measure _Prps1/2_ mRNA levels (m, n). Data are the mean ± SD. N.S., not significant by One-way ANOVA post hoc test. (O) Endogenous
CLOCK-depleted Huh7 cells with reconstituted expression of the indicated CLOCK protein were transfected with HA-PRPS1/2 and treated with or without IGF1 for 1 h. Immunoprecipitation using
limited amount of HA antibody was performed. Source data EXTENDED DATA FIG. 5 CLOCK-MEDIATED PRPS1/2 STABILIZATION PROMOTES _DE NOVO_ NUCLEOTIDE SYNTHESIS. (G-L) Immunoprecipitation and
immunoblotting with the indicated antibodies was performed. All experiments were repeated at least twice independently. (A-G) Data are the mean ± SD, n = 6, *_P_ < 0.01; **_P_ < 0.001;
***_P_ < 0.0001; N.S., not significant by One-way ANOVA post hoc test. (A-F) The indicated cells expressing CLOCK shRNA with reconstituted expression of the indicated CLOCK proteins (A,
B, E) or the indicated clones with knock-in expression of PRPS1/2 K29R (C, D, F) were transfected with or without active IGF1R-CA, followed by labeling with D-[6-14C] glucose for 30 min. The
amounts of 14C-RNA (A, C) and 14C-DNA (B, D) were measured. The 13C-labeled PRPP, IMP, AMP, GMP, UMP, and CMP were measured by LC/MS-MS (E, F). (G) Purified GST–CLOCK was incubated with or
without His-CK2α in the presence of ATP for an _in vitro_ kinase assay, followed by incubation with or without the indicated His-PRPS1/2 proteins in the presence of acetyl-CoA. The PRPS1/2
activity was measured. (H, I) Huh7 cells expressing CLOCK shRNA with reconstituted expression of the indicated Flag-rCLOCK were serum-starved for 12 h in the presence of CQ and then treated
with or without EGF (H) or FGF1 (I) for 1 h. Cytosolic and whole cell lysates were harvested. (J) Huh7 cells were stimulated with EGF (100 ng/ml) or FGF1 (25 ng/ml) for the indicated time.
(K) Huh7 cells stably transfected with constitutively active EGFR-vIII or FGFR1-CA were harvested. (L) Huh7 cells expressing CLOCK shRNA and constitutively active EGFR-vIII or FGFR1-CA with
reconstituted expression of the indicated Flag-rCLOCK were treated with CHX for the indicated time. The quantification of PRPS1/2 protein levels is shown. Data are the mean ± SD, **_P_ <
0.001 by One-way ANOVA post hoc test. Source data EXTENDED DATA FIG. 6 OVEREXPRESSED CK2Α IN HCC CELLS IS CRITICAL FOR CLOCK-ENHANCED PRPS1/2 STABILITY. (A, B, D-L) Immunoprecipitation and
immunoblotting with the indicated antibodies was performed. All experiments were repeated three times independently. (C, D, G, I) Data are the mean ± SD. n = 6, *_P_ < 0.01; **_P_ <
0.001; ***_P_ < 0.0001 by One-way ANOVA post hoc test. (A, B) The indicated cells expressing CLOCK shRNA with reconstituted expression of the indicated shRNA-resistant CLOCK were
constructed (A) and serum-starved for 12 h in the presence of CQ before treatment with or without IGF1 for 1 h (B). Cytosolic and whole cell lysates were harvested. (C) The indicated cells
with reconstituted expression of the indicated CLOCK proteins were treated with or without IGF1 for 12 h, followed by labeling with D-[6-14C] glucose for 30 min. The amounts of 14C-RNA and
14C-DNA were measured. (D) The indicated cells expressing CLOCK shRNA and IGF1R-CA with reconstituted expression of Flag-rCLOCK were treated with CHX for the indicated time. The
quantification of PRPS1/2 levels is shown. (E, F) The indicated cells were treated with or without IGF1 for 1 h. Whole cell lysates were harvested. (G) L02 cells transfected with the
indicated plasmids were treated with CHX for the indicated time in the presence or absence of IGF1. The quantification of PRPS1/2 levels is shown. (H) Huh7 cells transfected with the
indicated shRNA were treated with or without IGF1 for 1 h. (I) Huh7 cells transfected with the indicated shRNA were treated with CHX for the indicated time in the presence or absence of
IGF1. The quantification of PRPS1/2 levels is shown. Source data EXTENDED DATA FIG. 7 BOTH NUCLEAR TRANSCRIPTIONAL ACTIVITY AND CYTOSOLIC FUNCTION OF CLOCK CONTRIBUTE TO HCC CELL
PROLIFERATION. (A, D, E, I, J, L, M) Immunoprecipitation and immunoblotting with the indicated antibodies was performed. All experiments were repeated at least twice independently. (A-C,
E-G, H, J, K, N) Data are the mean ± SD. *_P_ < 0.01; **_P_ < 0.001; ***_P_ < 0.0001 by One-way ANOVA post hoc test. (A-C) The indicated cells stably transfected with the indicated
plasmids and shRNA were constructed (A) and transiently expressed a Per1-driven luciferase reporter before IGF1 treatment for 12 h. The luciferase activity is shown (n = 6). The mRNA levels
of CLOCK-downstream target genes were measured using qPCR (n = 6) (C). Cytosolic and whole cell lysates from Huh7 cells were harvested (D). (E) Huh7 cells transfected with the indicated
plasmids and shRNA were treated with CHX. PRPS1/2 protein was quantified (n = 6). (F, G) The indicated cells transfected with the indicated plasmids and shRNA were labeled with D-[6-14C]
glucose. The amounts of 14C-RNA (F) and 14C-DNA (G) were measured (n = 6). (H) The indicated cells transfected with the indicated plasmids and shRNA were plated in complete culture medium
and counted (n = 6). (I) Huh7 cells were pretreated with or without different dose of Sorafenib for 30 min before IGF1 treatment for 1 h. (J, K) The indicated cells expressing CLOCK shRNA
and active IGF1R-CA with reconstituted expression of Flag-rCLOCK WT or S106D were treated with CHX (J) or without CHX (K) in the presence or absence of Sorafenib for the indicted time.
PRPS1/2 protein quantification (J) and cell counting (K) were performed (n = 6). (L-N) The indicated cells with reconstituted expression of Flag-rCLOCK WT or S106D were harvested (L) or
treated with CHX (M) or without CHX (N) for the indicated time. PRPS1/2 protein quantification (M) and cell counting (N) were performed (n = 6). Source data EXTENDED DATA FIG. 8
CLOCK-MEDIATED PRPS1/2 STABILIZATION PROMOTES HCC CELL PROLIFERATION AND LIVER TUMOR GROWTH. (A, B) Huh7 cells (1 × 106) expressing CLOCK shRNA with reconstituted expression of the indicated
CLOCK proteins (A) expressing WT PRPS1/2 or PRPS1/2 K29R knock-in mutants (B) were subcutaneously injected into athymic nude mice (n = 6 per group). The mice were euthanized and examined
for tumor growth 28 days after injection. Tumor volumes were calculated, and the tumors were weighed. Data are the mean ± SD, n = 7, *_P_ < 0.01; **_P_ < 0.001 by One-way ANOVA post
hoc test. (C, D) IHC analyses of the indicated tumor samples were performed with an anti-Ki67 antibody. Ki67-positive cells were quantified. Data are the mean ± SD, n = 10, ***_P_ <
0.0001 by One-way ANOVA post hoc test. (E, F) TUNEL analyses of the indicated tumor samples were performed (upper). Apoptotic cells were stained brown and quantified in n = 10 microscopic
fields (lower). Data are the mean ± SD, ***_P_ < 0.0001 by One-way ANOVA post hoc test. (G) Huh7 cells (1 × 106) expressing CLOCK shRNA with reconstituted expression of the indicated
CLOCK proteins were intrahepatically injected into athymic nude mice (n = 6 per group). The mice were euthanized and examined for tumor growth 22 days after injection. The arrows indicate
tumors (left). The tumor volumes were measured (right). Data are the mean ± SD, n = 6, *_P_ < 0.01 by two-tailed Student’s t test. (H) Huh7 cells (1 × 106) expressing CLOCK shRNA with
reconstituted expression of the indicated CLOCK proteins were subcutaneously injected into athymic nude mice (n = 7 per group). The resulting tumors were resected 22 days after injection
(left). The growth of xenografted tumors in the mice was measured (middle) and the tumors were weighed (right). Data are the mean ± SD, **_P_ < 0.001 by two-tailed Student’s t test.
Source data SUPPLEMENTARY INFORMATION REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1 Unprocessed WBs. SOURCE DATA FIG. 2 Unprocessed WBs. SOURCE DATA FIG. 3 Unprocessed WBs. SOURCE DATA
FIG. 4 Unprocessed WBs. SOURCE DATA EXTENDED DATA FIG. 1 Unprocessed WB. SOURCE DATA EXTENDED DATA FIG. 2 Unprocessed WBs. SOURCE DATA EXTENDED DATA FIG. 3 Unprocessed WB. SOURCE DATA
EXTENDED DATA FIG. 4 Unprocessed WBs. SOURCE DATA EXTENDED DATA FIG. 5 Unprocessed WB. SOURCE DATA EXTENDED DATA FIG. 6 Unprocessed WBs. SOURCE DATA EXTENDED DATA FIG. 7 Unprocessed WBs.
SOURCE DATA TABLE FIG. 1 Statistical source data. SOURCE DATA TABLE FIG. 2 Statistical source data. SOURCE DATA TABLE FIG. 4 Statistical source data. SOURCE DATA TABLE FIG. 5 Statistical
source data. SOURCE DATA TABLE FIG. 6 Statistical source data. SOURCE DATA TABLE FIG. 7 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 1 Statistical source data for Extended Data
Fig. 1. SOURCE DATA EXTENDED DATA FIG. 4 Statistical source data for Extended Data Fig. 4. SOURCE DATA EXTENDED DATA FIG. 5 Statistical source data for Extended Data Fig. 5. SOURCE DATA
EXTENDED DATA FIG. 6 Statistical source data for Extended Data Fig. 6. SOURCE DATA EXTENDED DATA FIG. 7 Statistical source data for Extended Data Fig. 7. SOURCE DATA EXTENDED DATA FIG. 8
Statistical source data for Extended Data Fig. 8. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, T., Wang, Z., Ye, L. _et al._ Nucleus-exported CLOCK acetylates PRPS to promote de novo
nucleotide synthesis and liver tumour growth. _Nat Cell Biol_ 25, 273–284 (2023). https://doi.org/10.1038/s41556-022-01061-0 Download citation * Received: 28 February 2022 * Accepted: 24
November 2022 * Published: 16 January 2023 * Issue Date: February 2023 * DOI: https://doi.org/10.1038/s41556-022-01061-0 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
