Gallbladder-derived retinoic acid signalling drives reconstruction of the damaged intrahepatic biliary ducts
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Severe damage to the intrahepatic biliary duct (IHBD) network occurs in multiple human advanced cholangiopathies, such as primary sclerosing cholangitis, biliary atresia and
end-stage primary biliary cholangitis. Whether and how a severely damaged IHBD network could reconstruct has remained unclear. Here we show that, although the gallbladder is not directly
connected to the IHBD, there is a common hepatic duct (CHD) in between, and severe damage to the IHBD network induces migration of gallbladder smooth muscle cells (SMCs) to coat the CHD in
mouse and zebrafish models. These gallbladder-derived, CHD-coating SMCs produce retinoic acid to activate Sox9b in the CHD, which drives proliferation and ingrowth of CHD cells into the
inner liver to reconstruct the IHBD network. This study reveals a hitherto unappreciated function of the gallbladder in the recovery of injured liver, and characterizes mechanisms involved
in how the gallbladder and liver communicate through inter-organ cell migration to drive tissue regeneration. Carrying out cholecystectomy will thus cause previously unexpected impairments
to liver health. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print
issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS MULTIDIMENSIONAL IMAGING OF LIVER INJURY REPAIR IN MICE REVEALS FUNDAMENTAL ROLE OF THE DUCTULAR REACTION Article Open access 05 June 2020 BILIARY NIK PROMOTES
DUCTULAR REACTION AND LIVER INJURY AND FIBROSIS IN MICE Article Open access 30 August 2022 IMPACT OF GALLBLADDER HYPOPLASIA ON HILAR HEPATIC DUCTS IN BILIARY ATRESIA Article Open access 11
June 2024 DATA AVAILABILITY All zebrafish lines and plasmids generated in this study will be made available on reasonable request, but we may require a payment or a completed material
transfer agreement if there is potential for commercial application. Data supporting the findings of this study are available from the corresponding author on reasonable request. Source data
are provided with this paper. REFERENCES * Tabibian, J. H., Masyuk, A. I., Masyuk, T. V., O’Hara, S. P. & LaRusso, N. F. Physiology of cholangiocytes. _Compr. Physiol._ 3, 541–565
(2013). PubMed Google Scholar * De Assuncao, T. M., Jalan-Sakrikar, N. & Huebert, R. C. Regenerative medicine and the biliary tree. _Semin. Liver Dis._ 37, 17–27 (2017). PubMed PubMed
Central Google Scholar * Banales, J. M. et al. Cholangiocyte pathobiology. _Nat. Rev. Gastroenterol. Hepatol._ 16, 269–281 (2019). PubMed PubMed Central Google Scholar * Hirschfield,
G. M. & Gershwin, M. E. The immunobiology and pathophysiology of primary biliary cirrhosis. _Annu. Rev. Pathol._ 8, 303–330 (2013). CAS PubMed Google Scholar * Lazaridis, K. N. &
LaRusso, N. F. Primary sclerosing cholangitis. _N. Engl. J. Med._ 375, 1161–1170 (2016). PubMed PubMed Central Google Scholar * Dyson, J. K., Beuers, U., Jones, D. E. J., Lohse, A. W.
& Hudson, M. Primary sclerosing cholangitis. _Lancet_ 391, 2547–2559 (2018). PubMed Google Scholar * Hartley, J. L., Davenport, M. & Kelly, D. A. Biliary atresia. _Lancet_ 374,
1704–1713 (2009). PubMed Google Scholar * Lazaridis, K. N. & LaRusso, N. F. The cholangiopathies. _Mayo Clinic Proc._ 90, 791–800 (2015). CAS Google Scholar * Lemaigre, F. P.
Development of the intrahepatic and extrahepatic biliary tract: a framework for understanding congenital diseases. _Annu. Rev. Pathol._ 15, 1–22 (2020). CAS PubMed Google Scholar * Van
Eyken, P., Sciot, R. & Desmet, V. J. A cytokeratin immunohistochemical study of cholestatic liver disease: evidence that hepatocytes can express ‘bile duct-type’ cytokeratins.
_Histopathology_ 15, 125–135 (1989). PubMed Google Scholar * Ernst, L. M., Spinner, N. B., Piccoli, D. A., Mauger, J. & Russo, P. Interlobular bile duct loss in pediatric cholestatic
disease is associated with aberrant cytokeratin 7 expression by hepatocytes. _Pediatr. Dev. Pathol._ 10, 383–390 (2007). PubMed Google Scholar * Kamimoto, K. et al. Heterogeneity and
stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. _eLife_ 5, e15034 (2016). PubMed PubMed Central Google Scholar * Michalopoulos, G.
K., Barua, L. & Bowen, W. C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. _Hepatology_ 41, 535–544 (2005). CAS PubMed
Google Scholar * Yanger, K. et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. _Genes Dev._ 27, 719–724 (2013). CAS PubMed PubMed Central Google Scholar
* Nagahama, Y. et al. Contributions of hepatocytes and bile ductular cells in ductular reactions and remodeling of the biliary system after chronic liver injury. _Am. J. Pathol._ 184,
3001–3012 (2014). CAS PubMed Google Scholar * Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. _Cell Stem Cell_ 15,
605–618 (2014). CAS PubMed PubMed Central Google Scholar * Sekiya, S. & Suzuki, A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the
chronically injured mouse liver. _Am. J. Pathol._ 184, 1468–1478 (2014). CAS PubMed Google Scholar * Gadd, V. L., Aleksieva, N. & Forbes, S. J. Epithelial plasticity during liver
injury and regeneration. _Cell Stem Cell_ 27, 557–573 (2020). CAS PubMed Google Scholar * Kwon, S. B. et al. Time- and dose-based gene expression profiles produced by a bile-duct-damaging
chemical, 4,4′-methylene dianiline, in mouse liver in an acute phase. _Toxicol. Pathol._ 36, 660–673 (2008). CAS PubMed Google Scholar * Sampaziotis, F. et al. Cholangiocyte organoids
can repair bile ducts after transplantation in the human liver. _Science_ 371, 839–846 (2021). CAS PubMed PubMed Central Google Scholar * Pastore, N. et al. TFEB regulates murine liver
cell fate during development and regeneration. _Nat. Commun._ 11, 2461 (2020). CAS PubMed PubMed Central Google Scholar * Schaub, J. R. et al. De novo formation of the biliary system by
TGFβ-mediated hepatocyte transdifferentiation. _Nature_ 557, 247–251 (2018). CAS PubMed PubMed Central Google Scholar * Kaneko, K., Kamimoto, K., Miyajima, A. & Itoh, T. Adaptive
remodeling of the biliary architecture underlies liver homeostasis. _Hepatology_ 61, 2056–2066 (2015). CAS PubMed Google Scholar * Mariotti, V., Strazzabosco, M., Fabris, L. &
Calvisi, D. F. Animal models of biliary injury and altered bile acid metabolism. _Biochim. Biophys. Acta Mol. Basis Dis._ 1864, 1254–1261 (2018). CAS PubMed Google Scholar * Cheung, I. D.
et al. Regulation of intrahepatic biliary duct morphogenesis by Claudin 15-like b. _Dev. Biol._ 361, 68–78 (2012). CAS PubMed Google Scholar * Zhang, W. et al. Formimidoyltransferase
cyclodeaminase prevents the starvation-induced liver hepatomegaly and dysfunction through downregulating mTORC1. _PLoS Genet._ 17, e1009980 (2021). CAS PubMed PubMed Central Google
Scholar * Zhang, D. et al. Identification of Annexin A4 as a hepatopancreas factor involved in liver cell survival. _Dev. Biol._ 395, 96–110 (2014). CAS PubMed PubMed Central Google
Scholar * Housset, C., Chrétien, Y., Debray, D. & Chignard, N. Functions of the gallbladder. _Compr. Physiol._ 6, 1549–1577 (2016). PubMed Google Scholar * He, L. et al. Enhancing the
precision of genetic lineage tracing using dual recombinases. _Nat. Med._ 23, 1488–1498 (2017). CAS PubMed PubMed Central Google Scholar * Kikuchi, K. et al. Retinoic acid production by
endocardium and epicardium is an injury response essential for zebrafish heart regeneration. _Dev. Cell_ 20, 397–404 (2011). CAS PubMed PubMed Central Google Scholar * Huang, W. et al.
Sox9b is a mediator of retinoic acid signaling restricting endocrine progenitor differentiation. _Dev. Biol._ 418, 28–39 (2016). CAS PubMed PubMed Central Google Scholar * Manfroid, I.
et al. Zebrafish sox9b is crucial for hepatopancreatic duct development and pancreatic endocrine cell regeneration. _Dev. Biol._ 366, 268–278 (2012). CAS PubMed PubMed Central Google
Scholar * Singh, S. et al. Heterogeneous murine peribiliary glands orchestrate compartmentalized epithelial renewal. _Dev. Cell_ 58, 2732–2745.e2735 (2023). CAS PubMed PubMed Central
Google Scholar * Lam, R. et al. Gallbladder disorders: a comprehensive review. _Dis. Mon._ 67, 101130 (2021). PubMed Google Scholar * Baron, T. H., Grimm, I. S. & Swanstrom, L. L.
Interventional approaches to gallbladder disease. _N. Engl. J. Med._ 373, 357–365 (2015). CAS PubMed Google Scholar * Sutton, H., Karpen, S. J. & Kamath, B. M. Pediatric cholestatic
diseases: common and unique pathogenic mechanisms. _Annu. Rev. Pathol._ 19, 319–344 (2024). CAS PubMed Google Scholar * Wang, X. et al. Comparative analysis of cell lineage
differentiation during hepatogenesis in humans and mice at the single-cell transcriptome level. _Cell Res._ 30, 1109–1126 (2020). CAS PubMed PubMed Central Google Scholar * Bertrand, J.
Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. _Nature_ 464, 108–111 (2010). CAS PubMed PubMed Central Google Scholar * Kikuchi, K. et
al. Primary contribution to zebrafish heart regeneration by _gata4__+_ cardiomyocytes. _Nature_ 464, 601–605 (2010). CAS PubMed PubMed Central Google Scholar * Delous, M. et al. Sox9b is
a key regulator of pancreaticobiliary ductal system development. _PLoS Genet._ 8, e1002754 (2012). CAS PubMed PubMed Central Google Scholar * Whitesell, T. R. et al. An α-smooth muscle
actin (acta2/αsma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. _PLoS ONE_ 9, e90590 (2014). PubMed PubMed Central Google Scholar * Suster, M.
L., Abe, G., Schouw, A. & Kawakami, K. Transposon-mediated BAC transgenesis in zebrafish. _Nat. Protoc._ 6, 1998–2021 (2011). CAS PubMed Google Scholar * Ma, J. et al. Rngtt governs
biliary-derived liver regeneration initiation by transcriptional regulation of mTORC1 and Dnmt1 in zebrafish. _Hepatology_ 78, 167–178 (2023). PubMed Google Scholar * He, J., Lu, H., Zou,
Q. & Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. _Gastroenterology_ 146, 789–800 (2014). CAS PubMed Google
Scholar * Liu, X. et al. NF-kB and Snail1a coordinate the cell cycle with gastrulation. _J. Cell Biol._ 184, 805–815 (2009). CAS PubMed PubMed Central Google Scholar * Lu, H., Ma, J.,
Yang, Y., Shi, W. & Luo, L. EpCAM is an endoderm-specific Wnt derepressor that licenses hepatic development. _Dev. Cell_ 24, 543–553 (2013). CAS PubMed Google Scholar * Liu, C. et al.
Macrophages mediate the repair of brain vascular rupture through direct physical adhesion and mechanical traction. _Immunity_ 44, 1162–1176 (2016). CAS PubMed Google Scholar * Chen, J.
et al. Cerebrovascular injuries induce lymphatic invasion into brain parenchyma to guide vascular regeneration in zebrafish. _Dev. Cell_ 49, 697–710 (2019). CAS PubMed Google Scholar *
Chen, J. et al. Acute brain vascular regeneration occurs via lymphatic transdifferentiation. _Dev. Cell_ 56, 3115–3127 (2021). CAS PubMed Google Scholar * He, J., Mo, D., Chen, J. &
Luo, L. Combined whole-mount fluorescence in situ hybridization and antibody staining in zebrafish embryos and larvae. _Nat. Protoc._ 15, 3361–3379 (2020). CAS PubMed Google Scholar * He,
J. et al. Mammalian target of rapamycin complex 1 signaling is required for the dedifferentiation from biliary cell to bipotential progenitor cell in zebrafish liver regeneration.
_Hepatology_ 70, 2092–2106 (2019). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank M. Parsons and S. Childs for plasmids, J. Peng for antibodies, C. Xu and B. Zhou
for transgenic mice. This work was supported by the National Natural Science Foundation of China (grants 32430032 (L. Luo), 32192400 (L. Luo), 32122033 (J.H.), 32470881 (J.H.) and 31970784
(J.H.)), the National Key R&D Program of China (grant 2021YFA0805000, L. Luo), the Natural Science Foundation of Chongqing (grant CSTB2023NSCQ-JQX0004, J.H.) and funds from Southwest
University (grants SWU-XJLJ202302 and SWU-XDPY22008, J.H.). AUTHOR INFORMATION Author notes * These authors contributed equally: Jianbo He, Shuang Li. AUTHORS AND AFFILIATIONS * State Key
laboratory of Genetic Engineering, School of Life Sciences, Liver Cancer Institute of Zhongshan Hospital, Fudan University, Shanghai, China Jianbo He, Jianlong Ma, Jingying Chen, Yunfan Sun,
Tianyu Zhao & Lingfei Luo * Institute of Developmental Biology and Regenerative Medicine, Southwest University, Chongqing, China Jianbo He, Shuang Li, Zhuolin Yang, Chuanfang Qian,
Zhuofu Huang, Linke Li, Yun Yang & Lingfei Luo Authors * Jianbo He View author publications You can also search for this author inPubMed Google Scholar * Shuang Li View author
publications You can also search for this author inPubMed Google Scholar * Zhuolin Yang View author publications You can also search for this author inPubMed Google Scholar * Jianlong Ma
View author publications You can also search for this author inPubMed Google Scholar * Chuanfang Qian View author publications You can also search for this author inPubMed Google Scholar *
Zhuofu Huang View author publications You can also search for this author inPubMed Google Scholar * Linke Li View author publications You can also search for this author inPubMed Google
Scholar * Yun Yang View author publications You can also search for this author inPubMed Google Scholar * Jingying Chen View author publications You can also search for this author inPubMed
Google Scholar * Yunfan Sun View author publications You can also search for this author inPubMed Google Scholar * Tianyu Zhao View author publications You can also search for this author
inPubMed Google Scholar * Lingfei Luo View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L. Luo, J.H., J.M., Y.S. and J.C. designed the
experimental strategy, analysed data and wrote the manuscript. S.L. performed and J.M. and T.Z. helped perform all the mice experiments. Z.Y. performed FISH. C.Q. performed zebrafish lineage
tracing. Z.H. and L. Li crossed and identified mice. Y.Y. helped with analyses. J.H. performed all the other experiments. CORRESPONDING AUTHOR Correspondence to Lingfei Luo. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Cell Biology_ thanks Wolfram Goessling and the other, anonymous,
reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published
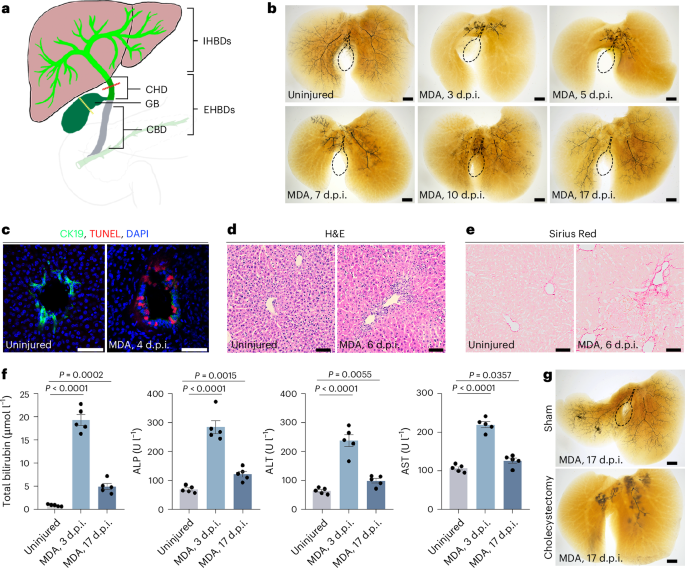
maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 THE RECONSTRUCTION OF SEVERELY DAMAGED IHBD NETWORK AND INVOLVEMENTS OF GALLBLADDER IN THE ANIT-INJURED MOUSE. A, IHBD
system visualized by retrograde ink injection into CBD of α-naphthylisothiocyanate (ANIT)-treated mice at 3 dpi (n = 7/10), 7 dpi (n = 7/11), 12 dpi (n = 8/10), and 28 dpi (n = 8/11). The
dashed lines indicate the gallbladder. B, IHBD system visualized by retrograde ink injection into CBD of ANIT-treated mice at 12 dpi in cholecystectomy group (n = 6/8). C, IHBD system
visualized by retrograde ink injection into CBD of ANIT-treated mice at 12 dpi in BMS493-treated group (n = 5/7). The dashed lines indicate the gallbladder. Scale bars, 200 μm. EXTENDED DATA
FIG. 2 THE GALLBLADDER IS ESSENTIAL FOR IHBD NETWORK RECONSTRUCTION AFTER SEVERE IHBD DAMAGES IN ADULT ZEBRAFISH. A, Experimental strategies of IHBD injury and reconstruction in adult
zebrafish. B, Confocal projection images for Tomato+ IHBD network reconstruction of uninjured group (n = 5/5) and MTZ-treated groups at 2 dpi (n = 8/8), 6 dpi (n = 9/12), 21 dpi (n = 12/15),
56 dpi (n = 11/14), and 84 dpi (n = 8/10). The dashed lines indicate the migration of IHBD cells and the asterisk indicates the pancreatic region outside of the liver. C, Diagram indicating
the IHBD network reconstruction starts from the region near gallbladder, fulfills in the left lobe and extends to the middle lobe, further extends to the right lobe, and completely
accomplishes in the whole liver. D, Experimental strategies of cholecystectomy. The undistinguished gallbladders (n = 10/10) labeled with methyl blue (n = 8/8) and subjected to
cholecystectomy (n = 12/12). E, Confocal projection images for Tomato+ IHBD reconstruction from sham-treated (n = 8/11) and cholecystectomy-treated (n = 11/12) zebrafish at 10 dpi. The
dashed lines indicate new regenerating IHBD cells. Scale bars, 500 μm. EXTENDED DATA FIG. 3 CHOLECYSTECTOMY IN ZEBRAFISH. A, Graphic images illustrate the procedures of cholecystectomy in
zebrafish adult. B, Graphic images illustrate the procedures of cholecystectomy in zebrafish larva. C, Extrahepatic duct system including CHD, cystic duct (CD), and GB visualized under the
_Tg(anxa4:GFP)_ transgenic background after Sham (n = 5/5) or cholecystectomy (n = 7/7) in adult zebrafish. Note that cholecystectomy removes GB and CD, but leaves CHD intact. D,
Extrahepatic duct system including CHD and GB visualized under the _Tg(anxa4:GFP)_ transgenic background at 5 dpf/0 dpc, 6 dpf/1 dpc, and 10 dpf/5 dpc after Sham (n = 10/10) or
cholecystectomy (n = 10/10) in larval zebrafish. The larvae were subjected to cholecystectomy at 5 dpf. Note that cholecystectomy removes GB, but leaves CHD intact. dpc, days post
cholecystectomy. Scale bars, 2 mm (C); 100 μm (D). The models (A, B) were Created in BioRender. EXTENDED DATA FIG. 4 THE MTZ INDUCES SEVERE AND SPECIFIC INJURY TO IHBDS IN ZEBRAFISH LARVAE.
A, Experimental strategies of MTZ treatment and analyses. B, The confocal projection images and quantifications for the Tomato+ IHBD cells at 1 dpi in the larvae subjected to treatments of
DMSO (n = 5 larvae), 5 mM MTZ (n = 12 larvae), 8 mM MTZ (n = 13 larvae), and 10 mM MTZ (n = 7 larvae). The dashed lines indicate the liver. C, Confocal images and quantifications for the
TUNEL assays at 0 dpi after DMSO (n = 6 larvae) or MTZ (n = 6 larvae) treatment. Note that the TUNEL+ cells are exclusively present in the inter-hepatocyte space, which means apoptotic BECs.
The anti-Bhmt indicate hepatocytes. D, The confocal images of anti-Anxa4, GFP, and Tomato in the DMSO- and MTZ-treated groups at 1 dpi. The dashed lines indicate the liver. Quantifications
indicate the number of Anxa4+Tomato+ BECs in the liver at 1 dpi after DMSO (n = 6 larvae) or MTZ (n = 5 larvae) treatment. E, The confocal projection images for anti-SPGP at 1 dpi after DMSO
(n = 10/10) and MTZ (n = 7/11) treatment. The dashed lines indicate the liver. F, The confocal projection images for anti-Anxa4 at 1 dpi. The dashed lines indicate the gallbladder.
Quantifications indicate the areas of gallbladder at 1 dpi after DMSO (n = 6 larvae) or MTZ (n = 10 larvae) treatment. G, The bright field (BF) and fluorescent images of larval morphologies
and Tomato expression at 1 dpi after DMSO (n = 15/15) or 10 mM MTZ (n = 20/20) treatment. The arrows indicate the liver region. Data are mean±s.e.m.; unpaired _t_-test. Scale bars, 100 μm
(B, D, E, F, G); 50 μm (C). Source data EXTENDED DATA FIG. 5 THE LABELING EFFICIENCY OF CHD CELLS BY THE CRE/LOXP SYSTEM IS AVERAGELY 88% IN ZEBRAFISH LARVAE. A, Experimental strategies of
CHD labeling with _Cre/loxP_ system. B, Antibody staining confocal images for Anxa4 and GFP in DMSO- and 4-OHT-treated groups at 7 dpf. Quantification of the ratio of GFP+ cells among CHD
after DMSO (n = 5 zebrafish) or 4-OHT (n = 6 zebrafish) treatment at 7 dpf. Data are mean±s.e.m.; unpaired _t_-test. Scale bars, 100 μm. Source data EXTENDED DATA FIG. 6 THE LABELING
EFFICIENCY OF CHD CELLS BY THE CRE/LOX-DRE/ROX SYSTEM IS AVERAGELY 80% IN MOUSE. A, Experimental strategies of CHD labeling in Krt19: DreER x Alb: Cre x DeaLT-IR triple transgenic mice after
7 times of 4-OHT intraperitoneal injection and analysis at 20 dpi. B, Diagram showing the section region of CHD (Red dashed line). C, tdTomato, CK19, and ZsGreen immunofluorescent confocal
images of CHD in oil-treated and 4-OHT-treated mice at 20 dpi. D, Quantification of the ratio of tdTomato+ among CK19+ CHD cells at 7 dpi after oil (n = 5 mice) and 4-OHT (n = 5 mice)
treatment. Data are mean±s.e.m.; unpaired _t_-test. Scale bars, 50 μm. Source data EXTENDED DATA FIG. 7 THE _RARGA_ IS TRANSCRIPTIONALLY ACTIVATED IN THE CHD AFTER IHBD INJURIES IN
ZEBRAFISH. FISH combined with anti-Anxa4 antibody staining showing the expressions of _raraa_ (A; DMSO, n = 14/14; MTZ, n = 15/15), _rarab_ (B; DMSO, n = 15/15; MTZ, n = 14/14), _rarga_ (C;
DMSO, n = 16/16; MTZ, n = 10/16), and _rargb_ (D; DMSO, n = 15/15; MTZ, n = 16/16) in the CHD at 1 dpi after MTZ treatment. Note that only _rarga_ was transcriptionally activated in CHD
after MTZ treatment. The dashed lines indicate the CHD region. Scale bars, 100 μm. EXTENDED DATA FIG. 8 RA DRIVES CHD CELL PROLIFERATION AND INGROWTH THROUGH SOX9B IN ZEBRAFISH. A,
Experimental strategies of RA signaling inhibition plus IHBD injury and analysis in zebrafish larvae. FISH combined with antibody staining images for _sox9b_ expressions in Anxa4+ CHD after
MTZ treatment with _hsp70l_- (n = 12/13), _cyp26a1_+ (n = 7/10), and _DnRAR_+ (n = 12/16) overexpression and DMSO-treated control (n = 8/8) groups at 1 dpi in zebrafish. The dashed lines
indicate the CHD. B, Antibody staining confocal images for Anxa4 and PCNA in _sox9b_ sibling and mutant CHD at 1 dpi. Quantification of the ratio of PCNA+ among CHD cells in _sox9b_ sibling
(n = 6 larvae) and mutant (n = 5 larvae) at 1 dpi. The dashed lines indicate the CHD. C, Confocal projection images for new regenerating Tomato+ IHBD cells in _sox9b_ sibling (n = 36/50) and
mutant (n = 40/59) at 20 dpi. Quantification of the ratio of IHBD regenerated larvae in _sox9b_ sibling (n = 3 groups) and mutant (n = 3 groups) at 20 dpi. The dashed line indicates the
IHBD network. Data are mean±s.e.m.; unpaired _t_-test. Scale bars, 100 μm. Source data EXTENDED DATA FIG. 9 THE PROLIFERATION OF IHBD CELL OCCURS AFTER THE MDA-INDUCED INJURY. A, EpCAM and
PCNA immunofluorescent confocal images of IHBD cells in uninjured, MDA-treated, and MDA plus BMS493-treated groups at 6 dpi. Note that the proliferation of IHBD was partially repressed after
BMS493 treatment at 6 dpi. B, The quantification of the ratios of PCNA+ cells among all the EpCAM+ IHBD cells in the uninjured (n = 5 mice), MDA-treated (n = 5 mice), and MDA plus
BMS493-treated (n = 5 mice) groups at 6 dpi. Data are mean±s.e.m.; unpaired _t_-test. Scale bars, 25 μm. Source data SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLE 1
Antibodies and primers used in this study. SUPPLEMENTARY VIDEO 1 Adult zebrafish cholecystectomy. SUPPLEMENTARY VIDEO 2 Larval zebrafish cholecystectomy. SOURCE DATA SOURCE DATA FIG. 1
Statistical source data. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA EXTENDED DATA
FIG./TABLE 4 Statistical source data. SOURCE DATA EXTENDED DATA FIG./TABLE 5 Statistical source data. SOURCE DATA EXTENDED DATA FIG./TABLE 6 Statistical source data. SOURCE DATA EXTENDED
DATA FIG./TABLE 8 Statistical source data. SOURCE DATA EXTENDED DATA FIG./TABLE 9 Statistical source data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other
partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this
article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE He, J., Li, S., Yang, Z. _et al._
Gallbladder-derived retinoic acid signalling drives reconstruction of the damaged intrahepatic biliary ducts. _Nat Cell Biol_ 27, 39–47 (2025). https://doi.org/10.1038/s41556-024-01568-8
Download citation * Received: 09 January 2024 * Accepted: 25 October 2024 * Published: 08 January 2025 * Issue Date: January 2025 * DOI: https://doi.org/10.1038/s41556-024-01568-8 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
