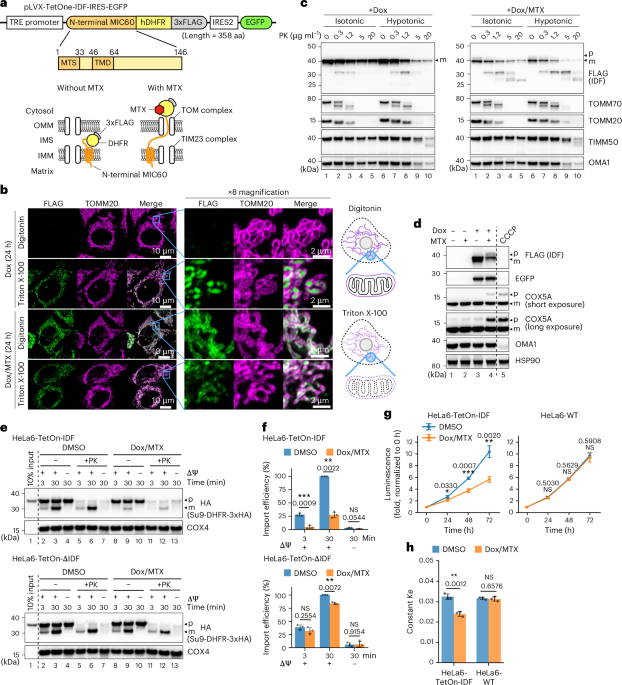
Mitochondrial yme1l1 governs unoccupied protein translocase channels
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Mitochondrial protein import through the outer and inner membranes is key to mitochondrial biogenesis. Recent studies have explored how cells respond when import is impaired by a
variety of different insults. Here, we developed a mammalian import blocking system using dihydrofolate reductase fused to the N terminus of the inner membrane protein MIC60. While
stabilization of the dihydrofolate reductase domain by methotrexate inhibited endogenous mitochondrial protein import, it neither activated the transcription factor ATF4, nor was affected by
ATAD1 expression or by VCP/p97 inhibition. On the other hand, notably, plugging the channel of translocase of the outer membrane) induced YME1L1, an ATP-dependent protease, to eliminate
translocase of the inner membrane (TIM23) channel components TIMM17A and TIMM23. The data suggest that unoccupied TIM23 complexes expose a C-terminal degron on TIMM17A to YME1L1 for
degradation. Import plugging caused a cell growth defect and loss of YME1L1 exacerbated the growth inhibition, showing the protective effect of YME1L1 activity. YME1L1 seems to play a
crucial role in mitochondrial quality control to counteract precursor stalling in the translocase of the outer membrane complex and unoccupied TIM23 channels. Access through your institution
Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS DYRK1A SIGNALLING
SYNCHRONIZES THE MITOCHONDRIAL IMPORT PATHWAYS FOR METABOLIC REWIRING Article Open access 20 June 2024 MOLECULAR PATHWAY OF MITOCHONDRIAL PREPROTEIN IMPORT THROUGH THE TOM–TIM23 SUPERCOMPLEX
Article 11 September 2023 STRUCTURAL BASIS OF MITOCHONDRIAL PROTEIN IMPORT BY THE TIM23 COMPLEX Article 21 June 2023 DATA AVAILABILITY Source data and mass spectrometry data are provided
with this paper. All other data supporting the finding of this study are available from the corresponding authors on reasonable request. The MitoCarta 3.0 database
(https://www.broadinstitute.org/mitocarta/mitocarta30-inventory-mammalian-mitochondrial-proteins-and-pathways) was used for proteomics data analysis. Proteomics from SILAC mass spectrometry
has been deposited to the ProteomeXchange consortium with the dataset identifier PXD057163. Source data are provided with this paper. REFERENCES * Yamano, K., Kinefuchi, H. & Kojima, W.
Mitochondrial quality control via organelle and protein degradation. _J. Biochem_. https://doi.org/10.1093/jb/mvad106 (2024). * den Brave, F., Pfanner, N. & Becker, T. Mitochondrial
entry gate as regulatory hub. _Biochim. Biophys. Acta Mol. Cell. Res._ 1871, 119529 (2024). Article CAS PubMed Google Scholar * Weidberg, H. & Amon, A. MitoCPR: a surveillance
pathway that protects mitochondria in response to protein import stress. _Science_ 360, eaan4146 (2018). Article PubMed PubMed Central Google Scholar * Martensson, C. U. et al.
Mitochondrial protein translocation-associated degradation. _Nature_ 569, 679–683 (2019). Article CAS PubMed Google Scholar * Basch, M. et al. Msp1 cooperates with the proteasome for
extraction of arrested mitochondrial import intermediates. _Mol. Biol. Cell_ 31, 753–767 (2020). Article CAS PubMed PubMed Central Google Scholar * Boos, F. et al. Mitochondrial
protein-induced stress triggers a global adaptive transcriptional programme. _Nat. Cell Biol._ 21, 442–451 (2019). Article CAS PubMed Google Scholar * Sim, S. I., Chen, Y., Lynch, D. L.,
Gumbart, J. C. & Park, E. Structural basis of mitochondrial protein import by the TIM23 complex. _Nature_ 621, 620–626 (2023). Article CAS PubMed PubMed Central Google Scholar *
Fielden, L. F. et al. Central role of Tim17 in mitochondrial presequence protein translocation. _Nature_ 621, 627–634 (2023). Article CAS PubMed PubMed Central Google Scholar * Jin, S.
M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. _J. Cell Biol._ 191, 933–942 (2010). Article CAS PubMed PubMed Central Google
Scholar * Baker, M. J. et al. Stress-induced OMA1 activation and autocatalytic turnover regulate OPA1-dependent mitochondrial dynamics. _EMBO J._ 33, 578–593 (2014). Article CAS PubMed
PubMed Central Google Scholar * Pfanner, N., Muller, H. K., Harmey, M. A. & Neupert, W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in
the binding to receptor sites. _EMBO J._ 6, 3449–3454 (1987). Article CAS PubMed PubMed Central Google Scholar * Metzger, M. B., Scales, J. L., Dunklebarger, M. F., Loncarek, J. &
Weissman, A. M. A protein quality control pathway at the mitochondrial outer membrane. _Elife_ 9, e51065 (2020). Article CAS PubMed PubMed Central Google Scholar * Tanaka, A. et al.
Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. _J. Cell Biol._ 191, 1367–1380 (2010). Article CAS PubMed PubMed Central Google Scholar * Eilers,
M. & Schatz, G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. _Nature_ 322, 228–232 (1986). Article CAS PubMed Google Scholar *
Chen, Y. C. et al. Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. _EMBO J._ 33, 1548–1564 (2014). Article CAS PubMed
PubMed Central Google Scholar * Okreglak, V. & Walter, P. The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. _Proc. Natl Acad. Sci. USA_ 111,
8019–8024 (2014). Article CAS PubMed PubMed Central Google Scholar * Nuebel, E. et al. The biochemical basis of mitochondrial dysfunction in Zellweger Spectrum Disorder. _EMBO Rep._ 22,
e51991 (2021). Article CAS PubMed PubMed Central Google Scholar * Kim, J., Goldstein, M., Zecchel, L. & Weidberg, H. ATAD1 and the integrated stress response prevent clogging of
TOM and damage caused by un-imported mitochondrial proteins. _Cell Rep._ 43, 114473 (2024). * Pickles, S., Vigie, P. & Youle, R. J. Mitophagy and quality control mechanisms in
mitochondrial maintenance. _Curr. Biol._ 28, R170–R185 (2018). Article CAS PubMed PubMed Central Google Scholar * Guo, X. et al. Mitochondrial stress is relayed to the cytosol by an
OMA1-DELE1-HRI pathway. _Nature_ 579, 427–432 (2020). Article CAS PubMed PubMed Central Google Scholar * Fessler, E. et al. A pathway coordinated by DELE1 relays mitochondrial stress to
the cytosol. _Nature_ 579, 433–437 (2020). Article CAS PubMed PubMed Central Google Scholar * Deshwal, S., Fiedler, K. U. & Langer, T. Mitochondrial proteases: multifaceted
regulators of mitochondrial plasticity. _Annu. Rev. Biochem._ 89, 501–528 (2020). Article CAS PubMed Google Scholar * MacVicar, T. et al. Lipid signalling drives proteolytic rewiring of
mitochondria by YME1L. _Nature_ 575, 361–365 (2019). Article CAS PubMed Google Scholar * Rainbolt, T. K., Atanassova, N., Genereux, J. C. & Wiseman, R. L. Stress-regulated
translational attenuation adapts mitochondrial protein import through Tim17A degradation. _Cell Metab._ 18, 908–919 (2013). Article CAS PubMed PubMed Central Google Scholar * Kaldi, K.,
Bauer, M. F., Sirrenberg, C., Neupert, W. & Brunner, M. Biogenesis of Tim23 and Tim17, integral components of the TIM machinery for matrix-targeted preproteins. _EMBO J._ 17, 1569–1576
(1998). Article CAS PubMed PubMed Central Google Scholar * Sinha, D., Srivastava, S., Krishna, L. & D’Silva, P. Unraveling the intricate organization of mammalian mitochondrial
presequence translocases: existence of multiple translocases for maintenance of mitochondrial function. _Mol. Cell. Biol._ 34, 1757–1775 (2014). Article CAS PubMed PubMed Central Google
Scholar * Busch, J. D., Fielden, L. F., Pfanner, N. & Wiedemann, N. Mitochondrial protein transport: versatility of translocases and mechanisms. _Mol. Cell_ 83, 890–910 (2023). Article
CAS PubMed Google Scholar * Youle, R. J. Mitochondria—striking a balance between host and endosymbiont. _Science_ 365, eaaw9855 (2019). Article CAS PubMed Google Scholar * Song, J.,
Herrmann, J. M. & Becker, T. Quality control of the mitochondrial proteome. _Nat. Rev. Mol. Cell Biol._ 22, 54–70 (2021). Article CAS PubMed Google Scholar * Itakura, E. et al.
Ubiquilins chaperone and triage mitochondrial membrane proteins for degradation. _Mol. Cell_ 63, 21–33 (2016). Article CAS PubMed PubMed Central Google Scholar * Krakowczyk, M. et al.
OMA1 protease eliminates arrested protein import intermediates upon mitochondrial depolarization. _J. Cell Biol._ 223, e202306051 (2024). Article CAS PubMed Google Scholar * Zhou, X. et
al. Molecular pathway of mitochondrial preprotein import through the TOM-TIM23 supercomplex. _Nat. Struct. Mol. Biol._ 30, 1996–2008 (2023). Article CAS PubMed Google Scholar * Rainbolt,
T. K., Lebeau, J., Puchades, C. & Wiseman, R. L. Reciprocal degradation of YME1L and OMA1 adapts mitochondrial proteolytic activity during Stress. _Cell Rep._ 14, 2041–2049 (2016).
Article CAS PubMed PubMed Central Google Scholar * Yamano, K. et al. Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy. _J. Cell Biol._ 219, e201912144
(2020). Article CAS PubMed PubMed Central Google Scholar * Tian, R. et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. _Neuron_ 104,
239–255 e212 (2019). Article CAS PubMed PubMed Central Google Scholar * Kanfer, G. et al. Image-based pooled whole-genome CRISPRi screening for subcellular phenotypes. _J. Cell Biol._
220, e202006180 (2021). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank D. Narendra for thoughtful reading of the manuscript and the Youle
laboratory for feedback. We also thank the National Institute of Neurological Disorders and Stroke (NINDS) Light Imaging Facility, National Heart, Lung, and Blood Institute Flow Cytometry
Core Facility and NINDS Protein/Peptide Sequencing Facility for technical assistance. We thank T. Langer for the _YME1L1_ KO HeLa cells and H. Takahashi for the pEU-E01-MCS(C1)-His vector.
This work was supported by the NINDS intramural program, the National Taiwan University startup funding (grant number 111L7475, 2022) (to M.-C.H.), the National Science and Technology
Council Grant (112-2320-B-002-061 to M.-C.H.), Nanken-Kyoten TMDU (2024-kokusai12) (to K.Y.) and the JSPS KAKENHI grants 22H02577 and 23H04923 (to K.Y.). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Biochemistry Section, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA Meng-Chieh Hsu,
Linlin Lei & Richard J. Youle * Department of Animal Science and Technology, National Taiwan University, Taipei City, Taiwan Meng-Chieh Hsu * Department of Biomolecular Pathogenesis,
Medical Research Institute, Tokyo Medical and Dental University, Tokyo, Japan Hiroki Kinefuchi, Reika Kikuchi & Koji Yamano * Department of Biomolecular Pathogenesis, Medical Research
Laboratory, Institute of Integrated Research, Institute of Science Tokyo, Tokyo, Japan Hiroki Kinefuchi, Reika Kikuchi & Koji Yamano Authors * Meng-Chieh Hsu View author publications You
can also search for this author inPubMed Google Scholar * Hiroki Kinefuchi View author publications You can also search for this author inPubMed Google Scholar * Linlin Lei View author
publications You can also search for this author inPubMed Google Scholar * Reika Kikuchi View author publications You can also search for this author inPubMed Google Scholar * Koji Yamano
View author publications You can also search for this author inPubMed Google Scholar * Richard J. Youle View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS M.-C.H., K.Y. and R.J.Y. designed the study. M.-C.H., H.K., L.L., R.K. and K.Y. performed the experiments. M.-C.H., H.K., L.L., R.K. and K.Y. analysed the data and/or its
significance. M.-C.H., K.Y. and R.J.Y. wrote the paper with contributions from H.K. and L.L. M.-C.H., K.Y. and R.J.Y. acquired funding. CORRESPONDING AUTHORS Correspondence to Koji Yamano or
Richard J. Youle. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Cell Biology_ thanks Cole Haynes, Thomas
Becker, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 THE IMMUNOFLUORESCENT STAINING OF IDF WITH
ANTI-DHFR ANTIBODY. HeLa6-TetOn-IDF cells prepared as in Fig. 1b were analysed by immunofluorescent staining with anti-DHFR antibody. All images are representative of at least two
independent experiments and were shown as Z-projected results. EXTENDED DATA FIG. 2 IDF-MTX COMPLEX DOES NOT DISSIPATE THE MITOCHONDRIAL MEMBRANE POTENTIAL. (A) HeLa6-TetOn-IDF cells were
treated with DMSO or Dox/MTX for 24 hours or further treated with CCCP for the last 1 hour. Mitochondrial membrane potential was visualized with TMRE staining. DIC, differential interference
contrast. Bars, 50 μm. All images are representative of at least two independent experiments. (B) Quantification of TMRE signals in (A). The TMRE-positive area values per cell were shown as
jittered dots. The data were obtained from two independent experiments (_n_=207 for DMSO, _n_=225 for Dox/MTX, _n_=206 for Dox/MTX+CCCP=206). Horizontal lines are displayed as the median.
Statistical significance was assessed by one-way ANOVA with Dunnett’s multiple comparisons test (ns, not significant; *** p<0.001). Source numerical data are available in source data.
Source data EXTENDED DATA FIG. 3 VCP/P97 DOES NOT REMOVE THE MATURE IDF-MTX COMPLEX FROM MITOCHONDRIA. (A) HeLa6-TetOn-IDF cells were treated with Dox or Dox/MTX for the indicated times. The
cell lysates were analysed by IB. p, precursor; m, mature form. All blots are representative of three independent experiments. (B) HeLa6-TetOn-IDF cells were treated with the indicated
reagents and times. The cells (Total) were fractionated into mitochondrial (Mito) and cytosolic (Cyto) fractions, and analysed by IB. d, degraded intermediate of IDF. NMS-873, a VCP/p97
inhibitor. All blots are representative of two independent experiments. (C) HeLa6-TetOn-IDF cells were treated with the indicated reagents and times. The cell lysates were analysed by IB.
All blots are representative of two independent experiments. Unprocessed blots are available in source data. Source data EXTENDED DATA FIG. 4 IDF PLUGGING DOES NOT INDUCE PINK1
STABILIZATION. HeLa6-WT and HeLa6-TetOn-IDF cells were treated with the indicated reagents and times. The cell lysates were analysed by IB. All blots are representative of two independent
experiments. O/A, oligomycin and antimycin A; Epo, epoxomicin. Unprocessed blots are available in source data. Source data EXTENDED DATA FIG. 5 IDF PLUGGING INDUCES SELECTIVE DEGRADATION OF
TIMM17A AND TIMM23. (A) The cell lysates prepared as in Fig. 5b were analysed by IB with antibodies against TOM subunits and TIMM50. All blots are representative of four independent
experiments. (B) Volcano plot representation of the change in the putative YME1L1 substrates upon IDF plugging. The quantitative proteomic data was the same as in Fig. 5a but with different
annotations. Class I proteins are the 29 putative YME1L1 substrates that are downregulated under hypoxic conditions; Class II proteins are the remaining 35 putative YME1L1 substrates that
accumulated in _YME1L1_ KO MEF cells during normoxia. The annotation of YME1L1 substrates was retrieved from the previous report23. Unprocessed blots are available in source data. Source
data EXTENDED DATA FIG. 6 RELATIONSHIP BETWEEN IDF-DEPENDENT TIMM17A DEGRADATION AND MTOR ACTIVITY OR THE IMBALANCE OF MITOCHONDRIA DNA- AND NUCLEAR-ENCODED PROTEINS. (A) HeLa6-TetOn-IDF
cells were treated with the indicated reagents for 24 hours. The cell lysates were analysed by IB. All blots are representative of three independent experiments. (B) Quantification of p-S6K
(Ser371), TIMM17A and TIMM23 in (A) determined by densitometry. For p-S6K (Ser371), the blot densities were normalized first to total S6K and subsequently to DMSO treatment (lane 1). For
TIMM17A and TIMM23, the blot densities were normalized first to HSP90 and subsequently to DMSO treatment (lane 1). The bars are displayed as mean ± SD from _n_=3 independent experiments.
Statistical analysis was assessed by one-way ANOVA with Dunnett’s multiple comparisons test (* p<0.05; ** p<0.01; ns, not significant). (C) HeLa6-TetOn-IDF cells were treated with
Dox/MTX or chloramphenicol (Cam) for 24 hours. The cell lysates were analysed by IB. All blots are representative of three biological replicates. (D) Quantification of YME1L1, TIMM17A,
TIMM23 and ATF4 in (C) determined by densitometry, normalized first to HSP90 and subsequently to untreatment (lane 1). The bars are displayed as mean ± SD from _n_=3 independent experiments.
Statistical analysis was assessed by one-way ANOVA with Dunnett’s multiple comparisons test (** p<0.01; *** p<0.001; ns, not significant). Source numerical data and unprocessed blots
are available in source data. Source data EXTENDED DATA FIG. 7 MITOCHONDRIAL LOCALIZATION OF FLAG-TAGGED TIMM17A AND TIMM17B. pBABE-EGFP-P2A-TIMM17 stable HeLa cells were analysed by
immunofluorescent staining with anti-FLAG and TOMM20 antibodies. Bars, 10 μm. All images are representative of two independent experiments. EXTENDED DATA FIG. 8 OVEREXPRESSION OF
MITOCHONDRIAL BIPARTITE SIGNALS FUSED TO DHFR INDUCE YME1L1-DEPENDENT TIMM17A DEGRADATION. (A) Schematic illustration of Dox-inducible proteins consisting of hDHFR and the indicated
N-terminal mitochondrial precursors that contain a bipartite signal of mitochondrial targeting sequence (MTS) and a single transmembrane domain (TMD). MIC60 is identical to IDF (Fig. 1a).
PISD is a mitochondria-localized enzyme that converts phosphatidylserine to phosphatidylethanolamine (UniProt: Q9UG56). DHODH has an uncleavable MTS at the N terminus followed by TMD
anchored to IMM (UniProt: Q02127), and SCO2 is known as a cytochrome c oxidase assembly factor (UniProt: O43819). (B) WT and _YME1L1_ KO (KO) HeLa cells stably expressing the indicated
construct were treated with or without Dox/MTX for 24 hours. The cell lysates were analysed by IB. All blots are representative of three independent experiments. p, precursor; m, mature.
Asterisks denote DHFR moieties partially degraded or translated from a second methionine or later. (C) Quantification of TIMM17A (_n_=3 independent experiments) in (B) determined by
densitometry, normalized first to Actin and subsequently to untreated for each substrate. The bars are displayed as mean ± SD. Statistical analysis was assessed by one-way ANOVA with
Dunnett’s multiple comparisons test (* p<0.05; ** p<0.01; *** p<0.001). Source numerical data and unprocessed blots are available in source data. Source data SUPPLEMENTARY
INFORMATION REPORTING SUMMARY PEER REVIEW FILE SUPPLEMENTARY TABLE 1 MS data file related to Fig. 5a. SUPPLEMENTARY TABLE 2 Materials including antibodies, cells, plasmids etc. SOURCE DATA
SOURCE DATA FIG. 1 Unprocessed western blots. SOURCE DATA FIG. 1 Statistical source data. SOURCE DATA FIG. 2 Unprocessed western blots. SOURCE DATA FIG. 2 Statistical source data. SOURCE
DATA FIG. 3 Unprocessed western blots. SOURCE DATA FIG. 3 Statistical source data. SOURCE DATA FIG. 4 Unprocessed western blots. SOURCE DATA FIG. 4 Statistical source data. SOURCE DATA FIG.
5 Unprocessed western blots. SOURCE DATA FIG. 5 Statistical source data. SOURCE DATA FIG. 6 Unprocessed western blots. SOURCE DATA FIG. 6 Statistical source data. SOURCE DATA FIG. 7
Unprocessed western blots. SOURCE DATA FIG. 7 Statistical source data. SOURCE DATA FIG. 8 Unprocessed western blots. SOURCE DATA FIG. 8 Statistical source data. SOURCE DATA EXTENDED DATA
FIG. 2 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 3 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 4 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 5
Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 6 Unprocessed western blots. SOURCE DATA EXTENDED DATA FIG. 6 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 8 Unprocessed
western blots. SOURCE DATA EXTENDED DATA FIG. 8 Statistical source data. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hsu, MC., Kinefuchi, H., Lei, L.
_et al._ Mitochondrial YME1L1 governs unoccupied protein translocase channels. _Nat Cell Biol_ 27, 309–321 (2025). https://doi.org/10.1038/s41556-024-01571-z Download citation * Received:
26 January 2024 * Accepted: 04 November 2024 * Published: 07 January 2025 * Issue Date: February 2025 * DOI: https://doi.org/10.1038/s41556-024-01571-z SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
