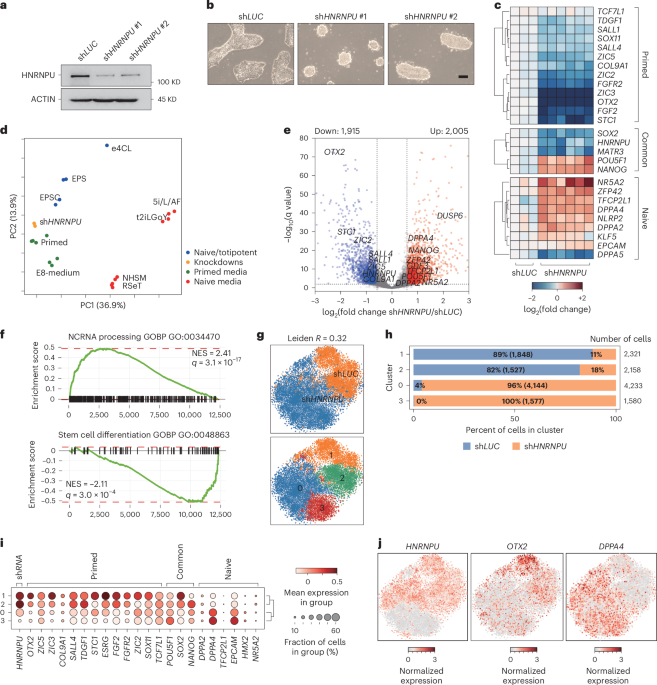
The nuclear matrix stabilizes primed-specific genes in human pluripotent stem cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The nuclear matrix, a proteinaceous gel composed of proteins and RNA, is an important nuclear structure that supports chromatin architecture, but its role in human pluripotent stem
cells (hPSCs) has not been described. Here we show that by disrupting heterogeneous nuclear ribonucleoprotein U (HNRNPU) or the nuclear matrix protein, Matrin-3, primed hPSCs adopted
features of the naive pluripotent state, including morphology and upregulation of naive-specific marker genes. We demonstrate that _HNRNPU_ depletion leads to increased chromatin
accessibility, reduced DNA contacts and increased nuclear size. Mechanistically, HNRNPU acts as a transcriptional co-factor that anchors promoters of primed-specific genes to the nuclear
matrix with POLII to promote their expression and their RNA stability. Overall, HNRNPU promotes cell-type stability and when reduced promotes conversion to earlier embryonic states. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other
Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online
access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
WIDESPREAD REORGANISATION OF PLURIPOTENT FACTOR BINDING AND GENE REGULATORY INTERACTIONS BETWEEN HUMAN PLURIPOTENT STATES Article Open access 07 April 2021 B1 SINE-BINDING ZFP266 IMPEDES
MOUSE IPSC GENERATION THROUGH SUPPRESSION OF CHROMATIN OPENING MEDIATED BY REPROGRAMMING FACTORS Article Open access 30 January 2023 RNA IS ESSENTIAL FOR PRC2 CHROMATIN OCCUPANCY AND
FUNCTION IN HUMAN PLURIPOTENT STEM CELLS Article 06 July 2020 DATA AVAILABILITY Sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus
under accession code GSE242351. Previously published data that were re-analysed here are available as follows. Naive and primed RNA-seq data are from GSE93241 (ref. 80), GSE75868, GSE85689
(ref. 28), PRJNA383735 (ref. 38), PRJNA397941 (ref. 29) and CNP0001454 (ref. 46). Aggregate ChIP-seq data are from Cistrome106; MAR-seq data were from GSE87671 (ref. 12). Other images
supporting the findings of this study are available from Figshare at https://doi.org/10.6084/m9.figshare.24739365 (ref. 115). Data supporting the findings of this study are available from
the corresponding author on reasonable request. Source data are provided with this paper. REFERENCES * Cantone, I. & Fisher, A. G. Epigenetic programming and reprogramming during
development. _Nat. Struct. Mol. Biol._ 20, 282–289 (2013). Article CAS PubMed Google Scholar * Wu, J. et al. Chromatin analysis in human early development reveals epigenetic transition
during ZGA. _Nature_ 557, 256–260 (2018). Article CAS PubMed Google Scholar * Sun, L., Fu, X., Ma, G. & Hutchins, A. P. Chromatin and epigenetic rearrangements in embryonic stem cell
fate transitions. _Front. Cell Dev. Biol._ 9, 637309 (2021). Article PubMed PubMed Central Google Scholar * Xu, Q. & Xie, W. Epigenome in early mammalian development: inheritance,
reprogramming and establishment. _Trends Cell Biol._ 28, 237–253 (2018). Article CAS PubMed Google Scholar * Wu, J. et al. The landscape of accessible chromatin in mammalian
preimplantation embryos. _Nature_ 534, 652–657 (2016). Article CAS PubMed Google Scholar * Liu, L. et al. An integrated chromatin accessibility and transcriptome landscape of human
pre-implantation embryos. _Nat. Commun._ 10, 364 (2019). Article PubMed PubMed Central Google Scholar * Chen, X. et al. Key role for CTCF in establishing chromatin structure in human
embryos. _Nature_ 576, 306–310 (2019). Article CAS PubMed Google Scholar * Ke, Y. et al. 3D chromatin structures of mature gametes and structural reprogramming during mammalian
embryogenesis. _Cell_ 170, 367–381.e20 (2017). Article CAS PubMed Google Scholar * Du, Z. et al. Allelic reprogramming of 3D chromatin architecture during early mammalian development.
_Nature_ 547, 232–235 (2017). Article CAS PubMed Google Scholar * Podgornaya, O. I. Nuclear organization by satellite DNA, SAF-A/hnRNPU and matrix attachment regions. _Semin. Cell Dev.
Biol._ 128, 61–68 (2022). Article CAS PubMed Google Scholar * Cha, H. J. et al. Inner nuclear protein matrin-3 coordinates cell differentiation by stabilizing chromatin architecture.
_Nat. Commun._ 12, 6241 (2021). Article CAS PubMed PubMed Central Google Scholar * Dobson, J. R. et al. Identifying nuclear matrix-attached DNA across the genome. _J. Cell. Physiol._
232, 1295–1305 (2017). Article CAS PubMed PubMed Central Google Scholar * Creamer, K. M., Kolpa, H. J. & Lawrence, J. B. Nascent RNA scaffolds contribute to chromosome territory
architecture and counter chromatin compaction. _Mol. Cell_ 81, 3509–3525.e5 (2021). Article CAS PubMed PubMed Central Google Scholar * Nozawa, R. S. et al. SAF-A regulates interphase
chromosome structure through oligomerization with chromatin-associated RNAs. _Cell_ 169, 1214–1227.e18 (2017). Article CAS PubMed PubMed Central Google Scholar * Kolpa, H. J., Creamer,
K. M., Hall, L. L. & Lawrence, J. B. SAF-A mutants disrupt chromatin structure through dominant negative effects on RNAs associated with chromatin. _Mamm. Genome_ 33, 366–381 (2022).
Article CAS PubMed Google Scholar * Xiao, R. et al. Nuclear matrix factor hnRNP U/SAF-A exerts a global control of alternative splicing by regulating U2 snRNP maturation. _Mol. Cell_ 45,
656–668 (2012). Article CAS PubMed PubMed Central Google Scholar * Ye, J. et al. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function.
_Proc. Natl Acad. Sci. USA_ 112, E3020–E3029 (2015). Article CAS PubMed PubMed Central Google Scholar * Sapir, T. & Reiner, O. HNRNPU’s multi-tasking is essential for proper
cortical development. _Bioessays_ 45, e2300039 (2023). Article PubMed Google Scholar * Fan, H. et al. The nuclear matrix protein HNRNPU maintains 3D genome architecture globally in mouse
hepatocytes. _Genome Res._ 28, 192–202 (2018). Article CAS PubMed PubMed Central Google Scholar * Hasegawa, Y. et al. The matrix protein hnRNP U is required for chromosomal localization
of Xist RNA. _Dev. Cell_ 19, 469–476 (2010). Article CAS PubMed Google Scholar * Marenda, M., Lazarova, E. & Gilbert, N. The role of SAF-A/hnRNP U in regulating chromatin structure.
_Curr. Opin. Genet. Dev._ 72, 38–44 (2022). Article CAS PubMed Google Scholar * Hacisuleyman, E. et al. Topological organization of multichromosomal regions by the long intergenic
noncoding RNA Firre. _Nat. Struct. Mol. Biol._ 21, 198–206 (2014). Article CAS PubMed PubMed Central Google Scholar * Kukalev, A., Nord, Y., Palmberg, C., Bergman, T. & Percipalle,
P. Actin and hnRNP U cooperate for productive transcription by RNA polymerase II. _Nat. Struct. Mol. Biol._ 12, 238–244 (2005). Article CAS PubMed Google Scholar * Obrdlik, A. et al. The
histone acetyltransferase PCAF associates with actin and hnRNP U for RNA polymerase II transcription. _Mol. Cell. Biol._ 28, 6342–6357 (2008). Article CAS PubMed PubMed Central Google
Scholar * Cao, L. et al. The nuclear matrix protein SAFA surveils viral RNA and facilitates immunity by activating antiviral enhancers and super-enhancers. _Cell Host Microbe_ 26,
369–384.e8 (2019). Article CAS PubMed Google Scholar * Bayerl, J. et al. Principles of signaling pathway modulation for enhancing human naive pluripotency induction. _Cell Stem Cell_ 28,
1549–1565.e12 (2021). Article CAS PubMed PubMed Central Google Scholar * Battle, S. L. et al. Enhancer chromatin and 3D genome architecture changes from naive to primed human embryonic
stem cell states. _Stem Cell Rep._ 12, 1129–1144 (2019). Article CAS Google Scholar * Theunissen, T. W. et al. Molecular criteria for defining the naive human pluripotent state. _Cell
Stem Cell_ 19, 502–515 (2016). Article CAS PubMed PubMed Central Google Scholar * Liu, X. et al. Comprehensive characterization of distinct states of human naive pluripotency generated
by reprogramming. _Nat. Methods_ 14, 1055–1062 (2017). Article CAS PubMed Google Scholar * Du, P. & Wu, J. Hallmarks of totipotent and pluripotent stem cell states. _Cell Stem Cell_
31, 312–333 (2024). Article CAS PubMed Google Scholar * Chan, Y. S. et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation
epiblast. _Cell Stem Cell_ 13, 663–675 (2013). Article CAS PubMed Google Scholar * Gafni, O. et al. Derivation of novel human ground state naive pluripotent stem cells. _Nature_ 504,
282–286 (2013). Article CAS PubMed Google Scholar * Guo, G. et al. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. _Stem Cell Rep._ 6,
437–446 (2016). Article CAS Google Scholar * Pastor, W. A. et al. Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. _Cell Stem Cell_
18, 323–329 (2016). Article CAS PubMed PubMed Central Google Scholar * Szczerbinska, I. et al. A chemically defined feeder-free system for the establishment and maintenance of the human
naive pluripotent state. _Stem Cell Rep._ 13, 612–626 (2019). Article CAS Google Scholar * Takashima, Y. et al. Resetting transcription factor control circuitry toward ground-state
pluripotency in human. _Cell_ 162, 452–453 (2015). Article CAS PubMed PubMed Central Google Scholar * Ware, C. B. et al. Derivation of naive human embryonic stem cells. _Proc. Natl
Acad. Sci. USA_ 111, 4484–4489 (2014). Article CAS PubMed PubMed Central Google Scholar * Cornacchia, D. et al. Lipid deprivation induces a stable, naive-to-primed intermediate state of
pluripotency in human PSCs. _Cell Stem Cell_ 25, 120–136.e10 (2019). Article CAS PubMed PubMed Central Google Scholar * Cha, Y. et al. Metabolic control of primed human pluripotent
stem cell fate and function by the miR-200c-SIRT2 axis. _Nat. Cell Biol._ 19, 445–456 (2017). Article CAS PubMed PubMed Central Google Scholar * Chovanec, P. et al. Widespread
reorganisation of pluripotent factor binding and gene regulatory interactions between human pluripotent states. _Nat. Commun._ 12, 2098 (2021). Article CAS PubMed PubMed Central Google
Scholar * Li, N. et al. Single-cell 3D genome structure reveals distinct human pluripotent states. _Genome Biol._ 25, 122 (2024). Article PubMed PubMed Central Google Scholar * Xu, R.,
Li, C., Liu, X. & Gao, S. Insights into epigenetic patterns in mammalian early embryos. _Protein Cell_ 12, 7–28 (2021). Article PubMed Google Scholar * Sapir, T. et al. Heterogeneous
nuclear ribonucleoprotein U (HNRNPU) safeguards the developing mouse cortex. _Nat. Commun._ 13, 4209 (2022). Article CAS PubMed PubMed Central Google Scholar * Hanna, J. et al. Human
embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. _Proc. Natl Acad. Sci. USA_ 107, 9222–9227 (2010). Article CAS PubMed PubMed Central
Google Scholar * Babarinde, I. A. et al. Transposable element sequence fragments incorporated into coding and noncoding transcripts modulate the transcriptome of human pluripotent stem
cells. _Nucleic Acids Res._ 49, 9132–9153 (2021). Article CAS PubMed PubMed Central Google Scholar * Mazid, M. A. et al. Rolling back human pluripotent stem cells to an eight-cell
embryo-like stage. _Nature_ 605, 315–324 (2022). Article CAS PubMed Google Scholar * Li, D. et al. c-Jun as a one-way valve at the naive to primed interface. _Cell Biosci._ 13, 191
(2023). Article CAS PubMed PubMed Central Google Scholar * Zhuang, Q. et al. NCoR/SMRT co-repressors cooperate with c-MYC to create an epigenetic barrier to somatic cell reprogramming.
_Nat. Cell Biol._ 20, 400–412 (2018). Article CAS PubMed Google Scholar * Fu, X. et al. Restricting epigenetic activity promotes the reprogramming of transformed cells to pluripotency in
a line-specific manner. _Cell Death Discov._ 9, 245 (2023). Article CAS PubMed PubMed Central Google Scholar * Taubenschmid-Stowers, J. et al. 8C-like cells capture the human zygotic
genome activation program in vitro. _Cell Stem Cell_ 29, 449–459.e6 (2022). Article CAS PubMed PubMed Central Google Scholar * Li, R. et al. A mesenchymal-to-epithelial transition
initiates and is required for the nuclear reprogramming of mouse fibroblasts. _Cell Stem Cell_ 7, 51–63 (2010). Article CAS PubMed Google Scholar * Li, Y. et al. Real-time 3D
single-molecule localization using experimental point spread functions. _Nat. Methods_ 15, 367–369 (2018). Article CAS PubMed PubMed Central Google Scholar * Li, Y. et al. Global
fitting for high-accuracy multi-channel single-molecule localization. _Nat. Commun._ 13, 3133 (2022). Article CAS PubMed PubMed Central Google Scholar * Ricci, M. A., Manzo, C.,
Garcia-Parajo, M. F., Lakadamyali, M. & Cosma, M. P. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. _Cell_ 160, 1145–1158 (2015). Article CAS PubMed
Google Scholar * Haas, K. T., Lee, M., Esposito, A. & Venkitaraman, A. R. Single-molecule localization microscopy reveals molecular transactions during RAD51 filament assembly at
cellular DNA damage sites. _Nucleic Acids Res._ 46, 2398–2416 (2018). Article CAS PubMed PubMed Central Google Scholar * Pollini, D. et al. Multilayer and MATR3-dependent regulation of
mRNAs maintains pluripotency in human induced pluripotent stem cells. _iScience_ 24, 102197 (2021). Article CAS PubMed PubMed Central Google Scholar * Huo, X. et al. The nuclear matrix
protein SAFB cooperates with major satellite RNAs to stabilize heterochromatin architecture partially through phase separation. _Mol. Cell_ 77, 368–383.e7 (2020). Article CAS PubMed
Google Scholar * Tsichlaki, E. & FitzHarris, G. Nucleus downscaling in mouse embryos is regulated by cooperative developmental and geometric programs. _Sci. Rep._ 6, 28040 (2016).
Article CAS PubMed PubMed Central Google Scholar * Xu, J. et al. Super-resolution imaging of higher-order chromatin structures at different epigenomic states in single mammalian cells.
_Cell Rep._ 24, 873–882 (2018). Article CAS PubMed PubMed Central Google Scholar * Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single
cells. _Nat. Commun._ 10, 1930 (2019). Article PubMed PubMed Central Google Scholar * Consortium, E. P. An integrated encyclopedia of DNA elements in the human genome. _Nature_ 489,
57–74 (2012). Article Google Scholar * Dixon, J. R. et al. Chromatin architecture reorganization during stem cell differentiation. _Nature_ 518, 331–336 (2015). Article CAS PubMed
PubMed Central Google Scholar * Lyu, X., Rowley, M. J. & Corces, V. G. Architectural proteins and pluripotency factors cooperate to orchestrate the transcriptional response of hESCs to
temperature stress. _Mol. Cell_ 71, 940–955.e7 (2018). Article CAS PubMed PubMed Central Google Scholar * Gertz, J. et al. Distinct properties of cell-type-specific and shared
transcription factor binding sites. _Mol. Cell_ 52, 25–36 (2013). Article CAS PubMed Google Scholar * Marshall, N. F. & Price, D. H. Purification of P-TEFb, a transcription factor
required for the transition into productive elongation. _J. Biol. Chem._ 270, 12335–12338 (1995). Article CAS PubMed Google Scholar * Liu, L. et al. Transcriptional pause release is a
rate-limiting step for somatic cell reprogramming. _Cell Stem Cell_ 15, 574–588 (2014). Article CAS PubMed Google Scholar * Narwade, N. et al. Mapping of scaffold/matrix attachment
regions in human genome: a data mining exercise. _Nucleic Acids Res._ 47, 7247–7261 (2019). Article CAS PubMed PubMed Central Google Scholar * Liu, T. et al. Matrin3 mediates
differentiation through stabilizing chromatin loop-domain interactions and YY1 mediated enhancer-promoter interactions. _Nat. Commun._ 15, 1274 (2024). Article CAS PubMed PubMed Central
Google Scholar * Ernst, J. & Kellis, M. Chromatin-state discovery and genome annotation with ChromHMM. _Nat. Protoc._ 12, 2478–2492 (2017). Article CAS PubMed PubMed Central Google
Scholar * Roadmap Epigenomics, C. et al. Integrative analysis of 111 reference human epigenomes. _Nature_ 518, 317–330 (2015). Article Google Scholar * Attig, J. et al. Heteromeric RNP
assembly at LINEs controls lineage-specific RNA processing. _Cell_ 174, 1067–1081.e17 (2018). Article CAS PubMed PubMed Central Google Scholar * Yugami, M., Kabe, Y., Yamaguchi, Y.,
Wada, T. & Handa, H. hnRNP-U enhances the expression of specific genes by stabilizing mRNA. _FEBS Lett._ 581, 1–7 (2007). Article CAS PubMed Google Scholar * Zhao, W. et al. Nuclear
to cytoplasmic translocation of heterogeneous nuclear ribonucleoprotein U enhances TLR-induced proinflammatory cytokine production by stabilizing mRNAs in macrophages. _J. Immunol._ 188,
3179–3187 (2012). Article CAS PubMed Google Scholar * Wang, J. et al. A novel long intergenic noncoding RNA indispensable for the cleavage of mouse two-cell embryos. _EMBO Rep._ 17,
1452–1470 (2016). Article CAS PubMed PubMed Central Google Scholar * Roshon, M. J. & Ruley, H. E. Hypomorphic mutation in hnRNP U results in post-implantation lethality. _Transgenic
Res._ 14, 179–192 (2005). Article CAS PubMed Google Scholar * Wei, M. et al. Nuclear actin regulates inducible transcription by enhancing RNA polymerase II clustering. _Sci. Adv._ 6,
eaay6515 (2020). Article CAS PubMed PubMed Central Google Scholar * Xie, X. et al. β-Actin-dependent global chromatin organization and gene expression programs control cellular
identity. _FASEB J._ 32, 1296–1314 (2018). Article CAS PubMed Google Scholar * Mahmood, S. R., Said, N. H. E., Gunsalus, K. C. & Percipalle, P. β-Actin mediated H3K27ac changes
demonstrate the link between compartment switching and enhancer-dependent transcriptional regulation. _Genome Biol._ 24, 18 (2023). Article CAS PubMed PubMed Central Google Scholar *
Mahmood, S. R. et al. β-Actin dependent chromatin remodeling mediates compartment level changes in 3D genome architecture. _Nat. Commun._ 12, 5240 (2021). Article CAS PubMed PubMed
Central Google Scholar * Collier, A. J. et al. Comprehensive cell surface protein profiling identifies specific markers of human naive and primed pluripotent states. _Cell Stem Cell_ 20,
874–890.e7 (2017). Article CAS PubMed PubMed Central Google Scholar * Yang, Y. et al. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. _Cell_ 169,
243–257.e25 (2017). Article CAS PubMed PubMed Central Google Scholar * Gao, X. et al. Establishment of porcine and human expanded potential stem cells. _Nat. Cell Biol._ 21, 687–699
(2019). Article CAS PubMed PubMed Central Google Scholar * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2.
_Genome Biol._ 15, 550 (2014). Article PubMed PubMed Central Google Scholar * Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory
elements required for macrophage and B cell identities. _Mol. Cell_ 38, 576–589 (2010). Article CAS PubMed PubMed Central Google Scholar * Sun, L. et al. BRD8 guards the pluripotent
state by sensing and maintaining histone acetylation. _Adv. Sci._ https://doi.org/10.1002/advs.202409160 (2024). * Warlich, E. et al. Lentiviral vector design and imaging approaches to
visualize the early stages of cellular reprogramming. _Mol. Ther._ 19, 782–789 (2011). Article CAS PubMed PubMed Central Google Scholar * Zhang, M. et al. β-Catenin safeguards the
ground state of mousepluripotency by strengthening the robustness of the transcriptional apparatus. _Sci. Adv._ 6, eaba1593 (2020). Article CAS PubMed PubMed Central Google Scholar *
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. _Nat. Protoc._ 11, 2301–2319 (2016). Article CAS PubMed Google
Scholar * Hutchins, A. P., Jauch, R., Dyla, M. & Miranda-Saavedra, D. glbase: a framework for combining, analyzing and displaying heterogeneous genomic and high-throughput sequencing
data. _Cell Regen._ 3, 1 (2014). Article PubMed PubMed Central Google Scholar * Binder, J. X. et al. COMPARTMENTS: unification and visualization of protein subcellular localization
evidence. _Database_ 2014, bau012 (2014). Article PubMed PubMed Central Google Scholar * Hu, H. et al. AnimalTFDB 3.0: a comprehensive resource for annotation and prediction of animal
transcription factors. _Nucleic Acids Res._ 47, D33–D38 (2019). Article CAS PubMed Google Scholar * Medvedeva, Y. A. et al. EpiFactors: a comprehensive database of human epigenetic
factors and complexes. _Database_ 2015, bav067 (2015). Article PubMed PubMed Central Google Scholar * Hutchins, A. P. et al. Models of global gene expression define major domains of cell
type and tissue identity. _Nucleic Acids Res._ 45, 2354–2367 (2017). Article CAS PubMed PubMed Central Google Scholar * He, J. et al. Identifying transposable element expression
dynamics and heterogeneity during development at the single-cell level with a processing pipeline scTE. _Nat. Commun._ 12, 1456 (2021). Article CAS PubMed PubMed Central Google Scholar
* Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS PubMed Google Scholar * Risso, D., Schwartz, K., Sherlock, G. & Dudoit,
S. GC-content normalization for RNA-seq data. _BMC Bioinformatics_ 12, 480 (2011). Article CAS PubMed PubMed Central Google Scholar * Young, M. D., Wakefield, M. J., Smyth, G. K. &
Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. _Genome Biol._ 11, R14 (2010). Article PubMed PubMed Central Google Scholar * Sergushichev, A. A. Fast gene
set enrichment analysis. Preprint at _bioRxiv_ https://doi.org/10.1101/060012 (2016). * Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data
analysis. _Genome Biol._ 19, 15 (2018). Article PubMed PubMed Central Google Scholar * Dahm, G. M. et al. Method for the isolation and identification of mRNAs, microRNAs and protein
components of ribonucleoprotein complexes from cell extracts using RIP-Chip. J. Vis. Exp. https://doi.org/10.3791/3851 (2012). * Li, D. et al. Chromatin accessibility dynamics during iPSC
reprogramming. _Cell Stem Cell_ 21, 819–833.e6 (2017). Article CAS PubMed Google Scholar * Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying
chromatin accessibility genome-wide. _Curr. Protoc. Mol. Biol._ 109, 21.29.1–21.29.9 (2015). Article PubMed Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment
with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). _Genome Biol._ 9, R137
(2008). Article PubMed PubMed Central Google Scholar * Ma, G., Babarinde, I. A., Zhuang, Q. & Hutchins, A. P. Unified analysis of multiple ChIP-seq datasets. _Methods Mol. Biol._
2198, 451–465 (2021). Article CAS PubMed Google Scholar * Zheng, R. et al. Cistrome Data Browser: expanded datasets and new tools for gene regulatory analysis. _Nucleic Acids Res._ 47,
D729–D735 (2019). Article CAS PubMed Google Scholar * Rao, S. S. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. _Cell_ 159, 1665–1680
(2014). Article CAS PubMed PubMed Central Google Scholar * Imakaev, M. et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. _Nat. Methods_ 9, 999–1003
(2012). Article CAS PubMed PubMed Central Google Scholar * Abdennur, N. & Mirny, L. A. Cooler: scalable storage for Hi-C data and other genomically labeled arrays. _Bioinformatics_
36, 311–316 (2020). Article CAS PubMed Google Scholar * Salameh, T. J. et al. A supervised learning framework for chromatin loop detection in genome-wide contact maps. _Nat. Commun._
11, 3428 (2020). Article CAS PubMed PubMed Central Google Scholar * Flyamer, I. M., Illingworth, R. S. & Bickmore, W. A. Coolpup.py: versatile pile-up analysis of Hi-C data.
_Bioinformatics_ 36, 2980–2985 (2020). Article CAS PubMed PubMed Central Google Scholar * Ries, J. SMAP: a modular super-resolution microscopy analysis platform for SMLM data. _Nat.
Methods_ 17, 870–872 (2020). Article CAS PubMed Google Scholar * Thevathasan, J. V. et al. Nuclear pores as versatile reference standards for quantitative superresolution microscopy.
_Nat. Methods_ 16, 1045–1053 (2019). Article CAS PubMed PubMed Central Google Scholar * Haas, K. T. & Peaucelle, A. Protocol for multicolor three-dimensional dSTORM data analysis
using MATLAB-based script package Grafeo. _STAR Protoc._ 2, 100808 (2021). Article PubMed PubMed Central Google Scholar * Ma, G. et al. Source data for the article ‘The nuclear matrix
stabilizes primed-specific genes in human pluripotent stem cells’. _Figshare_ https://doi.org/10.6084/m9.figshare.24739365 (2024). * Yang, Y. et al. Metabolic and epigenetic dysfunctions
underlie the arrest of in vitro fertilized human embryos in a senescent-like state. _PLoS Biol._ 20, e3001682 (2022). Article CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (32150710521 and 32270597 to A.P.H., 62375116 to Y.L. and 32270574 to D.L.), Shenzhen
Medical Research Fund (B2302038 to Y.L.), Key Technology Research and Development Program of Shandong Province (2021CXGC010212 to Y.L.), Shenzhen Science and Technology Innovation Program
(JCYJ20220818100416036 and KQTD20200820113012029 to Y.L.), Basic and Applied Basic Research Fund of Guangdong Province (2024A1515011565 to Y.L.) and the Science and Technology Projects in
Guangzhou (2023A03J0045 to D.L.). Additional support was rendered by the Center for Computational Science and Engineering of the Southern University of Science and Technology. We acknowledge
the assistance of SUSTech Core Research Facilities. The H9 TPRX1-GFP cells were a kind gift from M. A. Esteban (Beijing Genomics Institute, Shenzhen), and the EpiSCs were a kind gift from
C. Jiekai (Guangzhou Institutes of Biomedicine and Health, Guangzhou). AUTHOR INFORMATION Author notes * These authors contributed equally: Gang Ma, Xiuling Fu, Lulu Zhou. * These authors
jointly supervised this work: Dongwei Li, Yiming Li, Andrew P. Hutchins. AUTHORS AND AFFILIATIONS * Department of Systems Biology, School of Life Sciences, Southern University of Science and
Technology, Shenzhen, China Gang Ma, Xiuling Fu, Isaac A. Babarinde, Liyang Shi, Jiao Chen, Zhen Xiao, Yu Qiao, Lisha Ma, Yuhao Ou, Yuhao Li, Chen Chang, Boping Deng, Li Sun & Andrew P.
Hutchins * Department of Biomedical Engineering, Southern University of Science and Technology, Shenzhen, China Lulu Zhou & Yiming Li * Department of Reproductive Medicine, The First
Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China Wenting Yang & Guoqing Tong * Key Laboratory of Biological Targeting Diagnosis, Therapy and Rehabilitation of Guangdong
Higher Education Institutes, The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China Ran Zhang & Dongwei Li Authors * Gang Ma View author publications You can
also search for this author inPubMed Google Scholar * Xiuling Fu View author publications You can also search for this author inPubMed Google Scholar * Lulu Zhou View author publications You
can also search for this author inPubMed Google Scholar * Isaac A. Babarinde View author publications You can also search for this author inPubMed Google Scholar * Liyang Shi View author
publications You can also search for this author inPubMed Google Scholar * Wenting Yang View author publications You can also search for this author inPubMed Google Scholar * Jiao Chen View
author publications You can also search for this author inPubMed Google Scholar * Zhen Xiao View author publications You can also search for this author inPubMed Google Scholar * Yu Qiao
View author publications You can also search for this author inPubMed Google Scholar * Lisha Ma View author publications You can also search for this author inPubMed Google Scholar * Yuhao
Ou View author publications You can also search for this author inPubMed Google Scholar * Yuhao Li View author publications You can also search for this author inPubMed Google Scholar * Chen
Chang View author publications You can also search for this author inPubMed Google Scholar * Boping Deng View author publications You can also search for this author inPubMed Google Scholar
* Ran Zhang View author publications You can also search for this author inPubMed Google Scholar * Li Sun View author publications You can also search for this author inPubMed Google
Scholar * Guoqing Tong View author publications You can also search for this author inPubMed Google Scholar * Dongwei Li View author publications You can also search for this author inPubMed
Google Scholar * Yiming Li View author publications You can also search for this author inPubMed Google Scholar * Andrew P. Hutchins View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS G.M. performed experiments, designed the project, interpreted data and wrote and revised the paper. X.L.F. performed experiments and revised the
paper. L.L.Z. performed the SMLM experiments and data analysis. L.Y.S. analysed the Hi-C data and performed aggregate ChIP-seq data analysis. I.A.B. and Y.H.L. assisted with the
bioinformatic analysis. W.Y., J.C., Z.X., Y.Q., L.M., Y.O., L.S., B.D. and R.Z. assisted with experiments. C.C. generated plasmids and key reagents. D.L. helped with analysis, revised the
paper and acquired funding. Y.L. supervised the super-resolution imaging, revised the paper and acquired funding. D.L., G.T. and Y.L. interpreted and analysed the findings. A.P.H. supervised
the project, performed some of the analysis, interpreted the data, wrote and revised the paper and acquired funding. All other authors contributed to the interpretation of results,
performed experiments or bioinformatic analysis and helped revise the paper. CORRESPONDING AUTHORS Correspondence to Dongwei Li, Yiming Li or Andrew P. Hutchins. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Cell Biology_ thanks the anonymous reviewers for their contribution to the peer
review of this work. Peer reviewer reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 NUCLEAR MATRIX GENES FORM A UNIQUE EXPRESSION PATTERN IN PRE-IMPLANTATION EMBRYOS. A Heatmap of the expression nuclear matrix
genes (GO:CC:0016363). Data is from https://github.com/oaxiom/human, and45. B. Boxplot showing the normalized expression of the nuclear matrix genes from panel A in embryonic (panel A,
orange labelled cell types) and somatic cell types (all other colours), as indicated in panel A. Note that the placental tissues were removed from the analysis, as were the hPSC samples, as
they reflect extraembryonic cells and _in vitro_ cells, respectively. Significance is from a two-sided Mann–Whitney U-test. Data is from the re-analysis performed in
https://github.com/oaxiom/human, and45,116. Data includes 3 replicates or more for each cell type or tissue, and the embryonic category contains 16 embryonic cell types or tissues and the
somatic category contains 69 cell types or tissues. C. Bar plot showing the expression of _HNRNPU_ in a selection of human cell types and pre-implantation embryonic stages. Data is from the
re-analysis performed in https://github.com/oaxiom/human, and45,116. D. Bar plot showing the expression of _Hnrnpu_ in a selection of mouse cell types and embryonic stages. Data is from the
re-analysis performed in93. E. Scatter plots showing the expression of _HNRNPU_ versus a selection of genes expressed in early embryonic stages (_DPPA3_, _DPPA5_, _TPRX1_, and _ZSCAN4_) in
various cell types from Extended Data Fig. 1a. Embryonic cell types are marked in orange, and somatic cell types are marked with the indicated colour according to their presumed germ
lineage, as indicated in the key in Extended Data Fig. 1a. Data is from https://github.com/oaxiom/human, and45. Correlation and significance are from Pearson’s correlation coefficient test
(R). F. Scatter plots showing the expression of _HNRNPU_ versus the nuclear matrix proteins _MATR3_ and _SAFB_ in various cell types from Extended Data Fig. 1a. Embryonic cell types are
marked in orange, and somatic cell types are marked according to their presumed germ lineage, as indicated in the key in Extended Data Fig. 1a. Data is from https://github.com/oaxiom/human,
and45. Correlation and significance are from Pearson’s correlation coefficient test (R). Source data EXTENDED DATA FIG. 2 KNOCKDOWN OF _HNRNPU_ CAUSES HPSCS TO ADOPT AN EARLIER NAÏVE-LIKE
EMBRYONIC STATE. A qRT-PCR data showing the RNA levels of _HNRNPU_ in hPSCs transfected with the indicated shRNAs. Data is from one replicate, each in technical triplicate. B. Line plot
showing the population doubling in hPSCs when transfected with the indicated shRNA. Data is from three biological replicates, with one technical replicate each. C. TUNEL (terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labelling) Apoptosis assay in WT hPSCs (H1) or DNase I treated cells as a positive control when transfected with the indicated shRNA.
Scale bar = 100 µm. The experiment was performed twice with similar results. D. Heatmap showing the pair-wise correlation (Pearson’s R) for RNA-seq data generated from hPSCs transfected with
the indicated shRNAs. rp = Repeat. E. qRT-PCR data for a selection of pluripotent and naïve marker genes in hPSCs transfected with shRNAs against _HNRNPU_ or _LUC_ (control). Data is from
three biological replicates, each in technical triplicate. F. qRT-qPCR data for a selection of naïve and primed-specific genes. Data is from three biological replicates, each in technical
triplicate. G. Bar chart showing significantly overrepresented gene ontology biological process terms in the down (left bar chart) and up-regulated (right bar chart) genes defined in Fig.
1e. Significance was determined by go-seq with a Bonferroni-Hochberg (BH)-corrected p-value (q-value) of less than 0.01. H. Volcano plot showing the differentially regulated TEs in the
_HNRNPU_ knockdown cells. Significantly different TEs were classified if their log2 fold change was >0.53 (1.5-fold) and they had a BH-corrected p-value (q-value) less than 0.01. Data is
from three biological replicates. I. qRT-PCR for _HNRNPU_ in primed hPSCs transfected with the indicated shRNA and grown in 4CL medium for the indicated number of days. Data is from one
biological replicate, each in technical triplicate. J. qRT-PCR for a selection of naïve and primed-specific genes in primed hPSCs transfected with the indicated shRNA and grown in 4CL medium
for the indicated number of days. Data is from one biological replicate, each in technical triplicate. K. Immunofluorescence images of KLF4 and HNRNPU in naïve hPSCs grown in 4CL medium for
6 days and transfected with the indicated shRNA. Scale bar = 10 µm. The experiment was performed twice with similar results. L. Grey-scale values for KLF17 was measured in at least 21 cells
from hPSCs grown in 4CL medium for six days transfected with the indicated shRNA. The box represents the first and third quartiles, the midpoint is the median and the whiskers are 1.5x the
interquartile mean. Significance is from a two-sided Welch’s t-test for panels b, e, f, l. EXTENDED DATA FIG. 3 REDUCED _HNRNPU_ PROMOTES CELL TRANSITIONS TO EARLIER EMBRYONIC-LIKE CELL
TYPES. A qRT-PCR for _Hnrnpu_ in primed EpiSCs transfected with the indicated shRNA. Data is from one biological replicates, each in technical triplicate. B. Schematic model of mouse primed
(EpiSC) to naïve (mESC) stem cell transition. C. Dot plot showing the percentages of GFP+ cells (GFP is driven from a Pou5f1::GFP reporter that is only expressed in naïve mESCs) in the
primed-to naïve conversion when transfected with the indicated shRNA. This experiment was performed three times. D. qRT-PCR results for pluripotency genes _Klf2_ and _Klf4_ in the conversion
of primed-to naïve stem cells when transfected with the indicated shRNA. The experiment was performed in biological triplicate. E. qRT-PCR results for naïve-specific genes _Tfcp2l1_ and
_Prdm14_ in the conversion of primed-to naïve stem cells when transfected with the indicated shRNA. The experiment was performed in biological triplicate. F. Schematic model of mouse naïve
mESCs to EpiLCs (Epiblast-like cell) differentiation. G. Percentage of GFP+ percentages cells in the naïve to EpiLC differentiation in cells transfected with the indicated shRNA. This
experiment was performed three times. H. qRT-PCR results in EpiLCs for selected pluripotency or primed-specific genes in cells transfected with the indicated shRNA. The experiment was
performed in biological duplicate. I. GSEA plot showing the enrichment of MORULA-specific genes in the ranked genes when _HNRNPU_ was knocked down. MORULA-specific genes are defined in ref.
116. J. Heatmap of the leading-edge genes specific to MORULA form the GSEA in panel I. Embryo data is from the re-analysis in ref. 116. Data is log2(fold change) relative to sh_LUC_ sample.
K. Schematic model of the induction of 8CLCs from primed hPSCs using 4CL and e4CL medium. P3 = passage 3. L. Left panel, representative FACS scatter plots and dotplots on passage 3 in the
conversion of hPSCs to 8CLCs. The y-axis represents _TPRX1_-GFP. NC is negative control (Primed hPSCs), compared to hPSCs on passage 3 in 4CL/e4CL medium transfected with the indicated
shRNA. The experiment was performed twice in biological duplicate. Right panel, Gating strategy for this experiment and all FSC related experiments. M. Percentages of TPRX1-GFP+ cells in
cells grown in 4CL/e4CL medium and transfected with the indicated shRNA. This experiment was performed two times. N. Schematic showing the reprogramming of mouse embryonic fibroblast (MEF)
into iPSCs. O. qRT-PCR for _Hnrnpu_ in MEFs transfected with the indicated shRNA. MEFs were transfected for 5 days. Data is from three biological replicates, each in technical triplicate. P.
AP (alkaline phosphatase) stained images of day 13 reprogramming MEFs transfected with OSKM reprogramming cassette and the indicated shRNA. Scale bar = 5 mm. Q. Number of AP+ colonies at
day 13 of a reprogramming time course, in MEFs transfected with the indicated shRNA. Data is from three biological replicates, each in technical singlicate. R. Representative bright-field
and GFP images of iPSCs transfected with the indicated shRNA. Scale bar = 100 µm. S. Number of GFP+ colonies at day 13 of a reprogramming time course, in MEFs transfected with the indicated
shRNA. Data is from three biological replicates, each in technical singlicate. T. qRT-PCR for selected mesenchymal-epithelial transition (MET) or epithelial-mesenchymal transition (EMT)
genes at day 5 of a reprogramming time course. The experiment was performed once with three technical replicates. Significance is from a two-sided Welch’s t-test for panels c, d, e, g.
EXTENDED DATA FIG. 4 HNRNPU INTERACTS WITH MATR3. A Heatmap of the Co-IP/MS intensity scores and the ‘call’ for present or absent. A protein was considered present if it had at least 1
unique peptide and a minimum intensity of 1,000,000 (Razor+Unique), and an intensity that was at least 2-fold higher than the anti-FLAG control. HNRNPU Co-IP/MS was performed three times,
the MATR3 Co-IP/MS was performed once. The list of proteins used here contains HNRNPU, MATR3, and proteins from the AnimalTFDB and the Epifactors databases91,92. The full list of interacting
proteins can be found in Supplementary Table 3. # indicates the biological repeat number. B. A network where each node is an interacting protein and each edge indicates an interaction with
HNRNPU or MATR3. The network includes all HNRNPU and MATR3 interacting proteins. See also Supplementary Table 3. C. Western blot of reciprocal co-IP for HNRNPU or MATR3. This experiment was
performed twice with similar results. D. Gene ontology analysis of overrepresented terms for proteins detected in the indicated Co-IP/MS. GO analysis was performed using go-seq, without
transcript length correction. A term was considered significant if its BH-corrected p-value (q-value) was less than 0.01. The top 10 terms are shown here, ranked by their q-value. EXTENDED
DATA FIG. 5 COLOUR ASSIGNMENT FOR RATIOMETRIC DUAL-COLOUR 3D SMLM IMAGING OF AF647 AND CF680, VORONOI DIAGRAMS, AND DELAUNAY TRIANGULATION AND KNOCKDOWN OF _MATR3._ A Scatter-plot of the
photons in two channels (transmission and reflection channels) analysed by global fit. The polygons were used to assign single molecules of AF647 and CF680 in different colours. B.
Cross-talk and rejected percentage for AF647 and CF680. C. Workflow for SMLM data filtering and clustering by Voronoi diagrams and Delaunay triangulation (DT) in Grafeo. D. qRT-PCR for
_MATR3_ expression after knockdown of _MATR3_ in hPSCs. Data is from three biological replicates, each in technical triplicate. Significance is from Welch’s t-test. E. Western blot for
HNRNPU and MATR3 in hPSCs transfected with an shRNA targeting _MATR3_. The experiment was performed twice with similar results. F. qRT-PCR for the core pluripotency genes _POU5F1_ and _SOX2_
in the indicated knockdowns. Data is from three biological replicates, each in technical triplicate. Significance is from Welch’s t-test. G. qRT-PCR for selected naïve and primed-specific
genes in the indicated knockdowns. Data is from three biological replicates, each in technical triplicate. Significance is from Welch’s t-test. EXTENDED DATA FIG. 6 HNRNPU IS INCREASED IN
SOMATIC CELLS, AND KNOCKDOWN DOES NOT DISRUPT SOME 3D CHROMATIN STRUCTURES. A Immunofluorescence images of hPSCs and hESF (human embryonic fibroblasts) stained with an antibody against
HNRNPU. Scale bar = 100 µm (top two panels) and 10 µm (bottom six panels). B. Western blot of HNRNPU and b-ACTIN In hPSCs and hESFs. This experiment was performed twice with similar results.
C. SMLM images of HNRNPU (stained with an anti-HNRNPU antibody) in primed hPSCs, and 293 T cells. For the upper panel scale bar = 2 µm, for the nuclear periphery, nucleoplasm, and the
z-axis, the scale bar = 200 nm. D. Boxplots showing the density for the HNRNPU clusters (top boxplot) and the localizations density within each cluster per volume for HNRNPU molecules
(bottom boxplot) in primed hPSCs, and 293 T cells. Significance is from a two-sided Welch’s t-test, for this panel and all subsequent panels in this figure. Statistical data relating to
cluster properties were derived from more than 10 cells in 3 independent experiments. The box represents the first and third quartiles, the midpoint is the median and the whiskers are 1.5x
the interquartile mean, for this panel and all subsequent boxplots. E. SMLM images of DNA (stained with an anti-DNA antibody) in primed hPSCs, and 293 T cells. For the upper panel scale bar
= 2 µm, and for the nuclear periphery, nucleoplasm, and the z-axis, the scale bar = 200 nm. F. Boxplots showing the density for the DNA clusters (top boxplot) and the localizations density
within each cluster per volume for HNRNPU (bottom boxplot) in primed hPSCs, and 293 T cells. The box represents the first and third quartiles, the midpoint is the median and the whiskers are
1.5x the interquartile mean. G. SMLM images of HNRNPU (stained with an anti-HNRNPU antibody) in hPSCs transfected with shRNAs targeting _LUC_ or _HNRNPU_. For the upper panel scale bar = 2
µm, for the nuclear periphery, nucleoplasm, and the z-axis, the scale bar = 200 nm. H. Boxplots showing the density for the HNRNPU clusters (top boxplot) and the localizations density within
each cluster per volume for HNRNPU (bottom boxplot) in primed hPSCs transfected with the indicated shRNA. I. SMLM images of DNA (stained with an anti-DNA antibody) in hPSCs transfected with
a shRNA targeting _LUC_ or _HNRNPU_. For the upper panel scale bar = 2 µm, and for the nuclear periphery, nucleoplasm, and the z-axis, the scale bar = 200 nm. J. Boxplots showing the
density for the DNA clusters (top boxplot) and the localizations density within each cluster per volume for HNRNPU (bottom boxplot) in primed hPSCs transfected with the indicated shRNA. K.
Histogram of TAD size (in megabase pairs) in sh_LUC_ (control) and sh_HNRNPU_ transfected hPSCs. L. Pie charts of A/B compartment reassignment after _HNRNPU_ knockdown. M. Heatmap showing
the Pearson correlation scores for chromosome 2 in hPSCs transfected with the indicated shRNA. The bottom track shows the first principal component, orientated by GC content to assign A/B
compartments. N. Heatmaps showing normalized contact frequency for all of chromosome 2 for hPSCs transfected with the indicated shRNA. O. Differential contact frequency based on the heatmaps
in panel N. EXTENDED DATA FIG. 7 HNRNPU BINDS TO THE NUCLEAR MATRIX. A Genome distribution of the HNRNPU CUT&Tag peaks. Peaks were annotated to the nearest TSS and allocated to bins
either 5’ (negative numbers) or 3’ (positive) kbp relative to the TSS. A random background is shown in grey for comparison. B. Co-correlation heatmap for all hPSC ChIP-seq/CUT&Tag
experiments in primed hPSCs. Data is from Cistrome106, or this study. C. POLII CUT&Tag at the TSS and transcript bodies of housekeeping genes (Defined as genes with an expression
coefficient of variance < 0.25, and a mean expression > 29). Transcripts were scaled to a uniform length between the TSS and TTS, and the flanking 3kbp regions 5’ or 3’ are shown. D.
Heatmap showing a comparison of MAR-seq in hPSCs (this study) versus MAR-seq in MDAMB231 cells from accession GSE8767112. MAR-seq peaks are centred on their mid-points, and the flanking 2
kbp on either side of the peak centre is shown. E. Genome distribution of MAR-seq peaks in hPSCs (upper panel) or MDADMB231 cells (lower panel), relative to the transcription start site
(TSS). F. Venn diagram of the MAR-seq peaks in hPSCs transfected with shRNAs targeting _LUC_ or _HNRNPU_. G. Pileup heatmap of MAR-seq data for hPSCs transfected with shRNAs targeting _LUC_
or _HNRNPU_. H. Heatmap of the intersection of MAR-seq and HNRNPU CUT&Tag data in hPSCs. I. Proportional bar chart showing the A/T nucleotide percentages in the indicated categories of
HNRNPU or MAR-seq loci, as defined in panel E. A/T frequency was measured using a genomic window of 100 bp centred on the peak summit for each locus. J. Cumulative bar chart of the total
number of chromatin loops significantly up-regulated, unchanged, or downregulated in sh_HNRNPU_ transfected cells compared to sh_LUC_ control cells. Significantly different loops were
determined using DESeq2 based on the weighted normalized pixel value at the loop coordinate. Loops needed to have a q-value (Bonferroni-Hochberg corrected p-value) of less than 0.01 and an
absolute fold change of at least 2. K. Boxplots showing the average loop length for all loops, up- and downregulated loops, and in HNRNPU&MAR-seq, HNRNPU-only and MAR-seq-only loops.
Those loops were defined as at least one end of the loop is inside a HNRNPU or MAR-seq locus. L. Boxplots of the distance of any loop end to the nearest TSS. The top boxplot shows all loops
and up- and downregulated loops. The lower boxplots show only downregulated loops, and loops with at least one end of the loop in inside a HNRNPU or MAR-seq locus. M. Genome view of loops at
the _FGF2_ transcripts on chromosome 4. Loops with one or more ends originating at a HNRNPU&MAR-seq marked locus are indicated. EXTENDED DATA FIG. 8 HNRNPU LINKS CHROMATIN TO THE
NUCLEAR MATRIX TO DIFFERENTIALLY REGULATE CHROMATIN DIRECTLY AND INDIRECTLY. A Heatmap of the MAR-seq, HNRNPU, ATAC-seq, H3K27ac, and H3K4me1 CUT&Tag data in hPSCs or in hPSCs
transfected with the indicated shRNA. B. Pileups of the Hi-C data centred on the HNRNPU or MAR-seq loci, as defined in Extended Data Fig. 7h. The genomic window size is the flanking 500 kbp.
C. Volcano plots showing the differential genome loci for H3K27ac when _HNRNPU_ was knocked down. The three plots are divided based on whether they are bound by HNRNPU or are found in the
MAR-seq data. A locus was considered significantly different if it had a q-value of less than 0.05. D. Radial plot showing the genome distributions of H3K27ac downregulated loci marked by
HNRNPU&MAR-seq. E. Density binding plot of HNRNPU&MAR-seq-bound genes (left plot) or HNRNPU-only-bound genes (right plot) that have decreased H3K27ac upon _HNRNPU_ knockdown. HNRNPU
was determined as bound to a specific gene if it was within 1 kbp of a TSS of a transcribed isoform of that gene. HNRNPU binding density was estimated using a moving window of 1000 genes.
The grey lines are 10 scrambled (random) backgrounds. The black line indicates the mean of the scrambled backgrounds and the red line one standard deviation from the mean. For this and panel
I. F. Gene ontology of the genes that have reduced H3K27ac and are bound by HNRNPU&MAR-seq. G. Volcano plots showing the differential genome loci for ATAC-seq when _HNRNPU_ was knocked
down. The three plots are divided based on whether they are bound by HNRNPU or are found in the MAR-seq data. H. Radial plot showing the genome distributions of loci that have significantly
increased ATAC-seq accessibility and are also marked by HNRNPU&MAR-seq. I. Density binding plot of HNRNPU&MAR-seq-bound genes (left plot) or HNRNPU-only-bound genes (right plot) that
have increased ATAC-seq accessibility upon _HNRNPU_ knockdown. EXTENDED DATA FIG. 9 HNRNPU ANCHORS PRIMED-SPECIFIC GENES TO THE NUCLEAR MATRIX. A Genome view of the MATR3 locus in the hg38
genome. Read density tracks density for MAR-seq and HNRNPU CUT&Tag. Black circles beneath the tracks indicate a binding/enrichment site. Selected _MATR3_ and _SNHG4_ transcript isoforms
are shown. B. Heatmap showing a selection of naïve and primed-marker genes in primed/naive RNA-seq data. Samples are from GSE93241 (ref. 80), GSE75868, GSE85689 (ref. 28), PRJNA383735 (ref.
38), PRJNA397941 (ref. 29), and CNP0001454 (ref. 46). C. Volcano plot of differentially regulated genes in the naïve and primed states (as defined in panel B). Significantly differentially
regulated genes were defined as those genes that had a Bonferroni-Hochberg corrected p-value (q-value) of <0.01 and a fold change of at least 1.5. D. Genome contact falloff line plots.
Contact frequency plotted as a function of genome distance for all chromosomes. E. Aggregate heatmaps of the Hi-C data centred on the TSSs of naïve or primed-specific genes in hPSCs
transfected with shRNAs targeting _LUC_ or _HNRNPU_. The genomic window size is the flanking 500 kbp. F. Genome view and pileup of the MAR-seq data in hPSCs for a selection of
primed-specific genes. Read density tracks density for MAR-seq (black) and HNRNPU (red) CUT&Tag are shown. Black circles beneath the tracks indicate a binding/enrichment site. G. Genome
view and pileup of the MAR-seq data in hPSCs for a selection of naive-specific genes. Read density tracks density for MAR-seq (black) and HNRNPU (red) CUT&Tag are shown. Black circles
beneath the tracks indicate a binding/enrichment site. H. POLII CUT&Tag at the TSS and transcript bodies of primed-specific and naïve-specific genes (As defined in panel C). A transcript
was considered bound if an HNRNPU binding site was within 500 bp of the TSS. Transcripts were scaled to a uniform length between the TSS and TTS, and the flanking 3kbp regions 5’ or 3’ are
shown. EXTENDED DATA FIG. 10 HNRNPU IS BOUND TO PRIMED-SPECIFIC AND SOMATIC RNAS. A Pearson correlation heatmap for the pair-wise Pearson R scores for the RIP-seq data generated in this
study. # indicates the biological replicate number. B. Volcano plot showing the HNRNPU-bound RNAs. HNRNPU-bound RNA was defined as an RNA significantly enriched in the anti-HNRNPU RIP-seq
data versus the anti-IgG RIP-seq data. The volcano plot is shown for hPSCs. Selected RNAs are indicated. Significantly enriched genes were defined as those genes that had a
Bonferroni-Hochberg corrected p-value (q-value) of <0.01 and a fold change of > 1.5. C. Venn diagram showing the overlap of transcripts bound by HNRNPU in hPSCs and 293 T cells. D. Bar
chart showing significantly enriched gene ontology terms for the HNRNPU-bound RNAs in hPSC RIP-seq data. E. Genome view and pileup of the RIP-seq data in hPSCs and 293 T cells at the _OTX2_
genome locus. F. Line plot showing the log2(fold change) relative to hour 0 in hPSCs treated with Actinomycin D to block transcription. The line plots show primed-specific RNAs either bound
(right plot) or not bound (left plot) by HNRNPU. The line shows mean of all transcripts in class and the upper and lower thin lines are the 90% confidence intervals. Significance is from a
Mann–Whitney U-test of sh_LUC_ versus sh_HNRNPU_. SUPPLEMENTARY INFORMATION REPORTING SUMMARY PEER REVIEW FILE SUPPLEMENTARY TABLE 1 Lists of shRNA and primer sequences used in this study.
SUPPLEMENTARY TABLE 2 Lists of significantly differentially regulated genes in this study. SUPPLEMENTARY TABLE 3 Proteins detected in the Co-IP–MS experiments. SUPPLEMENTARY TABLE 4 RNAs
bound by HNRNPU identified in the RIP-seq in hPSCs and 293T cells. SOURCE DATA SOURCE DATA FIG. 1 Uncropped western blots. SOURCE DATA EXTENDED DATA FIG. 1 Statistical source data. RIGHTS
AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other
rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ma, G., Fu, X., Zhou, L. _et al._ The nuclear matrix stabilizes primed-specific genes in human pluripotent stem cells. _Nat Cell Biol_ 27,
232–245 (2025). https://doi.org/10.1038/s41556-024-01595-5 Download citation * Received: 06 December 2023 * Accepted: 11 December 2024 * Published: 09 January 2025 * Issue Date: February
2025 * DOI: https://doi.org/10.1038/s41556-024-01595-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
