Dual-function enzyme catalysis for enantioselective carbon–nitrogen bond formation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

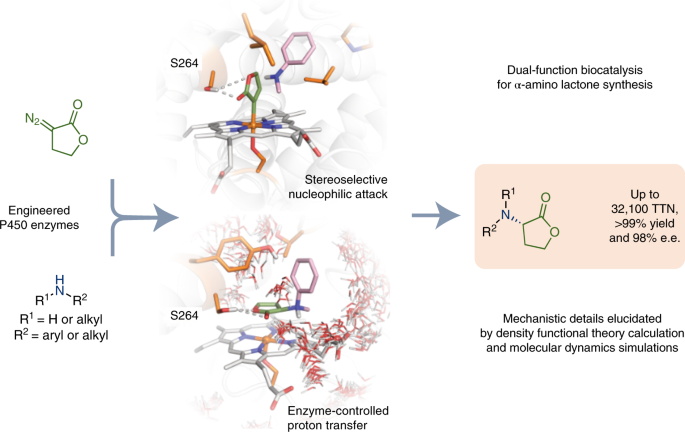
ABSTRACT Chiral amines can be made by insertion of a carbene into an N–H bond using two-catalyst systems that combine a transition metal-based carbene-transfer catalyst and a chiral
proton-transfer catalyst to enforce stereocontrol. Haem proteins can effect carbene N–H insertion, but asymmetric protonation in an active site replete with proton sources is challenging.
Here we describe engineered cytochrome P450 enzymes that catalyse carbene N–H insertion to prepare biologically relevant α-amino lactones with high activity and enantioselectivity (up to
32,100 total turnovers, >99% yield and 98% e.e.). These enzymes serve as dual-function catalysts, inducing carbene transfer and promoting the subsequent proton transfer with excellent
stereoselectivity in a single active site. Computational studies uncover the detailed mechanism of this new-to-nature enzymatic reaction and explain how active-site residues accelerate this
transformation and provide stereocontrol. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS BIOCATALYTIC, ENANTIOENRICHED PRIMARY AMINATION OF TERTIARY C–H BONDS Article 03 May 2024 CHEMODIVERGENT C(_SP_3)–H AND C(_SP_2)–H
CYANOMETHYLATION USING ENGINEERED CARBENE TRANSFERASES Article 19 January 2023 MULTIFUNCTIONAL BIOCATALYST FOR CONJUGATE REDUCTION AND REDUCTIVE AMINATION Article 06 April 2022 DATA
AVAILABILITY All data necessary to support the paper’s conclusions are available in the main text and the Supplementary Information. X-ray crystal structures of 3E (CCDC 2065484) and 3L
(CCDC 2065489) are available free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Plasmids encoding the enzymes reported in this study are
available for research purposes from F.H.A. under a material transfer agreement with the California Institute of Technology. Source data are provided with this paper. REFERENCES * Hili, R.
& Yudin, A. K. Making carbon–nitrogen bonds in biological and chemical synthesis. _Nat. Chem. Biol._ 2, 284–287 (2006). Article CAS Google Scholar * Froidevaux, V., Negrell, C.,
Caillol, S., Pascault, J.-P. & Boutevin, B. Biobased amines: from synthesis to polymers; present and future. _Chem. Rev._ 116, 14181–14224 (2016). Article CAS Google Scholar *
Bariwal, J. & Van der Eycken, E. C–N bond forming cross-coupling reactions: an overview. _Chem. Soc. Rev._ 42, 9283–9303 (2013). Article CAS Google Scholar * Kim, J. E., Choi, S.,
Balamurugan, M., Jang, J. H. & Nam, K. T. Electrochemical C–N bond formation for sustainable amine synthesis. _Trends Chem._ 2, 1004–1019 (2020). Article CAS Google Scholar * Kohls,
H., Steffen-Munsberg, F. & Höhne, M. Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. _Curr. Opin. Chem. Biol._ 19, 180–192 (2014). Article CAS
Google Scholar * Hauer, B. Embracing nature’s catalysts: a viewpoint on the future of biocatalysis. _ACS Catal._ 10, 8418–8427 (2020). Article CAS Google Scholar * Wu, S., Snajdrova, R.,
Moore, J. C., Baldenius, K. & Bornscheuer, U. T. Biocatalysis: enzymatic synthesis for industrial applications. _Angew. Chem. Int. Ed._ 60, 88–119 (2021). Article CAS Google Scholar
* Doyle, M. P. Catalytic methods for metal carbene transformations. _Chem. Rev._ 86, 919–939 (1986). Article CAS Google Scholar * Liu, B., Zhu, S.-F., Zhang, W., Chen, C. & Zhou,
Q.-L. Highly enantioselective insertion of carbenoids into N−H bonds catalyzed by copper complexes of chiral spiro bisoxazolines. _J. Am. Chem. Soc._ 129, 5834–5835 (2007). Article CAS
Google Scholar * Lee, E. C. & Fu, G. C. Copper-catalyzed asymmetric N−H insertion reactions: couplings of diazo compounds with carbamates to generate α-amino acids. _J. Am. Chem. Soc._
129, 12066–12067 (2007). Article CAS Google Scholar * Hou, Z. et al. Highly enantioselective insertion of carbenoids into N–H bonds catalyzed by copper(I) complexes of binol derivatives.
_Angew. Chem. Int. Ed._ 49, 4763–4766 (2010). Article CAS Google Scholar * Arredondo, V., Hiew, S. C., Gutman, E. S., Premachandra, I. D. U. A. & Van Vranken, D. L. Enantioselective
palladium-catalyzed carbene insertion into the N−H bonds of aromatic heterocycles. _Angew. Chem. Int. Ed._ 56, 4156–4159 (2017). Article CAS Google Scholar * Wang, Z. J., Peck, N. E.,
Renata, H. & Arnold, F. H. Cytochrome P450-catalyzed insertion of carbenoids into N–H bonds. _Chem. Sci._ 5, 598–601 (2014). Article CAS Google Scholar * Sreenilayam, G. & Fasan,
R. Myoglobin-catalyzed intermolecular carbene N–H insertion with arylamine substrates. _Chem. Commun._ 51, 1532–1534 (2015). Article CAS Google Scholar * Steck, V., Carminati, D. M.,
Johnson, N. R. & Fasan, R. Enantioselective synthesis of chiral amines via biocatalytic carbene N–H insertion. _ACS Catal._ 10, 10967–10977 (2020). Article CAS Google Scholar * Steck,
V., Sreenilayam, G. & Fasan, R. Selective functionalization of aliphatic amines via myoglobin-catalyzed carbene N–H insertion. _Synlett_ 31, 224–229 (2020). Article CAS Google Scholar
* Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. _Science_ 339, 307–310 (2013).
Article CAS Google Scholar * Zhang, R. K. et al. Enzymatic assembly of carbon–carbon bonds via iron-catalysed _sp__3_ C–H functionalization. _Nature_ 565, 67–72 (2019). Article CAS
Google Scholar * Liu, Z. & Arnold, F. H. New-to-nature chemistry from old protein machinery: carbene and nitrene transferases. _Curr. Opin. Biotechnol._ 69, 43–51 (2021). Article CAS
Google Scholar * Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. _Nat. Catal._ 3, 203–213 (2020). Article CAS Google Scholar * Ren, Y.-Y., Zhu, S.-F. &
Zhou, Q.-L. Chiral proton-transfer shuttle catalysts for carbene insertion reactions. _Org. Biomol. Chem._ 16, 3087–3094 (2018). Article CAS Google Scholar * Li, M.-L., Yu, J.-H., Li,
Y.-H., Zhu, S.-F. & Zhou, Q.-L. Highly enantioselective carbene insertion into N–H bonds of aliphatic amines. _Science_ 366, 990–994 (2019). Article CAS Google Scholar * Sharon, D.
A., Mallick, D., Wang, B. & Shaik, S. Computation sheds insight into iron porphyrin carbenes’ electronic structure, formation, and N–H insertion reactivity. _J. Am. Chem. Soc._ 138,
9597–9610 (2016). Article CAS Google Scholar * Pavlović, D. et al. Synthesis and structure–activity relationships of α-amino-γ-lactone ketolides: a novel class of macrolide antibiotics.
_ACS Med. Chem. Lett._ 5, 1133–1137 (2014). Article CAS Google Scholar * DeAngelis, A., Dmitrenko, O. & Fox, J. M. Rh-catalyzed intermolecular reactions of cyclic α-diazocarbonyl
compounds with selectivity over tertiary C–H bond migration. _J. Am. Chem. Soc._ 134, 11035–11043 (2012). Article CAS Google Scholar * Sattely, E. S., Meek, S. J., Malcolmson, S. J.,
Schrock, R. R. & Hoveyda, A. H. Design and stereoselective preparation of a new class of chiral olefin metathesis catalysts and application to enantioselective synthesis of
quebrachamine: catalyst development inspired by natural product synthesis. _J. Am. Chem. Soc._ 131, 943–953 (2009). Article CAS Google Scholar * Chen, K., Zhang, S.-Q., Brandenberg, O.
F., Hong, X. & Arnold, F. H. Alternate heme ligation steers activity and selectivity in engineered cytochrome P450-catalyzed carbene-transfer reactions. _J. Am. Chem. Soc._ 140,
16402–16407 (2018). Article CAS Google Scholar * Zhou, A. Z., Chen, K. & Arnold, F. H. Enzymatic lactone-carbene C–H insertion to build contiguous chiral centers. _ACS Catal._ 10,
5393–5398 (2020). Article CAS Google Scholar * Chen, K. et al. Engineered cytochrome _c_-catalyzed lactone-carbene B–H insertion. _Synlett_ 30, 378–382 (2019). Article CAS Google
Scholar * Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. _Nat. Chem. Biol._ 9, 485–487 (2013). Article CAS Google Scholar
* Brandenberg, O. F., Chen, K. & Arnold, F. H. Directed evolution of a cytochrome P450 carbene transferase for selective functionalization of cyclic compounds. _J. Am. Chem. Soc._ 141,
8989–8995 (2019). Article CAS Google Scholar * Garcia-Borràs, M. et al. Origin and control of chemoselectivity in cytochrome _c_ catalyzed carbene transfer into Si–H and N–H bonds. _J.
Am. Chem. Soc._ 143, 7114–7123 (2021). Article CAS Google Scholar * Khade, R. L. & Zhang, Y. Catalytic and biocatalytic iron porphyrin carbene formation: effects of binding mode,
carbene substituent, porphyrin substituent, and protein axial ligand. _J. Am. Chem. Soc._ 137, 7560–7563 (2015). Article CAS Google Scholar * Kalita, S., Shaik, S., Kisan, H. K. &
Dubey, K. D. A paradigm shift in the catalytic cycle of P450: the preparatory choreography during O2 binding and origins of the necessity for two protonation pathways. _ACS Catal._ 10,
11481–11492 (2020). Article CAS Google Scholar * Fisher, D. J. & Hayes, A. L. Mode of action of the systemic fungicides furalaxyl, metalaxyl and ofurace. _Pestic. Sci._ 13, 330–339
(1982). Article CAS Google Scholar * Kunz, W. & Kristinsson, H. Bildung von 4-, 5- und 6gliedrigen heterocyclen durch ambidoselektive ringschlüsse von enolat-ionen. _Helv. Chim. Acta_
62, 872–881 (1979). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Science Foundation Division of Molecular and Cellular
Biosciences (grant 2016137 to F.H.A.), the US Army Research Office Institute for Collaborative Biotechnologies (cooperative agreement W911NF-19-2-0026 to F.H.A.), the Spanish Ministry of
Science and Innovation MICINN (grant PID2019-111300GA-I00 to M.G.-B.) and the Generalitat de Catalunya AGAUR Beatriu de Pinós H2020 MSCA-Cofund (2018-BP-00204 project to M.G.-B.). K.C.
thanks the Resnick Sustainability Institute at Caltech for fellowship support. The computer resources at MinoTauro and the Barcelona Supercomputing Center BSC-RES are acknowledged
(RES-QSB-2020-2-0016). We thank D. C. Miller, S. Brinkmann-Chen, R. Lal and T. Zeng for helpful discussions and comments on the manuscript. We further thank M. Shahgholi for high-resolution
mass spectrometry analysis and M. K. Takase for X-ray crystallographic analysis. AUTHOR INFORMATION Author notes * Kai Chen Present address: Innovative Genomics Institute, University of
California, Berkeley, CA, USA AUTHORS AND AFFILIATIONS * Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, USA Zhen Liu, Andrew Z. Zhou, Kai
Chen & Frances H. Arnold * Institut de Química Computacional i Catàlisi and Departament de Química, Universitat de Girona, Girona, Spain Carla Calvó-Tusell & Marc Garcia-Borràs
Authors * Zhen Liu View author publications You can also search for this author inPubMed Google Scholar * Carla Calvó-Tusell View author publications You can also search for this author
inPubMed Google Scholar * Andrew Z. Zhou View author publications You can also search for this author inPubMed Google Scholar * Kai Chen View author publications You can also search for this
author inPubMed Google Scholar * Marc Garcia-Borràs View author publications You can also search for this author inPubMed Google Scholar * Frances H. Arnold View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.L. and K.C. conceived and designed the overall project with F.H.A. providing guidance. Z.L. and A.Z.Z. designed and
performed the initial screening of haem proteins and the substrate scope study. C.C.-T. and M.G.-B. carried out the computational studies. Z.L., K.C., M.G.-B. and F.H.A. wrote the manuscript
with the input of all authors. CORRESPONDING AUTHORS Correspondence to Kai Chen, Marc Garcia-Borràs or Frances H. Arnold. ETHICS DECLARATIONS COMPETING INTERESTS K.C., Z.L. and A.Z.Z. are
inventors on a US Patent Application (invention title, Diverse Carbene Transferase Enzyme Catalysts Derived from a P450 Enzyme; application no., 17/200,394) filed by the California Institute
of Technology, which covers lactone-carbene N–H insertion with engineered P450 enzymes. The patent was filed on 12 March 2021. The remaining authors declare no competing interests.
ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Chemistry_ thanks Sabine Flitsch, Sason Shaik and the other, anonymous, reviewer(s) for their contribution to the peer review of this
work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION Supplementary Figs. 1–21, Tables 1–17, experimental data, procedural details, synthesis and characterization data, NMR spectra, X-ray crystallographic data and computational
modelling data. SUPPLEMENTARY DATA 1 crystallographic data of compound 3E; CCDC 2065484. SUPPLEMENTARY DATA 2 crystallographic data of compound 3L; CCDC 2065489. SOURCE DATA SOURCE DATA FIG.
2 Statistical source data of 40 previously reported variants. SOURCE DATA FIG. 4 Statistical source data of substrate scope. SOURCE DATA FIG. 5 Statistical source data of OD test. RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, Z., Calvó-Tusell, C., Zhou, A.Z. _et al._ Dual-function enzyme catalysis for enantioselective
carbon–nitrogen bond formation. _Nat. Chem._ 13, 1166–1172 (2021). https://doi.org/10.1038/s41557-021-00794-z Download citation * Received: 14 April 2021 * Accepted: 23 August 2021 *
Published: 18 October 2021 * Issue Date: December 2021 * DOI: https://doi.org/10.1038/s41557-021-00794-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
