On the design of complex drug candidate syntheses in the pharmaceutical industry
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The overall goal of a process chemistry department within the pharmaceutical industry is to identify and develop a commercially viable approach to a drug candidate. However, the
high chemical complexity of many modern pharmaceuticals presents a challenge to process scientists. Delivering disruptive, rather than incremental, change is critical to maximizing synthetic
efficiency in complex settings. In this Review, we focus on the importance of synthetic strategy in delivering ‘disruptive innovation’ — innovation that delivers a step change in synthetic
efficiency using new chemistry, displacing any prior synthetic route. We argue that achieving this goal requires visionary retrosynthetic strategy and is tightly linked to the discovery and
development of new reactions and novel processes. Investing in high-risk innovation during the route design process can ultimately lead to safer, more robust and more efficient manufacturing
processes capable of addressing the challenge of high molecular complexity. Routinely delivering such innovation in a time-bound environment requires organizational focus and can be enabled
by the concepts of expansive ideation, strategy aggregation and strategy selection. Access through your institution Buy or subscribe This is a preview of subscription content, access via
your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days
cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on
SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about
institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS AUTOMATED SYNTHESIS OF PREXASERTIB AND DERIVATIVES ENABLED BY CONTINUOUS-FLOW
SOLID-PHASE SYNTHESIS Article 19 April 2021 THE LANDSCAPE OF SMALL-MOLECULE PRODRUGS Article 02 April 2024 MOLECULAR CHAMELEONS IN DRUG DISCOVERY Article 20 December 2023 REFERENCES *
Anderson, N. G. _Practical Process Research and Development_ (Academic Press, 2000). Google Scholar * Li, J. & Eastgate, M. D. Current complexity: a tool for assessing the complexity or
organic molecules. _Org. Biomol. Chem._ 13, 7164–7176 (2015). Article CAS Google Scholar * Chase, C. E. _et al_. Process development of Halaven®: synthesis of the C1–C13 fragment from
d-(−)-gulono-1,4-lactone. _Synlett_ 24, 323–326 (2013). Article CAS Google Scholar * Austad, B. C. _et al_. Process development of Halaven®: synthesis of the C14–C35 fragment via
iterative Nozaki–Hiyama–Kishi reaction–Williamson ether cyclization. _Synlett_ 24, 327–332 (2013). Article CAS Google Scholar * Austad, B. C. _et al_. Commercial manufacture of Halaven®:
chemoselective transformation en route to structurally complex macrocyclic ketones. _Synlett_ 24, 333–337 (2013). Article CAS Google Scholar * Stinson, S. C. Chemistry 2020: a myopic
vision? _Chem. Eng. News_ 75, 28 (1997). Google Scholar * Stinson, S. C. Counting on chiral drugs. _Chem. Eng. News_ 76, 83–104 (1998). Article Google Scholar * Mullin, R. Breaking down
barriers. _Chem. Eng. News_ 85, 11–17 (2007). Google Scholar * Christensen, C. M. _The Innovator's Dilemma: When New Technologies Cause Great Firms to Fail_ (Harvard Business School
Press, 1997). Google Scholar * Li, J., Simmons, E. M. & Eastgate, M. D. A data-driven strategy for predicting greenness scores, rationally comparing synthetic routes and benchmarking
PMI outcomes for the synthesis of molecules in the pharmaceutical industry. _Green Chem._ 19, 127–139 (2017). Article CAS Google Scholar * Kola, I. & Landis, J. Can the pharmaceutical
industry reduce attrition rates? _Nat. Rev. Drug Discov._ 3, 711–715 (2004). Article CAS Google Scholar * Fitzgerald, M. A. _et al_. Functionalization in the formation of a complex
heterocycle: synthesis of the potent JAK2 inhibitor BMS-911543. _J. Org. Chem._ 80, 6001–6011 (2015). Article CAS Google Scholar * Chen, K., Eastgate, M. D., Zheng, B. & Li, J. The
development of scalable and efficient methods for the preparation of dicyclopropylamine HCl salt. _Org. Process Res. Dev._ 15, 886–892 (2011). Article CAS Google Scholar * Mudryk, B.,
Zheng, B., Chen, K. & Eastgate, M. D. Development of a robust process for the preparation of high-quality dicyclopropylamine hydrochloride. _Org. Process Res. Dev._ 18, 520–527 (2014).
Article CAS Google Scholar * Schmidt, M. A. & Eastgate, M. D. Regioselective synthesis of 1,4-disubstituted imidazoles. _Org. Biomol. Chem._ 10, 1079–1087 (2012). Article CAS Google
Scholar * Beutner, G. L. _et al_. A method for heteroaromatic nitration demonstrating remarkable thermal stability. _Org. Process Res. Dev._ 18, 1812–1820 (2014). Article CAS Google
Scholar * Gallagher, W. P., Deshpande, P. P., Li, J. & Katipally, K. Method for producing festinavir using 5-methyluridine as a starting material. WO patent 2014172264 (2014). *
Gallagher, W. P., Deshpande, P. P., Li, J., Katipally, K. & Sausker, J. A Claisen approach to 4′-Ed4T. _Org. Lett._ 17, 14–17 (2015). Article CAS Google Scholar * Ortiz, A. _et al_.
Scalable synthesis of the potent HIV inhibitor BMS-986001 by non-enzymatic dynamic kinetic asymmetric transformation (DYKAT). _Angew. Chem. Int. Ed._ 54, 7185–7188 (2015). Article CAS
Google Scholar * Benkovics, T., Ortiz, A., Guo, Z., Goswami, A. & Deshpande, P. Enantioselective preparation of (_S_)-5-oxo-5,6-dihydro-2H-pyran-2-yl benzoate. _Org. Synth._ 91, 293–306
(2014). Article CAS Google Scholar * Ji, Y. _et al_. Mechanistic insights into the vanadium-catalyzed Achmatowicz rearrangement of furfurol. _J. Org. Chem._ 80, 1696–1702 (2015). Article
CAS Google Scholar * Chen, K., Risatti, C. & Eastgate, M. in _Strategies and Tactics in Organic Synthesis_ Vol. 11 (ed. Harmata, M. ) 171–233 (Elsevier, 2015). Google Scholar *
Wengryniuk, S. E. _et al_. Regioselective bromination of fused heterocyclic _N_-oxides. _Org. Lett._ 15, 792–795 (2013). Article CAS Google Scholar * Chen, K. _et al_. Synthesis of the
6-azaindole containing HIV-1 attachment inhibitor pro-drug, BMS-663068. _J. Org. Chem._ 79, 8757–8767 (2014). Article CAS Google Scholar * Tran, K. _et al_. Development of a
diastereoselective phosphorylation of a complex nucleoside via dynamic kinetic resolution. _J. Org. Chem._ 80, 4994–5003 (2015). Article CAS Google Scholar * Uchiyama, M., Aso, Y.,
Noyori, R. & Hayakawa, Y. O-Selective phosphorylation of nucleosides without N-protection. _J. Org. Chem._ 58, 373–379 (1993). Article CAS Google Scholar * Chamberlain, S., Igo, D.,
Bis, J. & Sukumar, S. Crystalline solvates of nucleoside phosphoroamidates, their stereoselective preparation, novel intermediates thereof, and their use in the treatment of viral
disease. WO patent 2013066991 (2013). * Goldberg, S. L. _et al_. Preparation of β-hydroxy-α-amino acid using recombinant d-threonine aldolase. _Org. Process Res. Dev._ 19, 1308–1316 (2015).
Article CAS Google Scholar * Schmidt, M. A. _et al_. Development of a two-step, enantioselective synthesis of an amino alcohol drug candidate. _Org. Process Res. Dev._ 19, 1317–1322
(2015). Article CAS Google Scholar * Bartolozzi, A. _et al_. Oxadiazole inhibitors of leukotriene production. WO patent 2012024150 (2012). * Marek, Y. _et al_. All-carbon quaternary
stereogenic centers in acyclic systems through the creation of several C–C Bonds per chemical step. _J. Am. Chem. Soc._ 136, 2682–2694 (2014). Article CAS Google Scholar * Zeng, X. _et
al_. Remarkable enhancement of enantioselectivity in the asymmetric conjugate addition of dimethylzinc to (_Z_)-nitroalkenes with a catalytic [(MeCN)4Cu]PF6–Hoveyda Ligand complex. _Angew.
Chem. Int. Ed._ 53, 12153–12157 (2014). Article CAS Google Scholar * Stymiest, J. L., Bagutski, V., French, R. M. & Aggarwal, V. K. Enantiodivergent conversion of chiral secondary
alcohols into tertiary alcohols. _Nature_ 456, 778–782 (2008). Article CAS Google Scholar * Bagutski, V., Ros, A. & Aggarwal, V. K. Improved method for the conversion of
pinacolboronic esters into trifluoroborate salts: facile synthesis of chiral secondary and tertiary trifluoroborates. _Tetrahedron_ 65, 9956–9960 (2009). Article CAS Google Scholar *
Bagutski, V., French, R. M. & Aggarwal, V. K. Full chirality transfer in the conversion of secondary alcohols into tertiary boronic esters and alcohols using lithiation–borylation
reactions. _Angew. Chem. Int. Ed._ 49, 5142–5145 (2010). Article CAS Google Scholar * Sonawane, R. P. _et al_. Enantioselective construction of quaternary stereogenic centers from
tertiary boronic esters: methodology and applications. _Angew. Chem. Int. Ed._ 50, 3760–3763 (2011). Article CAS Google Scholar * Rangaishenvi, M. V., Singaram, B. & Brown, H. C.
Chiral synthesis via organoboranes. 30. Facile synthesis, by the Matteson asymmetric homologation procedure, of α-methyl boronic acids not available from asymmetric hydroboration and their
conversion into the corresponding aldehydes, ketones, carboxylic acids and amines of high enantiomeric purity. _J. Org. Chem._ 56, 3286–3294 (1991). Article CAS Google Scholar * Scott, H.
K. & Aggarwal, V. K. Highly enantioselective synthesis of tertiary boronic esters and their stereospecific conversion to other functional groups and quaternary stereocentres. _Chem.
Eur. J._ 17, 13124–13132 (2011). Article CAS Google Scholar * Senanayake, C. H. & Krishnamurthy, D. Asymmetric synthesis for process research. _Curr. Opin. Drug Discov. Dev._ 2,
590–605 (1999). CAS Google Scholar * Farina, V., Reeves, J. T., Senanayake, C. H. & Song, J. J. Asymmetric synthesis of active pharmaceutical ingredients. _Chem. Rev._ 106, 2734–2793
(2006). Article CAS Google Scholar * Hoppe, D., Hintze, F. & Tebben, P. Chiral lithium-1-oxyalkanides by asymmetric seprotonation; enantioselective synthesis of 2-hydroalkanoic acids
and secondary alkanols. _Angew. Chem. Int. Ed. Engl._ 29, 1422–1424 (1990). Article Google Scholar * Hoppe, D. & Hense, T. Enantioselective synthesis with lithium/(−)-sparteine
carbanion pairs. _Angew. Chem. Int. Ed. Engl._ 36, 2282–2316 (1997). Article CAS Google Scholar * Matteson, D. S., Soundararajan, R., Ho, O. C. & Gatzweiler, W. (Alkoxyalkyl)boronic
ester intermediates for asymmetric synthesis. _Organometallics_ 15, 152–163 (1996). Article CAS Google Scholar * Fandrick, K. R. _et al_. Addressing the configuration stability of
lithiated secondary benzylic carbamates for the development of a noncryogenic stereospecific boronate rearrangement. _Org. Lett._ 16, 4360–4363 (2014). Article CAS Google Scholar *
Fandrick, K. R. _et al_. Development of an asymmetric synthesis of a chiral quaternary FLAP inhibitor. _J. Org. Chem._ 80, 1651–1660 (2015). Article CAS Google Scholar * Fandrick, K. R.,
Gao, J. J., Mulder, J. A., Patel, N. D. & Zheng, X. Process for the preparation of carboxamidine compounds. WO Patent 2013119751 (2013). * Munchhof, M. J. _et al_. Discovery of
PF-04449913, a potent and orally bioavailable inhibitor of smoothened. _ACS Med. Chem. Lett._ 3, 106–111 (2012). Article CAS Google Scholar * Gillard, J. _et al_. Preparation of
(2_S_,4_R_)-4-hydroxypipecolic acid and derivatives. _J. Org. Chem._ 61, 2226–2231 (1996). Article CAS Google Scholar * Peng, Z., Wong, J. W., Hansen, E. C., Puchlopek-Dermenci, A. L. A.
& Clarke, H. J. Development of a concise, asymmetric synthesis of a smoothened receptor (SMO) inhibitor: enzymatic transamination of a 4-piperidinone with dynamic kinetic resolution.
_Org. Lett._ 16, 860–863 (2014). Article CAS Google Scholar * Wiss, J., Fleury, C. & Onken, U. Safety improvement of chemical processes involving azides by online monitoring of the
hydrazoic acid concentration. _Org. Process Res. Dev._ 10, 349–353 (2006). AZIDE PROCESSES AND, IN PARTICULAR, THE FORMATION OF HYDRAZOIC ACID ARE AN IMPORTANT SAFETY CONCERN FOR LARGE-SCALE
SYNTHESIS. Article CAS Google Scholar * Wells, A. What is in a biocatalyst? _Org. Process Res. Dev._ 10, 678–681 (2006). Article CAS Google Scholar * Wells, A. S., Finch, G. L.,
Michels, P. C. & Wong, J. W. Use of enzymes in the manufacture of active pharmaceutical ingredients — a science and safety-based approach to ensure patient safety and drug quality. _Org.
Process Res. Dev._ 16, 1968–1993 (2012). Article Google Scholar * Wells, A. S. _et al_. Case studies illustrating a science and risk-based approach to ensuring drug quality when using
enzymes in the manufacture of active pharmaceuticals ingredients for oral dosage form. _Org. Process Res. Dev._ 20, 594–601 (2016). Article CAS Google Scholar * Saville, C. K. _et al_.
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. _Science_ 329, 305–309 (2010). THIS PAPER OFFERS A NOTABLE EXAMPLE OF THE USE OF A
TRANSAMINASE IN THE MANUFACTURE OF SITAGLIPTIN. Article Google Scholar * Bravo, F. _et al_. Development of a dynamic kinetic resolution for the isolation of an intermediate in the
synthesis of casopitant mesylate: application of QbD principles in the definition of the parameter ranges, issues in the scale-up and mitigation strategies. _Org. Process Res. Dev._ 14,
1162–1168 (2010). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank all of the collaborators and researchers who have worked on these fascinating molecules
during their development. M.D.E. and M.A.S. are especially grateful to P. Baran for insightful discussions and inspiration, along with S. Tummala, R. Waltermire, A. Ortiz and C. Guerrero
for helpful discussions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Chemical and Synthetic Development, Bristol-Myers Squibb, 1 Squibb Drive, New Brunswick, 08901, New Jersey, USA Martin
D. Eastgate & Michael A. Schmidt * Chemical Development, Boehringer Ingelheim Pharmaceuticals, Inc., 900 Ridgebury Road, Ridgefield, 06877, Connecticut, USA Keith R. Fandrick Authors *
Martin D. Eastgate View author publications You can also search for this author inPubMed Google Scholar * Michael A. Schmidt View author publications You can also search for this author
inPubMed Google Scholar * Keith R. Fandrick View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Martin D. Eastgate.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3
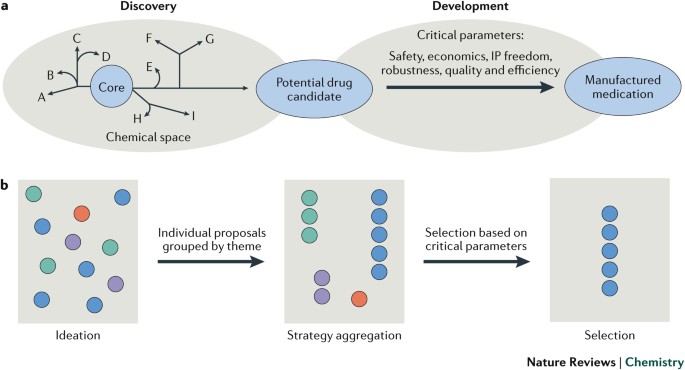
POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR FIG. 6 POWERPOINT SLIDE FOR FIG. 7 POWERPOINT SLIDE FOR FIG. 8 GLOSSARY * Ideation Ensuring the robust and
expansive evaluation of all key strategic bonds, developing a much fuller retrosynthetic analysis before entering the lab, and using the collective wisdom of multiple researchers to raise
and address concerns. * Strategy aggregation Taking the multiple synthetic proposals, or proposed disconnections, and collating them into clusters of aligned core disconnection strategies or
reactivities, not focusing on any individual technology or precedent. Key experiments can rapidly be explored in the lab to assist in the triaging of strategies. * Strategy selection
Aligning the team on selecting a strategy, not an individual synthesis proposal. The selected strategy should have multiple related synthetic options (for example, shared reactivity patterns
or common intermediates) such that high-risk disruptive approaches can be investigated, while data gained from the exploration can be applied to lower-risk proposals. Appropriate selection
can also lead to a more effective staged approach to synthesis development, which is often crucial in aligning work to the risk of the drug progressing to market. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Eastgate, M., Schmidt, M. & Fandrick, K. On the design of complex drug candidate syntheses in the pharmaceutical industry.
_Nat Rev Chem_ 1, 0016 (2017). https://doi.org/10.1038/s41570-017-0016 Download citation * Published: 08 February 2017 * DOI: https://doi.org/10.1038/s41570-017-0016 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative
