Taking fluorine to the bridge | Nature Reviews Chemistry
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Access through your institution Buy or subscribe While the benzene ring remains ubiquitous in synthetic and medicinal chemistry, its flat, _sp_2-rich nature doesn’t always provide the best
framework to develop effective, bioavailable drugs. As such, saturated analogues have gained attention as benzene replacement groups, especially bridged cyclic structures like
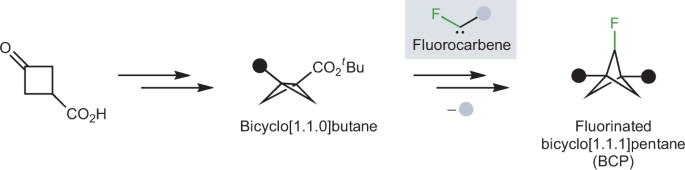
bicyclo[1.1.1]pentanes (BCPs). Now, writing in _Angewandte Chemie_, Roman Bychek and Pavel Mykhailiuk from Enamine Ltd describe the development of a new method to access BCPs with a fluorine
substituent on one of the bridging carbon atoms. BCPs have a cyclobutane structure, with an additional methylene group bridging across two non-adjacent carbons. Their bicyclic structure
promotes their use in medicinal chemistry as benzene replacement groups compared to other saturated rings, such as cyclohexane, due to their small size and restricted flexibility. In the
decade since their first use as benzene bioisosteres, synthetic efforts have focussed on accessing BCPs with new substitution patterns, able to mimic the various derivatives of benzene rings
in literature compounds. This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio
journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles
$119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES ORIGINAL ARTICLE * Bychek, R.
& Mykhailiuk, P. K. A practical and scalable approach to fluoro-substituted bicyclo[1.1.1]pentanes. _Angew. Chem. Int. Ed._ https://doi.org/10.1002/anie.202205103 (2022) Article Google
Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nature Reviews Chemistry http://www.nature.com/natrevchem/ Stephanie Greed Authors * Stephanie Greed View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Stephanie Greed. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Greed, S. Taking fluorine to the bridge. _Nat Rev Chem_ 6, 447 (2022). https://doi.org/10.1038/s41570-022-00405-6 Download citation * Published: 27 June 2022 *
Issue Date: July 2022 * DOI: https://doi.org/10.1038/s41570-022-00405-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
