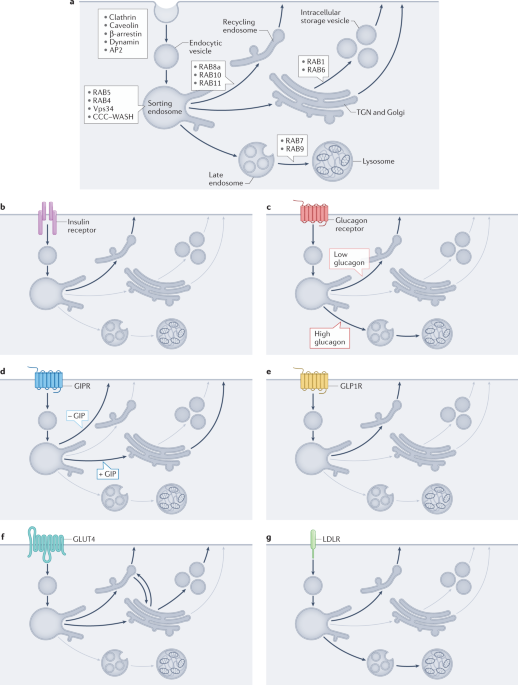
Endosomal trafficking in metabolic homeostasis and diseases
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The global prevalences of obesity and type 2 diabetes mellitus have reached epidemic status, presenting a heavy burden on society. It is therefore essential to find novel mechanisms
and targets that could be utilized in potential treatment strategies and, as such, intracellular membrane trafficking has re-emerged as a regulatory tool for controlling metabolic
homeostasis. Membrane trafficking is an essential physiological process that is responsible for the sorting and distribution of signalling receptors, membrane transporters and hormones or
other ligands between different intracellular compartments and the plasma membrane. Dysregulation of intracellular transport is associated with many human diseases, including cancer,
neurodegeneration, immune deficiencies and metabolic diseases, such as type 2 diabetes mellitus and its associated complications. This Review focuses on the latest advances on the role of
endosomal membrane trafficking in metabolic physiology and pathology in vivo, highlighting the importance of this research field in targeting metabolic diseases. KEY POINTS * The endosomal
system controls signalling involved in metabolic physiology by tuning the trafficking and distribution of key proteins. * Some of the core machineries involved in the endosomal system are
altered in metabolic diseases. * Tuning the endosomal system could improve metabolic parameters in metabolic diseases. * Altering receptor trafficking through ligand-biased agonism is a
potential novel therapeutic strategy for metabolic pathologies. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS METABOLIC MESSENGERS: SMALL EXTRACELLULAR VESICLES Article 07 February 2025 REGULATION OF CARGO SELECTION IN EXOSOME
BIOGENESIS AND ITS BIOMEDICAL APPLICATIONS IN CANCER Article Open access 05 April 2024 CONTEXT-SPECIFIC REGULATION OF EXTRACELLULAR VESICLE BIOGENESIS AND CARGO SELECTION Article 10 February
2023 CHANGE HISTORY * _ 31 OCTOBER 2022 A Correction to this paper has been published: https://doi.org/10.1038/s41574-022-00772-6 _ REFERENCES * Laaksonen, D. E. et al. Metabolic syndrome
and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. _Am. J. Epidemiol._ 156, 1070–1077
(2002). Article Google Scholar * Sattar, N. et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland
Coronary Prevention Study. _Circulation_ 108, 414–419 (2003). Article CAS Google Scholar * Noubiap, J. J. et al. Geographic distribution of metabolic syndrome and its components in the
general adult population: a meta-analysis of global data from 28 million individuals. _Diabetes Res. Clin. Pract._ 188, 109924 (2022). Article CAS Google Scholar * Lillich, F. F., Imig,
J. D. & Proschak, E. Multi-target approaches in metabolic syndrome. _Front. Pharmacol._ 11, 554961 (2020). Article CAS Google Scholar * Müller, T. D., Blüher, M., Tschöp, M. H. &
DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. _Nat. Rev. Drug Discov._ 21, 201–223 (2022). Article Google Scholar * Shaffner, J., Chen, B., Malhotra, D. K.,
Dworkin, L. D. & Gong, R. Therapeutic targeting of SGLT2: a new era in the treatment of diabetes and diabetic kidney disease. _Front. Endocrinol._ 12, 749010 (2021). Article Google
Scholar * Filipovic, B. et al. The new therapeutic approaches in the treatment of non-alcoholic fatty liver disease. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms222413219 (2021).
Article Google Scholar * Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. _N. Engl. J. Med._ 384, 989–1002 (2021). Article CAS Google Scholar *
Tak, Y. J. & Lee, S. Y. Anti-obesity drugs: long-term efficacy and safety: an updated review. _World J. Mens Health_ 39, 208–221 (2021). Article Google Scholar * Coskun, T. et al.
LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. _Mol. Metab._ 18, 3–14 (2018). Article CAS
Google Scholar * Müller, T. D. et al. Glucagon-like peptide 1 (GLP-1). _Mol. Metab._ 30, 72–130 (2019). Article Google Scholar * Cullen, P. J. & Steinberg, F. To degrade or not to
degrade: mechanisms and significance of endocytic recycling. _Nat. Rev. Mol. Cell Biol._ 19, 679–696 (2018). Article CAS Google Scholar * Villasenor, R., Kalaidzidis, Y. & Zerial, M.
Signal processing by the endosomal system. _Curr. Opin. Cell Biol._ 39, 53–60 (2016). Article CAS Google Scholar * Villasenor, R., Nonaka, H., Del Conte-Zerial, P., Kalaidzidis, Y. &
Zerial, M. Regulation of EGFR signal transduction by analogue-to-digital conversion in endosomes. _Elife_ https://doi.org/10.7554/eLife.06156 (2015). Article Google Scholar * Vazirani, R.
P. et al. Disruption of adipose Rab10-dependent insulin signaling causes hepatic insulin resistance. _Diabetes_ 65, 1577–1589 (2016). Article CAS Google Scholar * Zeigerer, A. et al.
Regulation of liver metabolism by the endosomal GTPase Rab5. _Cell Rep._ 11, 884–892 (2015). Article CAS Google Scholar * Bartuzi, P. et al. CCC- and WASH-mediated endosomal sorting of
LDLR is required for normal clearance of circulating LDL. _Nat. Commun._ 7, 10961 (2016). Article CAS Google Scholar * Fedoseienko, A. et al. The COMMD family regulates plasma LDL levels
and attenuates atherosclerosis through stabilizing the CCC complex in endosomal LDLR trafficking. _Circ. Res._ 122, 1648–1660 (2018). Article CAS Google Scholar * Seitz, S. et al. Hepatic
Rab24 controls blood glucose homeostasis via improving mitochondrial plasticity. _Nat. Metab._ 1, 1009–1026 (2019). Article CAS Google Scholar * Trelford, C. B. & Di Guglielmo, G. M.
Molecular mechanisms of mammalian autophagy. _Biochem. J._ 478, 3395–3421 (2021). Article CAS Google Scholar * Papandreou, M. E. & Tavernarakis, N. Crosstalk between endo/exocytosis
and autophagy in health and disease. _Biotechnol. J._ 15, e1900267 (2020). Article Google Scholar * Buratta, S. et al. Lysosomal exocytosis, exosome release and secretory autophagy: the
autophagic- and endo-lysosomal systems go extracellular. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms21072576 (2020). Article Google Scholar * Mayor, S., Parton, R. G. & Donaldson,
J. G. Clathrin-independent pathways of endocytosis. _Cold Spring Harb. Perspect. Biol._ https://doi.org/10.1101/cshperspect.a016758 (2014). Article Google Scholar * Merrifield, C. J.
& Kaksonen, M. Endocytic accessory factors and regulation of clathrin-mediated endocytosis. _Cold Spring Harb. Perspect. Biol._ 6, a016733 (2014). Article Google Scholar * Maxfield, F.
R. Role of endosomes and lysosomes in human disease. _Cold Spring Harb. Perspect. Biol._ 6, a016931 (2014). Article Google Scholar * Mellman, I. & Yarden, Y. Endocytosis and cancer.
_Cold Spring Harb. Perspect. Biol._ 5, a016949 (2013). Article Google Scholar * Gilleron, J., Gerdes, J. M. & Zeigerer, A. Metabolic regulation through the endosomal system. _Traffic_
https://doi.org/10.1111/tra.12670 (2019). Article Google Scholar * Grant, B. D. & Donaldson, J. G. Pathways and mechanisms of endocytic recycling. _Nat. Rev. Mol. Cell Biol._ 10,
597–608 (2009). Article CAS Google Scholar * Klip, A., McGraw, T. E. & James, D. E. Thirty sweet years of GLUT4. _J. Biol. Chem._ 294, 11369–11381 (2019). Article CAS Google Scholar
* Hunter, T. Tyrosine phosphorylation: thirty years and counting. _Curr. Opin. Cell Biol._ 21, 140–146 (2009). Article CAS Google Scholar * Popovic, D., Vucic, D. & Dikic, I.
Ubiquitination in disease pathogenesis and treatment. _Nat. Med._ 20, 1242–1253 (2014). Article CAS Google Scholar * Andreou, A. M. & Tavernarakis, N. SUMOylation and cell signalling.
_Biotechnol. J._ 4, 1740–1752 (2009). Article CAS Google Scholar * Sanger, A., Hirst, J., Davies, A. K. & Robinson, M. S. Adaptor protein complexes and disease at a glance. _J. Cell
Sci._ https://doi.org/10.1242/jcs.222992 (2019). Article Google Scholar * Bonifacino, J. S. & Lippincott-Schwartz, J. Coat proteins: shaping membrane transport. _Nat. Rev. Mol. Cell
Biol._ 4, 409–414 (2003). Article CAS Google Scholar * Koike, S. & Jahn, R. SNARE proteins: zip codes in vesicle targeting? _Biochem. J._ 479, 273–288 (2022). Article CAS Google
Scholar * Wandinger-Ness, A. & Zerial, M. Rab proteins and the compartmentalization of the endosomal system. _Cold Spring Harb. Perspect. Biol._ 6, a022616 (2014). Article Google
Scholar * Szymanska, E., Budick-Harmelin, N. & Miaczynska, M. Endosomal “sort” of signaling control: the role of ESCRT machinery in regulation of receptor-mediated signaling pathways.
_Semin. Cell Dev. Biol._ 74, 11–20 (2018). Article CAS Google Scholar * White, M. F. & Kahn, C. R. Insulin action at a molecular level–100 years of progress. _Mol. Metab._ 52, 101304
(2021). Article CAS Google Scholar * Fagerholm, S., Ortegren, U., Karlsson, M., Ruishalme, I. & Stralfors, P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae
in primary adipocytes. _PLoS ONE_ 4, e5985 (2009). Article Google Scholar * McClain, D. A. & Olefsky, J. M. Evidence for two independent pathways of insulin-receptor internalization in
hepatocytes and hepatoma cells. _Diabetes_ 37, 806–815 (1988). Article CAS Google Scholar * Couet, J., Li, S., Okamoto, T., Ikezu, T. & Lisanti, M. P. Identification of peptide and
protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. _J. Biol. Chem._ 272, 6525–6533 (1997). Article CAS
Google Scholar * Nystrom, F. H., Chen, H., Cong, L. N., Li, Y. & Quon, M. J. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in
transfected Cos-7 cells and rat adipose cells. _Mol. Endocrinol._ 13, 2013–2024 (1999). Article CAS Google Scholar * Carpentier, J. L. et al. Two steps of insulin receptor internalization
depend on different domains of the beta-subunit. _J. Cell Biol._ 122, 1243–1252 (1993). Article CAS Google Scholar * Ceresa, B. P., Kao, A. W., Santeler, S. R. & Pessin, J. E.
Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways. _Mol. Cell Biol._ 18, 3862–3870 (1998). Article CAS Google
Scholar * Iraburu, M. J., Garner, T. & Montiel-Duarte, C. Revising endosomal trafficking under insulin receptor activation. _Int. J. Mol. Sci._ https://doi.org/10.3390/ijms22136978
(2021). Article Google Scholar * Fan, J. Y. et al. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. _Proc. Natl Acad. Sci. USA_ 79, 7788–7791
(1982). Article CAS Google Scholar * Cohen, A. W. et al. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. _Am. J.
Physiol. Cell Physiol._ 285, C222–C235 (2003). Article CAS Google Scholar * Razani, B. et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of
caveolae. _Mol. Cell Biol._ 22, 2329–2344 (2002). Article CAS Google Scholar * Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against
obesity and the metabolic syndrome. _Pharmacol. Res._ 53, 482–491 (2006). Article CAS Google Scholar * Kim, C. A. et al. Association of a homozygous nonsense caveolin-1 mutation with
Berardinelli–Seip congenital lipodystrophy. _J. Clin. Endocrinol. Metab._ 93, 1129–1134 (2008). Article CAS Google Scholar * Mann, J. P. & Savage, D. B. What lipodystrophies teach us
about the metabolic syndrome. _J. Clin. Invest._ 129, 4009–4021 (2019). Article Google Scholar * Boucher, J. et al. Differential roles of insulin and IGF-1 receptors in adipose tissue
development and function. _Diabetes_ 65, 2201–2213 (2016). Article CAS Google Scholar * Ansarullah et al. Inceptor counteracts insulin signalling in β-cells to control glycaemia. _Nature_
590, 326–331 (2021). Article CAS Google Scholar * Rosen, O. M., Herrera, R., Olowe, Y., Petruzzelli, L. M. & Cobb, M. H. Phosphorylation activates the insulin receptor tyrosine
protein kinase. _Proc. Natl Acad. Sci. USA_ 80, 3237–3240 (1983). Article CAS Google Scholar * Bevan, A. P. et al. Selective activation of the rat hepatic endosomal insulin receptor
kinase. Role for the endosome in insulin signaling. _J. Biol. Chem._ 270, 10784–10791 (1995). Article CAS Google Scholar * Christoforidis, S., McBride, H. M., Burgoyne, R. D. &
Zerial, M. The Rab5 effector EEA1 is a core component of endosome docking. _Nature_ 397, 621–625 (1999). Article CAS Google Scholar * Schenck, A. et al. The endosomal protein Appl1
mediates Akt substrate specificity and cell survival in vertebrate development. _Cell_ 133, 486–497 (2008). Article CAS Google Scholar * Kalaidzidis, I. et al. APPL endosomes are not
obligatory endocytic intermediates but act as stable cargo-sorting compartments. _J. Cell Biol._ 211, 123–144 (2015). Article CAS Google Scholar * Mitsuuchi, Y. et al. Identification of a
chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. _Oncogene_ 18, 4891–4898 (1999). Article CAS Google
Scholar * Ryu, J. et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. _Cell Rep._ 7, 1227–1238 (2014). Article CAS Google Scholar
* Tan, Y., You, H., Coffey, F. J., Wiest, D. L. & Testa, J. R. Appl1 is dispensable for Akt signaling in vivo and mouse T-cell development. _Genesis_ 48, 531–539 (2010). Article CAS
Google Scholar * Cheng, K. K. et al. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. _Cell Metab._ 9, 417–427
(2009). Article CAS Google Scholar * Alliouachene, S. et al. Inactivation of the class II PI3K-C2β potentiates insulin signaling and sensitivity. _Cell Rep._ 13, 1881–1894 (2015).
Article CAS Google Scholar * Hunker, C. M. et al. Role of Rab5 in insulin receptor-mediated endocytosis and signaling. _Arch. Biochem. Biophys._ 449, 130–142 (2006). Article CAS Google
Scholar * Haeusler, R. A., McGraw, T. E. & Accili, D. Biochemical and cellular properties of insulin receptor signalling. _Nat. Rev. Mol. Cell Biol._ 19, 31–44 (2018). Article CAS
Google Scholar * Zeigerer, A. et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. _Nature_ 485, 465–470 (2012). Article CAS Google Scholar * Tokarz, V. L.,
MacDonald, P. E. & Klip, A. The cell biology of systemic insulin function. _J. Cell Biol._ 217, 2273–2289 (2018). Article CAS Google Scholar * Seely, B. L. et al. Protein tyrosine
phosphatase 1B interacts with the activated insulin receptor. _Diabetes_ 45, 1379–1385 (1996). Article CAS Google Scholar * Byon, J. C., Kusari, A. B. & Kusari, J. Protein-tyrosine
phosphatase-1B acts as a negative regulator of insulin signal transduction. _Mol. Cell Biochem._ 182, 101–108 (1998). Article CAS Google Scholar * Carpentier, J. L., Fehlmann, M., Van
Obberghen, E., Gorden, P. & Orci, L. Insulin receptor internalization and recycling: mechanism and significance. _Biochimie_ 67, 1143–1145 (1985). Article CAS Google Scholar *
Gillingham, A. K. & Munro, S. The small G proteins of the Arf family and their regulators. _Annu. Rev. Cell Dev. Biol._ 23, 579–611 (2007). Article CAS Google Scholar * Rodiger, M. et
al. Adiponectin release and insulin receptor targeting share trans-Golgi-dependent endosomal trafficking routes. _Mol. Metab._ 8, 167–179 (2018). Article Google Scholar * Authier, F.
& Desbuquois, B. Glucagon receptors. _Cell Mol. Life Sci._ 65, 1880–1899 (2008). Article CAS Google Scholar * Zeigerer, A. et al. Glucagon’s metabolic action in health and disease.
_Compr. Physiol._ 11, 1759–1783 (2021). Article Google Scholar * Merlen, C., Fabrega, S., Desbuquois, B., Unson, C. G. & Authier, F. Glucagon-mediated internalization of
serine-phosphorylated glucagon receptor and Gsα in rat liver. _FEBS Lett._ 580, 5697–5704 (2006). Article CAS Google Scholar * Watanabe, J., Kanai, K. & Kanamura, S. Glucagon
receptors in endothelial and Kupffer cells of mouse liver. _J. Histochem. Cytochem._ 36, 1081–1089 (1988). Article CAS Google Scholar * Krilov, L. et al. Dual mode of glucagon receptor
internalization: role of PKCα, GRKs and β-arrestins. _Exp. Cell Res._ 317, 2981–2994 (2011). Article CAS Google Scholar * Authier, F., Janicot, M., Lederer, F. & Desbuquois, B. Fate
of injected glucagon taken up by rat liver in vivo. Degradation of internalized ligand in the endosomal compartment. _Biochem. J._ 272, 703–712 (1990). Article CAS Google Scholar *
Krilov, L., Nguyen, A., Miyazaki, T., Unson, C. G. & Bouscarel, B. Glucagon receptor recycling: role of carboxyl terminus, β-arrestins, and cytoskeleton. _Am. J. Physiol. Cell Physiol._
295, C1230–C1237 (2008). Article CAS Google Scholar * Bomholt, A. B. et al. Evaluation of commercially available glucagon receptor antibodies and glucagon receptor expression. _bioRxiv_
https://doi.org/10.1101/2021.12.21.473442 (2021). Article Google Scholar * Van Der Sluijs, P. et al. The small GTP-binding protein rab4 is associated with early endosomes. _Proc. Natl
Acad. Sci. USA_ 88, 6313–6317 (1991). Article Google Scholar * Perrin, L. et al. Rab4b controls an early endosome sorting event by interacting with the γ-subunit of the clathrin adaptor
complex 1. _J. Cell Sci._ 126, 4950–4962 (2013). CAS Google Scholar * Kaur, S., Chen, Y. & Shenoy, S. K. Agonist-activated glucagon receptors are deubiquitinated at early endosomes by
two distinct deubiquitinases to facilitate Rab4a-dependent recycling. _J. Biol. Chem._ 295, 16630–16642 (2020). Article CAS Google Scholar * Cegla, J. et al. RAMP2 influences glucagon
receptor pharmacology via trafficking and signaling. _Endocrinology_ 158, 2680–2693 (2017). Article CAS Google Scholar * McGlone, E. R. et al. Receptor activity-modifying protein 2
(RAMP2) alters glucagon receptor trafficking in hepatocytes with functional effects on receptor signalling. _Mol. Metab._ 53, 101296 (2021). Article CAS Google Scholar * Zhu, L. et al.
Hepatic β-arrestin 2 is essential for maintaining euglycemia. _J. Clin. Invest._ 127, 2941–2945 (2017). Article Google Scholar * Wang, Y. et al. Glucagon is associated with NAFLD
inflammatory progression in type 2 diabetes, not with NAFLD fibrotic progression. _Eur. J. Gastroenterol. Hepatol._ 33, e818–e823 (2021). Article CAS Google Scholar * Sorensen, H. et al.
Glucagon receptor knockout mice display increased insulin sensitivity and impaired β-cell function. _Diabetes_ 55, 3463–3469 (2006). Article Google Scholar * Conarello, S. L. et al.
Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. _Diabetologia_ 50, 142–150 (2007). Article CAS Google
Scholar * Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. _Lancet_ 368, 1696–1705
(2006). Article CAS Google Scholar * Campbell, J. E. Targeting the GIPR for obesity: to agonize or antagonize? Potential mechanisms. _Mol. Metab._ 46, 101139 (2021). Article CAS Google
Scholar * Morgan, L. M., Flatt, P. R. & Marks, V. Nutrient regulation of the enteroinsular axis and insulin secretion. _Nutr. Res. Rev._ 1, 79–97 (1988). Article CAS Google Scholar *
Yamada, Y. & Seino, Y. Physiology of GIP–a lesson from GIP receptor knockout mice. _Horm. Metab. Res._ 36, 771–774 (2004). Article CAS Google Scholar * El, K. & Campbell, J. E.
The role of GIP in α-cells and glucagon secretion. _Peptides_ 125, 170213 (2020). Article CAS Google Scholar * Zhang, Q. et al. The glucose-dependent insulinotropic polypeptide (GIP)
regulates body weight and food intake via CNS-GIPR signaling. _Cell Metab._ 33, 833–844.e5 (2021). Article CAS Google Scholar * Mohammad, S. et al. Gastric inhibitory peptide controls
adipose insulin sensitivity via activation of cAMP-response element-binding protein and p110β isoform of phosphatidylinositol 3-kinase. _J. Biol. Chem._ 286, 43062–43070 (2011). Article CAS
Google Scholar * Mohammad, S. et al. A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin
sensitivity. _Mol. Cell Biol._ 34, 3618–3629 (2014). Article Google Scholar * Gabe, M. B. N. et al. Human GIP(3-30)NH2 inhibits G protein-dependent as well as G protein-independent
signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. _Biochem. Pharmacol._ 150, 97–107 (2018). Article CAS Google Scholar *
Abdullah, N., Beg, M., Soares, D., Dittman, J. S. & McGraw, T. E. Downregulation of a GPCR by β-arrestin2-mediated switch from an endosomal to a TGN recycling pathway. _Cell Rep._ 17,
2966–2978 (2016). Article CAS Google Scholar * Ismail, S. et al. Internalized receptor for glucose-dependent insulinotropic peptide stimulates adenylyl cyclase on early endosomes.
_Biochem. Pharmacol._ 120, 33–45 (2016). Article CAS Google Scholar * Roussel, M., Mathieu, J. & Dalle, S. Molecular mechanisms redirecting the GLP-1 receptor signalling profile in
pancreatic β-cells during type 2 diabetes. _Horm. Mol. Biol. Clin. Investig._ 26, 87–95 (2016). CAS Google Scholar * Pierce, K. L., Premont, R. T. & Lefkowitz, R. J.
Seven-transmembrane receptors. _Nat. Rev. Mol. Cell Biol._ 3, 639–650 (2002). Article CAS Google Scholar * Girada, S. B. et al. Gαs regulates glucagon-like peptide 1 receptor-mediated
cyclic AMP generation at Rab5 endosomal compartment. _Mol. Metab._ 6, 1173–1185 (2017). Article CAS Google Scholar * Syme, C. A., Zhang, L. & Bisello, A. Caveolin-1 regulates cellular
trafficking and function of the glucagon-like peptide 1 receptor. _Mol. Endocrinol._ 20, 3400–3411 (2006). Article CAS Google Scholar * Buenaventura, T. et al. A targeted RNAi screen
identifies endocytic trafficking factors that control GLP-1 receptor signaling in pancreatic β-cells. _Diabetes_ 67, 385–399 (2018). Article CAS Google Scholar * Li, D. T. et al. GLUT4
storage vesicles: specialized organelles for regulated trafficking. _Yale J. Biol. Med._ 92, 453–470 (2019). CAS Google Scholar * Gould, G. W., Brodsky, F. M. & Bryant, N. J. Building
GLUT4 vesicles: CHC22 clathrin’s human touch. _Trends Cell Biol._ 30, 705–719 (2020). Article CAS Google Scholar * Batty, S. R. & Langlais, P. R. Microtubules in insulin action:
what’s on the tube? _Trends Endocrinol. Metab._ 32, 776–789 (2021). Article CAS Google Scholar * Jaldin-Fincati, J. R., Pavarotti, M., Frendo-Cumbo, S., Bilan, P. J. & Klip, A. Update
on GLUT4 vesicle traffic: a cornerstone of insulin action. _Trends Endocrinol. Metab._ 28, 597–611 (2017). Article CAS Google Scholar * Stockli, J., Fazakerley, D. J. & James, D. E.
GLUT4 exocytosis. _J. Cell Sci._ 124, 4147–4159 (2011). Article CAS Google Scholar * Leto, D. & Saltiel, A. R. Regulation of glucose transport by insulin: traffic control of GLUT4.
_Nat. Rev. Mol. Cell Biol._ 13, 383–396 (2012). Article CAS Google Scholar * Foley, K., Boguslavsky, S. & Klip, A. Endocytosis, recycling, and regulated exocytosis of glucose
transporter 4. _Biochemistry_ 50, 3048–3061 (2011). Article CAS Google Scholar * Bogan, J. S. Regulation of glucose transporter translocation in health and diabetes. _Annu. Rev. Biochem._
81, 507–532 (2012). Article CAS Google Scholar * Zeigerer, A. et al. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. _Mol. Biol. Cell_ 13,
2421–2435 (2002). Article CAS Google Scholar * Volchuk, A. et al. Perturbation of dynamin II with an amphiphysin SH3 domain increases GLUT4 glucose transporters at the plasma membrane in
3T3-L1 adipocytes. Dynamin II participates in GLUT4 endocytosis. _J. Biol. Chem._ 273, 8169–8176 (1998). Article CAS Google Scholar * Nishimura, H., Zarnowski, M. J. & Simpson, I. A.
Glucose transporter recycling in rat adipose cells. Effects of potassium depletion. _J. Biol. Chem._ 268, 19246–19253 (1993). Article CAS Google Scholar * Robinson, L. J., Pang, S.,
Harris, D. S., Heuser, J. & James, D. E. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S
and localization of GLUT4 to clathrin lattices. _J. Cell Biol._ 117, 1181–1196 (1992). Article CAS Google Scholar * Marsh, B. J., Alm, R. A., McIntosh, S. R. & James, D. E. Molecular
regulation of GLUT-4 targeting in 3T3-L1 adipocytes. _J. Cell Biol._ 130, 1081–1091 (1995). Article CAS Google Scholar * Pan, X., Zaarur, N., Singh, M., Morin, P. & Kandror, K. V.
Sortilin and retromer mediate retrograde transport of Glut4 in 3T3-L1 adipocytes. _Mol. Biol. Cell_ 28, 1667–1675 (2017). Article CAS Google Scholar * Yang, Z. et al. Functional
characterization of retromer in GLUT4 storage vesicle formation and adipocyte differentiation. _FASEB J._ 30, 1037–1050 (2016). Article CAS Google Scholar * Blot, V. & McGraw, T. E.
Molecular mechanisms controlling GLUT4 intracellular retention. _Mol. Biol. Cell_ 19, 3477–3487 (2008). Article CAS Google Scholar * Foley, K. P. & Klip, A. Dynamic GLUT4 sorting
through a syntaxin-6 compartment in muscle cells is derailed by insulin resistance-causing ceramide. _Biol. Open_ 3, 314–325 (2014). Article CAS Google Scholar * Shewan, A. M. et al.
GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. _Mol. Biol. Cell_ 14, 973–986 (2003). Article
CAS Google Scholar * Proctor, K. M., Miller, S. C., Bryant, N. J. & Gould, G. W. Syntaxin 16 controls the intracellular sequestration of GLUT4 in 3T3-L1 adipocytes. _Biochem.
Biophys. Res. Commun._ 347, 433–438 (2006). Article CAS Google Scholar * Li, L. V. & Kandror, K. V. Golgi-localized, γ-ear-containing, Arf-binding protein adaptors mediate
insulin-responsive trafficking of glucose transporter 4 in 3T3-L1 adipocytes. _Mol. Endocrinol._ 19, 2145–2153 (2005). Article CAS Google Scholar * Vollenweider, P. et al. The small
guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3-L1 cells. _Endocrinology_ 138, 4941–4949 (1997).
Article CAS Google Scholar * Cormont, M. et al. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. _Mol. Cell Biol._ 16, 6879–6886 (1996).
Article CAS Google Scholar * Mari, M. et al. The Rab4 effector Rabip4 plays a role in the endocytotic trafficking of Glut 4 in 3T3-L1 adipocytes. _J. Cell Sci._ 119, 1297–1306 (2006).
Article CAS Google Scholar * Lampson, M. A., Schmoranzer, J., Zeigerer, A., Simon, S. M. & McGraw, T. E. Insulin-regulated release from the endosomal recycling compartment is
regulated by budding of specialized vesicles. _Mol. Biol. Cell_ 12, 3489–3501 (2001). Article CAS Google Scholar * Hirshman, M. F., Goodyear, L. J., Wardzala, L. J., Horton, E. D. &
Horton, E. S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. _J. Biol. Chem._ 265, 987–991 (1990). Article CAS
Google Scholar * Tanti, J. F., Gremeaux, T., Van Obberghen, E. & Le Marchand-Brustel, Y. Insulin receptor substrate 1 is phosphorylated by the serine kinase activity of
phosphatidylinositol 3-kinase. _Biochem. J._ 304, 17–21 (1994). Article CAS Google Scholar * Hopkins, B. D., Goncalves, M. D. & Cantley, L. C. Insulin-PI3K signalling: an
evolutionarily insulated metabolic driver of cancer. _Nat. Rev. Endocrinol._ 16, 276–283 (2020). Article CAS Google Scholar * Bjornholm, M., Kawano, Y., Lehtihet, M. & Zierath, J. R.
Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. _Diabetes_ 46, 524–527
(1997). Article CAS Google Scholar * Dannhauser, P. N. et al. CHC22 and CHC17 clathrins have distinct biochemical properties and display differential regulation and function. _J. Biol.
Chem._ 292, 20834–20844 (2017). Article CAS Google Scholar * Camus, S. M. et al. CHC22 clathrin mediates traffic from early secretory compartments for human GLUT4 pathway biogenesis. _J.
Cell Biol._ https://doi.org/10.1083/jcb.201812135 (2020). Article Google Scholar * Garvey, W. T. et al. Evidence for defects in the trafficking and translocation of GLUT4 glucose
transporters in skeletal muscle as a cause of human insulin resistance. _J. Clin. Invest._ 101, 2377–2386 (1998). Article CAS Google Scholar * Maianu, L., Keller, S. R. & Garvey, W.
T. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus:
implications regarding defects in vesicle trafficking. _J. Clin. Endocrinol. Metab._ 86, 5450–5456 (2001). Article CAS Google Scholar * Zierath, J. R. et al. Insulin action on glucose
transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. _Diabetologia_ 39, 1180–1189 (1996). Article CAS Google Scholar * Benninghoff, T. et al. The
RabGAPs TBC1D1 and TBC1D4 control uptake of long-chain fatty acids into skeletal muscle via fatty acid transporter SLC27A4/FATP4. _Diabetes_ 69, 2281–2293 (2020). Article CAS Google
Scholar * Sano, H. et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. _J. Biol. Chem._ 278, 14599–14602 (2003). Article CAS Google
Scholar * Mafakheri, S. et al. AKT and AMP-activated protein kinase regulate TBC1D1 through phosphorylation and its interaction with the cytosolic tail of insulin-regulated aminopeptidase
IRAP. _J. Biol. Chem._ 293, 17853–17862 (2018). Article CAS Google Scholar * Sakamoto, K. & Holman, G. D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic.
_Am. J. Physiol. Endocrinol. Metab._ 295, E29–E37 (2008). Article CAS Google Scholar * Hatakeyama, H., Morino, T., Ishii, T. & Kanzaki, M. Cooperative actions of Tbc1d1 and
AS160/Tbc1d4 in GLUT4-trafficking activities. _J. Biol. Chem._ 294, 1161–1172 (2019). Article CAS Google Scholar * Dash, S. et al. A truncation mutation in TBC1D4 in a family with
acanthosis nigricans and postprandial hyperinsulinemia. _Proc. Natl Acad. Sci. USA_ 106, 9350–9355 (2009). Article CAS Google Scholar * Miinea, C. P. et al. AS160, the Akt substrate
regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. _Biochem. J._ 391, 87–93 (2005). Article CAS Google Scholar * Roach, W. G., Chavez, J. A., Miinea,
C. P. & Lienhard, G. E. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. _Biochem. J._ 403, 353–358 (2007). Article CAS Google
Scholar * Stockli, J., Fazakerley, D. J., Coster, A. C., Holman, G. D. & James, D. E. Muscling in on GLUT4 kinetics. _Commun. Integr. Biol._ 3, 260–262 (2010). Article Google Scholar
* Fazakerley, D. J., Koumanov, F. & Holman, G. D. GLUT4 on the move. _Biochem. J._ 479, 445–462 (2022). Article CAS Google Scholar * Belman, J. P. et al. Acetylation of TUG protein
promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. _J. Biol. Chem._ 290, 4447–4463 (2015). Article CAS Google Scholar * Bogan, J.
S. et al. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. _J. Biol. Chem._ 287, 23932–23947 (2012). Article CAS Google Scholar * Habtemichael,
E. N. et al. Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes. _J. Biol. Chem._ 293, 10466–10486 (2018).
Article CAS Google Scholar * Habtemichael, E. N. et al. Insulin-stimulated endoproteolytic TUG cleavage links energy expenditure with glucose uptake. _Nat. Metab._ 3, 378–393 (2021).
Article CAS Google Scholar * Fischer, A. W. et al. The adaptor protein PID1 regulates receptor-dependent endocytosis of postprandial triglyceride-rich lipoproteins. _Mol. Metab._ 16,
88–99 (2018). Article CAS Google Scholar * Jedrychowski, M. P. et al. Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin
signaling. _J. Biol. Chem._ 285, 104–114 (2010). Article CAS Google Scholar * Fischer, A. W. et al. PID1 regulates insulin-dependent glucose uptake by controlling intracellular sorting of
GLUT4-storage vesicles. _Biochim. Biophys. Acta Mol. Basis Dis._ 1865, 1592–1603 (2019). Article CAS Google Scholar * Goldstein, J. L. & Brown, M. S. The LDL receptor. _Arterioscler.
Thromb. Vasc. Biol._ 29, 431–438 (2009). Article CAS Google Scholar * Go, G. W. & Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. _Yale
J. Biol. Med._ 85, 19–28 (2012). CAS Google Scholar * Mishra, S. K., Watkins, S. C. & Traub, L. M. The autosomal recessive hypercholesterolemia (ARH) protein interfaces directly with
the clathrin-coat machinery. _Proc. Natl Acad. Sci. USA_ 99, 16099–16104 (2002). Article CAS Google Scholar * He, G. et al. ARH is a modular adaptor protein that interacts with the LDL
receptor, clathrin, and AP-2. _J. Biol. Chem._ 277, 44044–44049 (2002). Article CAS Google Scholar * Garuti, R. et al. The modular adaptor protein autosomal recessive hypercholesterolemia
(ARH) promotes low density lipoprotein receptor clustering into clathrin-coated pits. _J. Biol. Chem._ 280, 40996–41004 (2005). Article CAS Google Scholar * Wu, J. H. et al. The adaptor
protein beta-arrestin2 enhances endocytosis of the low density lipoprotein receptor. _J. Biol. Chem._ 278, 44238–44245 (2003). Article CAS Google Scholar * Kim, J. et al. Beta-arrestins
regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. _Circ. Res._ 103, 70–79 (2008). Article CAS Google Scholar * Zhang, C.,
Hao, C., Shui, G. & Li, W. BLOS1 mediates kinesin switch during endosomal recycling of LDL receptor. _Elife_ https://doi.org/10.7554/eLife.58069 (2020). Article Google Scholar * Scott,
I., Wang, L., Wu, K., Thapa, D. & Sack, M. N. GCN5L1/BLOS1 links acetylation, organelle remodeling, and metabolism. _Trends Cell Biol._ 28, 346–355 (2018). Article CAS Google Scholar
* Vos, D. Y. & van de Sluis, B. Function of the endolysosomal network in cholesterol homeostasis and metabolic-associated fatty liver disease (MAFLD). _Mol. Metab._ 50, 101146 (2021).
Article CAS Google Scholar * Wijers, M. et al. The hepatic WASH complex is required for efficient plasma LDL and HDL cholesterol clearance. _JCI Insight_
https://doi.org/10.1172/jci.insight.126462 (2019). Article Google Scholar * Zelcer, N., Hong, C., Boyadjian, R. & Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent
ubiquitination of the LDL receptor. _Science_ 325, 100–104 (2009). Article CAS Google Scholar * Poirier, S. & Mayer, G. The biology of PCSK9 from the endoplasmic reticulum to
lysosomes: new and emerging therapeutics to control low-density lipoprotein cholesterol. _Drug Des. Devel Ther._ 7, 1135–1148 (2013). Google Scholar * Goldstein, J. L., Anderson, R. G. W.
& Brown, M. S. In _Ciba Foundation Symposium 92–Membrane Recycling_ Ch. 5 (eds Evered, D. & Collins, G.M.) 77–95 (Pitman, 1982). * Jang, H. D. et al. Cyclase-associated protein 1 is
a binding partner of proprotein convertase subtilisin/kexin type-9 and is required for the degradation of low-density lipoprotein receptors by proprotein convertase subtilisin/kexin type-9.
_Eur. Heart J._ 41, 239–252 (2020). Article CAS Google Scholar * Ruscica, M. et al. Liver fat accumulation is associated with circulating PCSK9. _Ann. Med._ 48, 384–391 (2016). Article
CAS Google Scholar * Krahmer, N. et al. Organellar proteomics and phospho-proteomics reveal subcellular reorganization in diet-induced hepatic steatosis. _Dev. Cell_ 47, 205–221.e7 (2018).
Article CAS Google Scholar * Luiken, J., Nabben, M., Neumann, D. & Glatz, J. F. C. Understanding the distinct subcellular trafficking of CD36 and GLUT4 during the development of
myocardial insulin resistance. _Biochim. Biophys. Acta Mol. Basis Dis._ 1866, 165775 (2020). Article CAS Google Scholar * Xue, B. et al. Effects of high fat feeding on adipose tissue gene
expression in diabetic Goto-Kakizaki rats. _Gene Regul. Syst. Bio_ 9, 15–26 (2015). CAS Google Scholar * Kita, Y. et al. Metformin prevents and reverses inflammation in a non-diabetic
mouse model of nonalcoholic steatohepatitis. _PLoS ONE_ 7, e43056 (2012). Article CAS Google Scholar * Gilleron, J. et al. Rab4b deficiency in T cells promotes adipose Treg/Th17
imbalance, adipose tissue dysfunction, and insulin resistance. _Cell Rep._ 25, 3329–3341.e25 (2018). Article CAS Google Scholar * Gandasi, N. R. et al. Glucose-dependent granule docking
limits insulin secretion and is decreased in human type 2 diabetes. _Cell Metab._ 27, 470–478.e4 (2018). Article CAS Google Scholar * Kaddai, V. et al. Involvement of TNF-α in abnormal
adipocyte and muscle sortilin expression in obese mice and humans. _Diabetologia_ 52, 932–940 (2009). Article CAS Google Scholar * Salani, B. et al. Caveolin-1 is essential for metformin
inhibitory effect on IGF1 action in non-small-cell lung cancer cells. _FASEB J._ 26, 788–798 (2012). Article CAS Google Scholar * Salis, O., Bedir, A., Ozdemir, T., Okuyucu, A. &
Alacam, H. The relationship between anticancer effect of metformin and the transcriptional regulation of certain genes (CHOP, CAV-1, HO-1, SGK-1 and Par-4) on MCF-7 cell line. _Eur. Rev.
Med. Pharmacol. Sci._ 18, 1602–1609 (2014). CAS Google Scholar * Strong, A., Patel, K. & Rader, D. J. Sortilin and lipoprotein metabolism: making sense out of complexity. _Curr. Opin.
Lipidol._ 25, 350–357 (2014). Article CAS Google Scholar * Musunuru, K. et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. _Nature_ 466, 714–719 (2010).
Article CAS Google Scholar * Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. _Nature_ 562, 203–209 (2018). Article CAS Google Scholar * Conlon, D. M.
et al. Sortilin restricts secretion of apolipoprotein B-100 by hepatocytes under stressed but not basal conditions. _J. Clin. Invest._ https://doi.org/10.1172/JCI144334 (2022). Article
Google Scholar * Kathiresan, S. et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. _Nat. Genet._
40, 189–197 (2008). Article CAS Google Scholar * Myocardial Infarction Genetics Consortiumet al. Genome-wide association of early-onset myocardial infarction with single nucleotide
polymorphisms and copy number variants. _Nat. Genet._ 41, 334–341 (2009). Article Google Scholar * Strong, A. et al. Hepatic sortilin regulates both apolipoprotein B secretion and LDL
catabolism. _J. Clin. Invest._ 122, 2807–2816 (2012). Article CAS Google Scholar * Conlon, D. M. Role of sortilin in lipid metabolism. _Curr. Opin. Lipidol._ 30, 198–204 (2019). Article
CAS Google Scholar * Rabinowich, L. et al. Sortilin deficiency improves the metabolic phenotype and reduces hepatic steatosis of mice subjected to diet-induced obesity. _J. Hepatol._ 62,
175–181 (2015). Article CAS Google Scholar * Chen, C., Li, J., Matye, D. J., Wang, Y. & Li, T. Hepatocyte sortilin 1 knockout and treatment with a sortilin 1 inhibitor reduced plasma
cholesterol in Western diet-fed mice. _J. Lipid Res._ 60, 539–549 (2019). Article CAS Google Scholar * Wu, L. et al. Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and
growth. _Dev. Cell_ 30, 378–393 (2014). Article CAS Google Scholar * Deus, C. M., Yambire, K. F., Oliveira, P. J. & Raimundo, N. Mitochondria-lysosome crosstalk: from physiology to
neurodegeneration. _Trends Mol. Med._ 26, 71–88 (2020). Article CAS Google Scholar * Hsu, F. et al. Rab5 and Alsin regulate stress-activated cytoprotective signaling on mitochondria.
_Elife_ https://doi.org/10.7554/eLife.32282 (2018). Article Google Scholar * Sugiura, A., McLelland, G. L., Fon, E. A. & McBride, H. M. A new pathway for mitochondrial quality control:
mitochondrial-derived vesicles. _EMBO J._ 33, 2142–2156 (2014). Article CAS Google Scholar * Shin, H. W. et al. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover
in the endocytic pathway. _J. Cell Biol._ 170, 607–618 (2005). Article CAS Google Scholar * Bilanges, B. et al. Vps34 PI 3-kinase inactivation enhances insulin sensitivity through
reprogramming of mitochondrial metabolism. _Nat. Commun._ 8, 1804 (2017). Article Google Scholar * Hamdi, A. et al. Erythroid cell mitochondria receive endosomal iron by a “kiss-and-run”
mechanism. _Biochim. Biophys. Acta_ 1863, 2859–2867 (2016). Article CAS Google Scholar * Wei, Z., Su, W., Lou, H., Duan, S. & Chen, G. Trafficking pathway between plasma membrane and
mitochondria via clathrin-mediated endocytosis. _J. Mol. Cell Biol._ 10, 539–548 (2018). Article CAS Google Scholar * Hothersall, J. D., Brown, A. J., Dale, I. & Rawlins, P. Can
residence time offer a useful strategy to target agonist drugs for sustained GPCR responses? _Drug Discov. Today_ 21, 90–96 (2016). Article CAS Google Scholar * Jones, B. et al. Targeting
GLP-1 receptor trafficking to improve agonist efficacy. _Nat. Commun._ 9, 1602 (2018). Article Google Scholar * Pickford, P. et al. Signalling, trafficking and glucoregulatory properties
of glucagon-like peptide-1 receptor agonists exendin-4 and lixisenatide. _Br. J. Pharmacol._ 177, 3905–3923 (2020). Article CAS Google Scholar * Novikoff, A. et al. Spatiotemporal GLP-1
and GIP receptor signaling and trafficking/recycling dynamics induced by selected receptor mono- and dual-agonists. _Mol. Metab._ 49, 101181 (2021). Article CAS Google Scholar * Wu, Q. et
al. Biased agonists with less glucagon-like peptide-1 receptor-mediated endocytosis prolong hypoglycaemic effects. _Eur. J. Pharmacol._ 907, 174203 (2021). Article CAS Google Scholar *
Marzook, A., Tomas, A. & Jones, B. The interplay of glucagon-like peptide-1 receptor trafficking and signalling in pancreatic beta cells. _Front. Endocrinol._ 12, 678055 (2021). Article
Google Scholar * Miaczynska, M. Effects of membrane trafficking on signaling by receptor tyrosine kinases. _Cold Spring Harb. Perspect. Biol._ 5, a009035 (2013). Article Google Scholar
* Eichel, K. & von Zastrow, M. Subcellular organization of GPCR signaling. _Trends Pharmacol. Sci._ 39, 200–208 (2018). Article CAS Google Scholar * Calebiro, D. et al. Persistent
cAMP-signals triggered by internalized G-protein-coupled receptors. _PLoS Biol._ 7, e1000172 (2009). Article Google Scholar * Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes
and their association with outcomes: a data-driven cluster analysis of six variables. _Lancet Diabetes Endocrinol._ 6, 361–369 (2018). Article Google Scholar * Zaharia, O. P. et al. Risk
of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. _Lancet Diabetes Endocrinol._ 7, 684–694 (2019). Article Google Scholar *
Picatoste, B. et al. Defective insulin-stimulated GLUT4 translocation in brown adipocytes induces systemic glucose homeostasis dysregulation independent of thermogenesis in female mice.
_Mol. Metab._ 53, 101305 (2021). Article CAS Google Scholar * Meier, J. J., Veldhuis, J. D. & Butler, P. C. Pulsatile insulin secretion dictates systemic insulin delivery by
regulating hepatic insulin extraction in humans. _Diabetes_ 54, 1649–1656 (2005). Article CAS Google Scholar * Farris, W. et al. Insulin-degrading enzyme regulates the levels of insulin,
amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. _Proc. Natl Acad. Sci. USA_ 100, 4162–4167 (2003). Article CAS Google Scholar * Abdul-Hay, S. O. et
al. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. _PLoS ONE_ 6, e20818 (2011). Article CAS Google Scholar * Steneberg, P.
et al. The type 2 diabetes-associated gene _Ide_ is required for insulin secretion and suppression of α-synuclein levels in β-cells. _Diabetes_ 62, 2004–2014 (2013). Article CAS Google
Scholar * Villasenor, R., Lampe, J., Schwaninger, M. & Collin, L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. _Cell Mol. Life Sci._ 76,
1081–1092 (2019). Article CAS Google Scholar * Storck, S. E. et al. Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. _J. Clin. Invest._ 126, 123–136 (2016).
Article Google Scholar * Nikolakopoulou, A. M. et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. _J. Exp. Med._ https://doi.org/10.1084/jem.20202207
(2021). Article Google Scholar * Zhou, A. L. et al. Apolipoprotein A-I crosses the blood-brain barrier through clathrin-independent and cholesterol-mediated endocytosis. _J. Pharmacol.
Exp. Ther._ 369, 481–488 (2019). Article CAS Google Scholar * Duquenne, M. et al. Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function.
_Nat. Metab._ 3, 1071–1090 (2021). Article CAS Google Scholar * Kaksonen, M. & Roux, A. Mechanisms of clathrin-mediated endocytosis. _Nat. Rev. Mol. Cell Biol._ 19, 313–326 (2018).
Article CAS Google Scholar * Chen, Z. & Schmid, S. L. Evolving models for assembling and shaping clathrin-coated pits. _J. Cell Biol._ https://doi.org/10.1083/jcb.202005126 (2020).
Article Google Scholar * Sorkin, A. Cargo recognition during clathrin-mediated endocytosis: a team effort. _Curr. Opin. Cell Biol._ 16, 392–399 (2004). Article CAS Google Scholar *
Kelly, B. T. et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. _Nature_ 456, 976–979 (2008). Article CAS Google Scholar * Lamaze, C.,
Tardif, N., Dewulf, M., Vassilopoulos, S. & Blouin, C. M. The caveolae dress code: structure and signaling. _Curr. Opin. Cell Biol._ 47, 117–125 (2017). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We thank R. Sekar, S. Herzig and T.D. Müller (all at The Institute for Diabetes and Cancer, Helmholtz Munich, Germany), J. Heeren (University Clinic
Hamburg Eppendorf, Hamburg, Germany), J.-F. Tanti and M. Cormont (both at the Université Côte d’Azur, INSERM UMR1065 C3M, Nice, France) and A. Schürmann (German Institute for Human Nutrition
Potsdam-Rehbruecke, Potsdam, Germany) for helpful discussions and critical comments to the review. We thank L. Harrison (The Institute for Diabetes and Cancer, Helmholtz Munich, Germany)
for creating the first drafts of the figures. The authors acknowledge the support of the DFG grants ZE1037/1-3 and ZE1037/3-2, H2020-MSCA-ITN 2020 Network grant ‘EndoConnect’ and the BMBF
grant 16LW0116K to A.Z., and of INSERM, the Université Côte d’Azur and the Young Investigator Program of the ANR to J.G. (ANR18-CE14-0035-01-GILLERON). AUTHOR INFORMATION Author notes *
These authors contributed equally: Jerome Gilleron, Anja Zeigerer. AUTHORS AND AFFILIATIONS * Université Côte d’Azur, Institut National de la Santé et de la Recherche Médicale (Inserm),
UMR1065 C3M, Team Cellular and Molecular Pathophysiology of Obesity, Nice, France Jerome Gilleron * Institute for Diabetes and Cancer, Helmholtz Center Munich, Neuherberg, Germany Anja
Zeigerer * German Center for Diabetes Research (DZD), Neuherberg, Germany Anja Zeigerer Authors * Jerome Gilleron View author publications You can also search for this author inPubMed Google
Scholar * Anja Zeigerer View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.G. and A.Z. contributed equally to all aspects of the article.
CORRESPONDING AUTHORS Correspondence to Jerome Gilleron or Anja Zeigerer. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interest. PEER REVIEW PEER REVIEW
INFORMATION _Nature Reviews Endocrinology_ thanks Jacqueline Stöckli, Amira Klip and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS PAN-UK BIOBANK:
https://pan.ukbb.broadinstitute.org/ UK BIOBANK: http://www.nealelab.is/uk-biobank GLOSSARY * Metabolic syndrome A cluster of conditions that occur together, increasing the risk of heart
disease, stroke and type 2 diabetes mellitus. * Non-alcoholic fatty liver disease A generic term for liver steatosis unrelated to excessive alcoholic consumption. * Endocytosis A process
requiring complex molecular machineries to internalize plasma membrane-containing cargo. * Exocytosis A cellular mechanism by which various molecules or proteins are transported from
intracellular compartments towards the plasma membrane. * Endosomal system A complex network of interacting organelles that comprise early, late and recycling endosomes. * Early endosomes
Also called sorting endosomes, these are endosomal compartments dedicated to cargo sorting to recycling or degradation pathways. * Late endosomes Also called multivesicular bodies, these are
intermediate endosomal compartments between early endosomes and lysosomes dedicated to concentrating cargo for degradation within intraluminal vesicles (vesicles generated within the lumen
of endolysosomal organelles). * Recycling endosomes Endosomal compartments dedicated to the recycling of cargo to the plasma membrane. * Lysosomes Specialized organelles dedicated to the
degradation of cargo. * Receptor desensitization A process leading to a decrease in the responsiveness of downstream signalling of receptors. * Endolysosomal system A complex network of
interacting organelles that comprise the endosomal system plus lysosomes. * Endocytic system A complex network of all organelles involved in endocytosis and endocytic trafficking. *
Endosomal tubules Tubules that arise from endosomes. * Ubiquitination Post-translational modification that covalently links ubiquitin molecules to a protein. * Trans-Golgi network A
tubulovesicular network of membrane compartments located at the _trans_-side of the Golgi apparatus. * Intracellular storage pool A pool of receptors and transmembrane proteins
intracellularly stored within compartments that can be recruited at the plasma membrane in response to a specific signal. * Adaptor proteins Proteins that act as connecting molecules. During
endocytosis, adaptor proteins connect cargo to be internalized with the molecular machinery performing endocytosis. * Cargo A generic term referring to transmembrane proteins (receptors,
transporters, etc.), signalling ligands (hormones, growth factors, etc.) and internalized extracellular proteins or macromolecules (transferrin, lipoproteins, etc.). * Coat proteins Proteins
that act to shape and/or form vesicles. * Soluble _N_-ethylmaleimide-sensitive factor attachment protein receptors A large protein family whose members mediate vesicle fusion. * Endosomal
sorting complexes required for transport A large protein family whose members mediate intraluminal vesicle formation. * GTPase-activating proteins Proteins able to activate the GTPase
activity of proteins. * Dominant-negative RAB A mutant form of RAB that preferentially binds GDP and out-competes the endogenous RAB pool for the binding of regulatory elements. *
Endoplasmic reticulum-to-Golgi intermediate compartment A tubule-vesicular network of membrane compartments located at the interface between the endoplasmic reticulum and the Golgi
apparatus. * Non-alcoholic steatohepatitis A form of NAFLD in which, in addition to liver steatosis, liver inflammation and damage appears. * Transcytosis Cellular mechanism by which various
molecules or proteins are transported across the cell. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under
a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such
publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gilleron, J., Zeigerer, A. Endosomal trafficking in metabolic homeostasis and diseases.
_Nat Rev Endocrinol_ 19, 28–45 (2023). https://doi.org/10.1038/s41574-022-00737-9 Download citation * Accepted: 09 August 2022 * Published: 10 October 2022 * Issue Date: January 2023 * DOI:
https://doi.org/10.1038/s41574-022-00737-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
