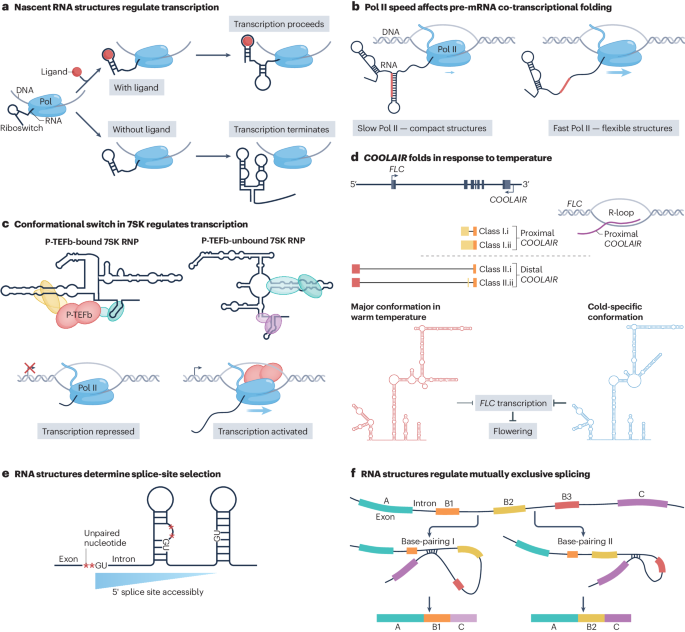
Identification of rna structures and their roles in rna functions
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The development of high-throughput RNA structure profiling methods in the past decade has greatly facilitated our ability to map and characterize different aspects of RNA structures
transcriptome-wide in cell populations, single cells and single molecules. The resulting high-resolution data have provided insights into the static and dynamic nature of RNA structures,
revealing their complexity as they perform their respective functions in the cell. In this Review, we discuss recent technical advances in the determination of RNA structures, and the roles
of RNA structures in RNA biogenesis and functions, including in transcription, processing, translation, degradation, localization and RNA structure-dependent condensates. We also discuss the
current understanding of how RNA structures could guide drug design for treating genetic diseases and battling pathogenic viruses, and highlight existing challenges and future directions in
RNA structure research. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS RNA STRUCTURE PROBING UNCOVERS RNA STRUCTURE-DEPENDENT BIOLOGICAL FUNCTIONS Article 25 June 2021 PROBING THE DYNAMIC RNA STRUCTUROME AND ITS FUNCTIONS
Article 08 November 2022 GLOBAL IN SITU PROFILING OF RNA-RNA SPATIAL INTERACTIONS WITH RIC-SEQ Article 21 May 2021 REFERENCES * Hingerty, B., Brown, R. S. & Jack, A. Further refinement
of the structure of yeast tRNAPhe. _J. Mol. Biol._ 124, 523–534 (1978). Article CAS PubMed Google Scholar * Chen, Y. & Pollack, L. SAXS studies of RNA: structures, dynamics, and
interactions with partners. _Wiley Interdiscip. Rev. RNA_ 7, 512–526 (2016). Article CAS PubMed PubMed Central Google Scholar * Dagenais, P., Desjardins, G. & Legault, P. An
integrative NMR–SAXS approach for structural determination of large RNAs defines the substrate-free state of a _trans_-cleaving _Neurospora_ Varkud Satellite ribozyme. _Nucleic Acids Res._
49, 11959–11973 (2021). Article CAS PubMed PubMed Central Google Scholar * Cheong, C. & Moore, P. B. Solution structure of an unusually stable RNA tetraplex containing G- and
U-quartet structures. _Biochemistry_ 31, 8406–8414 (1992). Article CAS PubMed Google Scholar * Barnwal, R. P., Yang, F. & Varani, G. Applications of NMR to structure determination of
RNAs large and small. _Arch. Biochem. Biophys._ 628, 42–56 (2017). Article CAS PubMed PubMed Central Google Scholar * Gabashvili, I. S. et al. Solution structure of the _E. coli_ 70S
ribosome at 11.5 Å resolution. _Cell_ 100, 537–549 (2000). Article CAS PubMed Google Scholar * Wrede, P., Wurst, R., Vournakis, J. & Rich, A. Conformational changes of yeast tRNAPhe
and _E. coli_ tRNA2Glu as indicated by different nuclease digestion patterns. _J. Biol. Chem._ 254, 9608–9616 (1979). Article CAS PubMed Google Scholar * Wurst, R. M., Vournakis, J. N.
& Maxam, A. M. Structure mapping of 5′-32P-labeled RNA with S1 nuclease. _Biochemistry_ 17, 4493–4499 (1978). Article CAS PubMed Google Scholar * Lockard, R. E. & Kumar, A.
Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. _Nucleic Acids Res._ 9, 5125–5140 (1981). Article CAS PubMed PubMed Central Google
Scholar * Lempereur, L. et al. Conformation of yeast 18S rRNA. Direct chemical probing of the 5′ domain in ribosomal subunits and in deproteinized RNA by reverse transcriptase mapping of
dimethyl sulfate-accessible. _Nucleic Acids Res._ 13, 8339–8357 (1985). Article CAS PubMed PubMed Central Google Scholar * Merino, E. J., Wilkinson, K. A., Coughlan, J. L. & Weeks,
K. M. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE). _J. Am. Chem. Soc._ 127, 4223–4231 (2005). Article CAS PubMed
Google Scholar * Wan, Y., Kertesz, M., Spitale, R. C., Segal, E. & Chang, H. Y. Understanding the transcriptome through RNA structure. _Nat. Rev. Genet._ 12, 641–655 (2011). Article
CAS PubMed Google Scholar * Kertesz, M. et al. Genome-wide measurement of RNA secondary structure in yeast. _Nature_ 467, 103–107 (2010). Article CAS PubMed Google Scholar *
Underwood, J. G. et al. FragSeq: transcriptome-wide RNA structure probing using high-throughput sequencing. _Nat. Methods_ 7, 995–1001 (2010). Article CAS PubMed PubMed Central Google
Scholar * Rouskin, S., Zubradt, M., Washietl, S., Kellis, M. & Weissman, J. S. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. _Nature_ 505,
701–705 (2014). Article CAS PubMed Google Scholar * Ding, Y. et al. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. _Nature_ 505, 696–700
(2014). Article CAS PubMed Google Scholar * Wan, Y. et al. Landscape and variation of RNA secondary structure across the human transcriptome. _Nature_ 505, 706–709 (2014). Article CAS
PubMed PubMed Central Google Scholar * Homan, P. J. et al. Single-molecule correlated chemical probing of RNA. _Proc. Natl Acad. Sci. USA_ 111, 13858–13863 (2014). Article CAS PubMed
PubMed Central Google Scholar * Spitale, R. C. & Incarnato, D. Probing the dynamic RNA structurome and its functions. _Nat. Rev. Genet._ 24, 178–196 (2023). Article CAS PubMed
Google Scholar * Strobel, E. J., Yu, A. M. & Lucks, J. B. High-throughput determination of RNA structures. _Nat. Rev. Genet._ 19, 615–634 (2018). Article CAS PubMed PubMed Central
Google Scholar * Corley, M. et al. Footprinting SHAPE-eCLIP reveals transcriptome-wide hydrogen bonds at RNA–protein interfaces. _Mol. Cell_ 80, 903–914.e8 (2020). Article CAS PubMed
PubMed Central Google Scholar * Lee, B. et al. Comparison of SHAPE reagents for mapping RNA structures inside living cells. _RNA_ 23, 169–174 (2017). Article CAS PubMed PubMed Central
Google Scholar * Spitale, R. C. et al. RNA SHAPE analysis in living cells. _Nat. Chem. Biol._ 9, 18–20 (2013). Article CAS PubMed Google Scholar * Marinus, T., Fessler, A. B., Ogle, C.
A. & Incarnato, D. A novel SHAPE reagent enables the analysis of RNA structure in living cells with unprecedented accuracy. _Nucleic Acids Res._ 49, e34 (2021). Article CAS PubMed
PubMed Central Google Scholar * Siegfried, N. A., Busan, S., Rice, G. M., Nelson, J. A. & Weeks, K. M. RNA motif discovery by SHAPE and mutational profiling (SHAPE-MaP). _Nat. Methods_
11, 959–965 (2014). Article CAS PubMed PubMed Central Google Scholar * Zubradt, M. et al. DMS-MaPseq for genome-wide or targeted RNA structure probing in vivo. _Nat. Methods_ 14, 75–82
(2017). Article CAS PubMed Google Scholar * Aviran, S. & Incarnato, D. Computational approaches for RNA structure ensemble deconvolution from structure probing data. _J. Mol. Biol._
434, 167635 (2022). Article CAS PubMed Google Scholar * Busan, S., Weidmann, C. A., Sengupta, A. & Weeks, K. M. Guidelines for SHAPE reagent choice and detection strategy for RNA
structure probing studies. _Biochemistry_ 58, 2655–2664 (2019). Article CAS PubMed Google Scholar * Guo, L. T. et al. Sequencing and structure probing of long RNAs using MarathonRT: a
next-generation reverse transcriptase. _J. Mol. Biol._ 432, 3338–3352 (2020). Article CAS PubMed PubMed Central Google Scholar * Mitchell, D., Cotter, J., Saleem, I. & Mustoe, A. M.
Mutation signature filtering enables high-fidelity RNA structure probing at all four nucleobases with DMS. _Nucleic Acids Res._ 51, 8744–8757 (2023). Article CAS PubMed PubMed Central
Google Scholar * Smola, M. J., Rice, G. M., Busan, S., Siegfried, N. A. & Weeks, K. M. Selective 2′-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP)
for direct, versatile and accurate RNA structure analysis. _Nat. Protoc._ 10, 1643–1669 (2015). Article CAS PubMed PubMed Central Google Scholar * Liu, Z. et al. In vivo nuclear RNA
structurome reveals RNA-structure regulation of mRNA processing in plants. _Genome Biol._ 22, 11 (2021). Article CAS PubMed PubMed Central Google Scholar * Sun, L. et al. RNA structure
maps across mammalian cellular compartments. _Nat. Struct. Mol. Biol._ 26, 322–330 (2019). Article CAS PubMed PubMed Central Google Scholar * Yamagami, R., Sieg, J. P., Assmann, S. M.
& Bevilacqua, P. C. Genome-wide analysis of the in vivo tRNA structurome reveals RNA structural and modification dynamics under heat stress. _Proc. Natl Acad. Sci. USA_ 119, e2201237119
(2022). Article CAS PubMed PubMed Central Google Scholar * Yang, M. et al. Intact RNA structurome reveals mRNA structure-mediated regulation of miRNA cleavage in vivo. _Nucleic Acids
Res._ 48, 8767–8781 (2020). Article CAS PubMed PubMed Central Google Scholar * Watters, K. E., Strobel, E. J., Yu, A. M., Lis, J. T. & Lucks, J. B. Cotranscriptional folding of a
riboswitch at nucleotide resolution. _Nat. Struct. Mol. Biol._ 23, 1124–1131 (2016). Article CAS PubMed PubMed Central Google Scholar * Incarnato, D. et al. In vivo probing of nascent
RNA structures reveals principles of cotranscriptional folding. _Nucleic Acids Res._ 45, 9716–9725 (2017). Article CAS PubMed PubMed Central Google Scholar * Saldi, T., Riemondy, K.,
Erickson, B. & Bentley, D. L. Alternative RNA structures formed during transcription depend on elongation rate and modify RNA processing. _Mol. Cell_ 81, 1789–1801.e5 (2021). Article
CAS PubMed PubMed Central Google Scholar * Yu, G. et al. Genome-wide probing of eukaryotic nascent RNA structure elucidates cotranscriptional folding and its antimutagenic effect. _Nat.
Commun._ 14, 5853 (2023). Article CAS PubMed PubMed Central Google Scholar * Yang, M. et al. In vivo single-molecule analysis reveals COOLAIR RNA structural diversity. _Nature_ 609,
394–399 (2022). Article CAS PubMed PubMed Central Google Scholar * Bohn, P., Gribling-Burrer, A. S., Ambi, U. B. & Smyth, R. P. Nano-DMS-MaP allows isoform-specific RNA structure
determination. _Nat. Methods_ 20, 849–859 (2023). Article CAS PubMed PubMed Central Google Scholar * Aw, J. G. A. et al. Determination of isoform-specific RNA structure with nanopore
long reads. _Nat. Biotechnol._ 39, 336–346 (2021). Article CAS PubMed Google Scholar * Bevilacqua, P. C., Ritchey, L. E., Su, Z. & Assmann, S. M. Genome-wide analysis of RNA
secondary structure. _Annu. Rev. Genet._ 50, 235–266 (2016). Article CAS PubMed Google Scholar * Wan, Y., Qu, K., Ouyang, Z. & Chang, H. Y. Genome-wide mapping of RNA structure using
nuclease digestion and high-throughput sequencing. _Nat. Protoc._ 8, 849–869 (2013). Article CAS PubMed Google Scholar * Mortimer, S. A. & Weeks, K. M. Time-resolved RNA SHAPE
chemistry: quantitative RNA structure analysis in one-second snapshots and at single-nucleotide resolution. _Nat. Protoc._ 4, 1413–1421 (2009). Article CAS PubMed PubMed Central Google
Scholar * Rabin, D. & Crothers, D. M. Analysis of RNA secondary structure by photochemical reversal of psoralen crosslinks. _Nucleic Acids Res._ 7, 689–703 (1979). Article CAS PubMed
PubMed Central Google Scholar * Cordero, P., Kladwang, W., VanLang, C. C. & Das, R. The mutate-and-map protocol for inferring base pairs in structured RNA. _Methods Mol. Biol._ 1086,
53–77 (2014). Article CAS PubMed PubMed Central Google Scholar * Lu, Z. et al. RNA duplex map in living cells reveals higher-order transcriptome structure. _Cell_ 165, 1267–1279
(2016). Article CAS PubMed PubMed Central Google Scholar * Aw, J. G. A. et al. In vivo mapping of eukaryotic RNA interactomes reveals principles of higher-order organization and
regulation. _Mol. Cell_ 62, 603–617 (2016). Article CAS PubMed Google Scholar * Sharma, E., Sterne-Weiler, T., O’Hanlon, D. & Blencowe, B. J. Global mapping of human RNA–RNA
interactions. _Mol. Cell_ 62, 618–626 (2016). Article CAS PubMed Google Scholar * Ziv, O. et al. COMRADES determines in vivo RNA structures and interactions. _Nat. Methods_ 15, 785–788
(2018). Article CAS PubMed PubMed Central Google Scholar * Kudla, G., Granneman, S., Hahn, D., Beggs, J. D. & Tollervey, D. Cross-linking, ligation, and sequencing of hybrids
reveals RNA–RNA interactions in yeast. _Proc. Natl Acad. Sci. USA_ 108, 10010–10015 (2011). Article CAS PubMed PubMed Central Google Scholar * Sugimoto, Y. et al. hiCLIP reveals the in
vivo atlas of mRNA secondary structures recognized by Staufen 1. _Nature_ 519, 491–494 (2015). Article CAS PubMed PubMed Central Google Scholar * Ye, R. et al. Capture RIC-seq reveals
positional rules of PTBP1-associated RNA loops in splicing regulation. _Mol. Cell_ 83, 1311–1327.e7 (2023). Article CAS PubMed Google Scholar * Cao, C. et al. Global in situ profiling of
RNA–RNA spatial interactions with RIC-seq. _Nat. Protoc._ 16, 2916–2946 (2021). Article CAS PubMed Google Scholar * Christy, T. W. et al. Direct mapping of higher-order RNA interactions
by SHAPE-JuMP. _Biochemistry_ 60, 1971–1982 (2021). Article CAS PubMed Google Scholar * Van Damme, R. et al. Chemical reversible crosslinking enables measurement of RNA 3D distances and
alternative conformations in cells. _Nat. Commun._ 13, 911 (2022). Article PubMed PubMed Central Google Scholar * Xu, B. et al. Recent advances in RNA structurome. _Sci. China Life
Sci._ 65, 1285–1324 (2022). Article CAS PubMed PubMed Central Google Scholar * Gabryelska, M. M. et al. Global mapping of RNA homodimers in living cells. _Genome Res._ 32, 956–967
(2022). PubMed PubMed Central Google Scholar * Zhang, M. et al. Classification and clustering of RNA crosslink-ligation data reveal complex structures and homodimers. _Genome Res._ 32,
968–985 (2022). PubMed PubMed Central Google Scholar * Tants, J.-N. & Schlundt, A. Advances, applications, and perspectives in small-angle X-ray scattering of RNA. _ChemBioChem_ 24,
e202300110 (2023). Article CAS PubMed Google Scholar * Zhang, K. et al. Cryo-EM structure of a 40 kDa SAM-IV riboswitch RNA at 3.7 Å resolution. _Nat. Commun._ 10, 5511 (2019). Article
CAS PubMed PubMed Central Google Scholar * Kappel, K. et al. Accelerated cryo-EM-guided determination of three-dimensional RNA-only structures. _Nat. Methods_ 17, 699–707 (2020). Article
CAS PubMed PubMed Central Google Scholar * Langeberg, C. J. & Kieft, J. S. A generalizable scaffold-based approach for structure determination of RNAs by cryo-EM. _Nucleic Acids
Res._ 51, e100 (2023). Article CAS PubMed PubMed Central Google Scholar * Reuter, J. S. & Mathews, D. H. RNAstructure: software for RNA secondary structure prediction and analysis.
_BMC Bioinforma._ 11, 129 (2010). Article Google Scholar * Lorenz, R. et al. ViennaRNA Package 2.0. _Algorithms Mol. Biol._ 6, 26 (2011). Article PubMed PubMed Central Google Scholar *
Hofacker, I. L. et al. Fast folding and comparison of RNA secondary structures. _Monatshefte fur Chem._ 125, 167–188 (1994). Article CAS Google Scholar * Hofacker, I. L., Fekete, M.
& Stadler, P. F. Secondary structure prediction for aligned RNA sequences. _J. Mol. Biol._ 319, 1059–1066 (2002). Article CAS PubMed Google Scholar * Washietl, S., Hofacker, I. L.
& Stadler, P. F. Fast and reliable prediction of noncoding RNAs. _Proc. Natl Acad. Sci. USA_ 102, 2454–2459 (2005). Article CAS PubMed PubMed Central Google Scholar * Rivas, E.,
Clements, J. & Eddy, S. R. A statistical test for conserved RNA structure shows lack of evidence for structure in lncRNAs. _Nat. Methods_ 14, 45–48 (2017). Article CAS PubMed Google
Scholar * Tavares, R. C. A., Pyle, A. M. & Somarowthu, S. Phylogenetic analysis with improved parameters reveals conservation in lncRNA structures. _J. Mol. Biol._ 431, 1592–1603
(2019). Article CAS PubMed PubMed Central Google Scholar * Yu, H., Qi, Y. & Ding, Y. Deep learning in RNA structure studies. _Front. Mol. Biosci._ 9, 869601 (2022). Article CAS
PubMed PubMed Central Google Scholar * Sato, K. & Hamada, M. Recent trends in RNA informatics: a review of machine learning and deep learning for RNA secondary structure prediction
and RNA drug discovery. _Brief. Bioinform_ 24, bbad186 (2023). Article PubMed PubMed Central Google Scholar * Zhang, J., Fei, Y., Sun, L. & Zhang, Q. C. Advances and opportunities in
RNA structure experimental determination and computational modeling. _Nat. Methods_ 19, 1193–1207 (2022). Article CAS PubMed Google Scholar * Aviran, S. et al. Modeling and automation
of sequencing-based characterization of RNA structure. _Proc. Natl Acad. Sci. USA_ 108, 11069–11074 (2011). Article CAS PubMed PubMed Central Google Scholar * Selega, A., Sirocchi, C.,
Iosub, I., Granneman, S. & Sanguinetti, G. Robust statistical modeling improves sensitivity of high-throughput RNA structure probing experiments. _Nat. Methods_ 14, 83–89 (2017). Article
CAS PubMed Google Scholar * Choudhary, K., Lai, Y. H., Tran, E. J. & Aviran, S. dStruct: identifying differentially reactive regions from RNA structurome profiling data. _Genome
Biol._ 20, 40 (2019). Article PubMed PubMed Central Google Scholar * Marangio, P., Law, K. Y. T., Sanguinetti, G. & Granneman, S. diffBUM-HMM: a robust statistical modeling approach
for detecting RNA flexibility changes in high-throughput structure probing data. _Genome Biol._ 22, 165 (2021). Article CAS PubMed PubMed Central Google Scholar * Yu, B., Li, P., Zhang,
Q. C. & Hou, L. Differential analysis of RNA structure probing experiments at nucleotide resolution: uncovering regulatory functions of RNA structure. _Nat. Commun._ 13, 4227 (2022).
Article CAS PubMed PubMed Central Google Scholar * Gong, J., Xu, K., Ma, Z., Lu, Z. J. & Zhang, Q. C. A deep learning method for recovering missing signals in transcriptome-wide RNA
structure profiles from probing experiments. _Nat. Mach. Intell._ 3, 995–1006 (2021). Article Google Scholar * Low, J. T. & Weeks, K. M. SHAPE-directed RNA secondary structure
prediction. _Methods_ 52, 150–158 (2010). Article CAS PubMed PubMed Central Google Scholar * Tomezsko, P. J. et al. Determination of RNA structural diversity and its role in HIV-1 RNA
splicing. _Nature_ 582, 438–442 (2020). Article CAS PubMed PubMed Central Google Scholar * Olson, S. W. et al. Discovery of a large-scale, cell-state-responsive allosteric switch in the
7SK RNA using DANCE-MaP. _Mol. Cell_ 82, 1708–1723.e10 (2022). Article CAS PubMed PubMed Central Google Scholar * Morandi, E. et al. Genome-scale deconvolution of RNA structure
ensembles. _Nat. Methods_ 18, 249–252 (2021). Article CAS PubMed Google Scholar * Goodarzi, H. et al. Systematic discovery of structural elements governing stability of mammalian
messenger RNAs. _Nature_ 485, 264–268 (2012). Article CAS PubMed PubMed Central Google Scholar * Fish, L. et al. A prometastatic splicing program regulated by SNRPA1 interactions with
structured RNA elements. _Science_ 372, eabc7531 (2021). Article CAS PubMed PubMed Central Google Scholar * Morandi, E., van Hemert, M. J. & Incarnato, D. SHAPE-guided RNA structure
homology search and motif discovery. _Nat. Commun._ 13, 1722 (2022). Article CAS PubMed PubMed Central Google Scholar * Yang, Z., Zeng, X., Zhao, Y. & Chen, R. AlphaFold2 and its
applications in the fields of biology and medicine. _Signal. Transduct. Target. Ther._ 8, 115 (2023). Article PubMed PubMed Central Google Scholar * Jumper, J. et al. Highly accurate
protein structure prediction with AlphaFold. _Nature_ 596, 583–589 (2021). Article CAS PubMed PubMed Central Google Scholar * Westhof, E. & Leontis, N. B. An RNA-centric historical
narrative around the Protein Data Bank. _J. Biol. Chem._ 296, 100555 (2021). Article CAS PubMed PubMed Central Google Scholar * Singh, J., Hanson, J., Paliwal, K. & Zhou, Y. RNA
secondary structure prediction using an ensemble of two-dimensional deep neural networks and transfer learning. _Nat. Commun._ 10, 5407 (2019). Article PubMed PubMed Central Google
Scholar * Danaee, P. et al. bpRNA: large-scale automated annotation and analysis of RNA secondary structure. _Nucleic Acids Res._ 46, 5381–5394 (2018). Article CAS PubMed PubMed Central
Google Scholar * Sato, K., Akiyama, M. & Sakakibara, Y. RNA secondary structure prediction using deep learning with thermodynamic integration. _Nat. Commun._ 12, 941 (2021). Article
CAS PubMed PubMed Central Google Scholar * Fu, L. et al. UFold: fast and accurate RNA secondary structure prediction with deep learning. _Nucleic Acids Res._ 50, e14 (2022). Article CAS
PubMed Google Scholar * Yang, E. et al. GCNfold: a novel lightweight model with valid extractors for RNA secondary structure prediction. _Comput. Biol. Med._ 164, 107246 (2023). Article
CAS PubMed Google Scholar * Li, Y. et al. Integrating end-to-end learning with deep geometrical potentials for ab initio RNA structure prediction. _Nat. Commun._ 14, 5745 (2023).
Article CAS PubMed PubMed Central Google Scholar * Wang, W. et al. trRosettaRNA: automated prediction of RNA 3D structure with transformer network. _Nat. Commun._ 14, 7266 (2023).
Article CAS PubMed PubMed Central Google Scholar * Das, R. & Baker, D. Automated de novo prediction of native-like RNA tertiary structures. _Proc. Natl Acad. Sci. USA_ 104,
14664–14669 (2007). Article CAS PubMed PubMed Central Google Scholar * Townshend, R. J. et al. Geometric deep learning of RNA structure. _Science_ 373, 1047–1051 (2021). Article CAS
PubMed PubMed Central Google Scholar * Watkins, A. M., Rangan, R. & Das, R. FARFAR2: improved de novo rosetta prediction of complex global RNA folds. _Structure_ 28, 963–976.e966
(2020). Article CAS PubMed PubMed Central Google Scholar * Pan, X., Rijnbeek, P., Yan, J. & Shen, H.-B. Prediction of RNA–protein sequence and structure binding preferences using
deep convolutional and recurrent neural networks. _BMC Genomics_ 19, 511 (2018). Article PubMed PubMed Central Google Scholar * Sun, L. et al. Predicting dynamic cellular protein–RNA
interactions by deep learning using in vivo RNA structures. _Cell Res._ 31, 495–516 (2021). Article CAS PubMed PubMed Central Google Scholar * Xu, Y. et al. PrismNet: predicting
protein–RNA interaction using in vivo RNA structural information. _Nucleic Acids Res._ 51, W468–W477 (2023). Article CAS PubMed PubMed Central Google Scholar * Baek, M. et al. Accurate
prediction of protein–nucleic acid complexes using RoseTTAFoldNA. _Nat. Methods_ 21, 117–121 (2024). Article CAS PubMed Google Scholar * Sun, W., Ding, L. & Zhang, H. The potential
role of RNA structure in crop molecular breeding. _Front. Plant. Sci._ 13, 868771 (2022). Article PubMed PubMed Central Google Scholar * Xiang, Y. et al. Pervasive downstream RNA
hairpins dynamically dictate start-codon selection. _Nature_ 621, 423–430 (2023). Article CAS PubMed PubMed Central Google Scholar * Flamm, C. et al. Caveats to deep learning approaches
to RNA secondary structure prediction. _Front. Bioinform._ 2, 835422 (2022). Article PubMed PubMed Central Google Scholar * Szikszai, M., Wise, M., Datta, A., Ward, M. & Mathews, D.
H. Deep learning models for RNA secondary structure prediction (probably) do not generalize across families. _Bioinformatics_ 38, 3892–3899 (2022). Article CAS PubMed PubMed Central
Google Scholar * Wayment-Steele, H. K. et al. RNA secondary structure packages evaluated and improved by high-throughput experiments. _Nat. Methods_ 19, 1234–1242 (2022). Article CAS
PubMed PubMed Central Google Scholar * Gusarov, I. & Nudler, E. The mechanism of intrinsic transcription termination. _Mol. Cell_ 3, 495–504 (1999). Article CAS PubMed Google
Scholar * Zhang, J. & Landick, R. A two-way street: regulatory interplay between RNA polymerase and nascent RNA structure. _Trends Biochem. Sci._ 41, 293–310 (2016). Article CAS
PubMed PubMed Central Google Scholar * Perdrizet, G. A. et al. Transcriptional pausing coordinates folding of the aptamer domain and the expression platform of a riboswitch. _Proc. Natl
Acad. Sci. USA_ 109, 3323–3328 (2012). Article CAS PubMed PubMed Central Google Scholar * Steinert, H. et al. Pausing guides RNA folding to populate transiently stable RNA structures
for riboswitch-based transcription regulation. _eLife_ 6, e21297 (2017). Article PubMed PubMed Central Google Scholar * Turowski, T. W. et al. Nascent transcript folding plays a major
role in determining RNA polymerase elongation rates. _Mol. Cell_ 79, 488–503.e11 (2020). Article CAS PubMed PubMed Central Google Scholar * Long, Y., Wang, X., Youmans, D. T. &
Cech, T. R. How do lncRNAs regulate transcription? _Sci. Adv._ 3, eaao2110 (2017). Article PubMed PubMed Central Google Scholar * Yang, F. et al. Shape of promoter antisense RNAs
regulates ligand-induced transcription activation. _Nature_ 595, 444–449 (2021). Article CAS PubMed PubMed Central Google Scholar * Liang, L. et al. Complementary Alu sequences mediate
enhancer–promoter selectivity. _Nature_ 619, 868–875 (2023). Article CAS PubMed Google Scholar * Peterlin, B. M., Brogie, J. E. & Price, D. H. 7SK snRNA: a noncoding RNA that plays a
major role in regulating eukaryotic transcription. _Wiley Interdiscip. Rev. RNA_ 3, 92–103 (2012). Article CAS PubMed Google Scholar * AJ, C. Q., Bugai, A. & Barboric, M. Cracking
the control of RNA polymerase II elongation by 7SK snRNP and P-TEFb. _Nucleic Acids Res._ 44, 7527–7539 (2016). Article Google Scholar * Whittaker, C. & Dean, C. The FLC locus: a
platform for discoveries in epigenetics and adaptation. _Annu. Rev. Cell Dev. Biol._ 33, 555–575 (2017). Article CAS PubMed Google Scholar * Roca, X., Krainer, A. R. & Eperon, I. C.
Pick one, but be quick: 5′ splice sites and the problems of too many choices. _Genes Dev._ 27, 129–144 (2013). Article CAS PubMed PubMed Central Google Scholar * Watakabe, A., Inoue,
K., Sakamoto, H. & Shimura, Y. A secondary structure at the 3′ splice site affects the in vitro splicing reaction of mouse immunoglobulin mu chain pre-mRNAs. _Nucleic Acids Res._ 17,
8159–8169 (1989). Article CAS PubMed PubMed Central Google Scholar * Varani, L. et al. Structure of tau exon 10 splicing regulatory element RNA and destabilization by mutations of
frontotemporal dementia and parkinsonism linked to chromosome 17. _Proc. Natl Acad. Sci. USA_ 96, 8229–8234 (1999). Article CAS PubMed PubMed Central Google Scholar * Singh, N. N.,
Singh, R. N. & Androphy, E. J. Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. _Nucleic Acids Res._ 35, 371–389 (2007).
Article CAS PubMed Google Scholar * Warf, M. B. & Berglund, J. A. Role of RNA structure in regulating pre-mRNA splicing. _Trends Biochem. Sci._ 35, 169–178 (2010). Article CAS
PubMed Google Scholar * Rubtsov, P. Role of pre-mRNA secondary structures in the regulation of alternative splicing. _Mol. Biol._ 50, 823–830 (2016). Article CAS Google Scholar *
Kubodera, T. et al. Thiamine‐regulated gene expression of _Aspergillus oryzae_ thiA requires splicing of the intron containing a riboswitch‐like domain in the 5′‐UTR. _FEBS Lett._ 555,
516–520 (2003). Article CAS PubMed Google Scholar * Cheah, M. T., Wachter, A., Sudarsan, N. & Breaker, R. R. Control of alternative RNA splicing and gene expression by eukaryotic
riboswitches. _Nature_ 447, 497–500 (2007). Article CAS PubMed Google Scholar * Wachter, A. et al. Riboswitch control of gene expression in plants by splicing and alternative 3′ end
processing of mRNAs. _Plant. Cell_ 19, 3437–3450 (2007). Article CAS PubMed PubMed Central Google Scholar * Warf, M. B., Diegel, J. V., von Hippel, P. H. & Berglund, J. A. The
protein factors MBNL1 and U2AF65 bind alternative RNA structures to regulate splicing. _Proc. Natl Acad. Sci. USA_ 106, 9203–9208 (2009). Article CAS PubMed PubMed Central Google Scholar
* Muro, A. F. et al. Regulation of fibronectin EDA exon alternative splicing: possible role of RNA secondary structure for enhancer display. _Mol. Cell. Biol._ 19, 2657–2671 (1999).
Article CAS PubMed PubMed Central Google Scholar * Buratti, E. et al. RNA folding affects the recruitment of SR proteins by mouse and human polypurinic enhancer elements in the
fibronectin EDA exon. _Mol. Cell. Biol._ 24, 1387–1400 (2004). Article CAS PubMed PubMed Central Google Scholar * McManus, C. J. & Graveley, B. R. RNA structure and the mechanisms
of alternative splicing. _Curr. Opin. Genet. Dev._ 21, 373–379 (2011). Article CAS PubMed PubMed Central Google Scholar * Lin, C. L., Taggart, A. J. & Fairbrother, W. G. RNA
structure in splicing: an evolutionary perspective. _RNA Biol._ 13, 766–771 (2016). Article PubMed PubMed Central Google Scholar * Graveley, B. R. Mutually exclusive splicing of the
insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. _Cell_ 123, 65–73 (2005). Article CAS PubMed PubMed Central Google Scholar * Anastassiou, D., Liu, H.
& Varadan, V. Variable window binding for mutually exclusive alternative splicing. _Genome Biol._ 7, 1–12 (2006). Article Google Scholar * Xu, B., Meng, Y. & Jin, Y. RNA structures
in alternative splicing and back-splicing. _Wiley Interdiscip. Rev. RNA_ 12, e1626 (2021). Article CAS PubMed Google Scholar * Lovci, M. T. et al. Rbfox proteins regulate alternative
mRNA splicing through evolutionarily conserved RNA bridges. _Nat. Struct. Mol. Biol._ 20, 1434–1442 (2013). Article CAS PubMed PubMed Central Google Scholar * Woodson, S. A., Panja, S.
& Santiago-Frangos, A. Proteins that chaperone RNA regulation. _Microbiol. Spectr_. 6 https://doi.org/10.1128/microbiolspec.RWR-0026-2018 (2018). * Wu, J. Y. & Maniatis, T. Specific
interactions between proteins implicated in splice site selection and regulated alternative splicing. _Cell_ 75, 1061–1070 (1993). Article CAS PubMed Google Scholar * Kalmykova, S. et
al. Conserved long-range base pairings are associated with pre-mRNA processing of human genes. _Nat. Commun._ 12, 2300 (2021). Article CAS PubMed PubMed Central Google Scholar * Zhang,
Y. et al. The biogenesis of nascent circular RNAs. _Cell Rep._ 15, 611–624 (2016). Article CAS PubMed Google Scholar * Zhang, X. O. et al. Complementary sequence-mediated exon
circularization. _Cell_ 159, 134–147 (2014). Article CAS PubMed Google Scholar * Johansson, J. et al. An RNA thermosensor controls expression of virulence genes in _Listeria
monocytogenes_. _Cell_ 110, 551–561 (2002). Article PubMed Google Scholar * Brito Querido, J., Diaz-Lopez, I. & Ramakrishnan, V. The molecular basis of translation initiation and its
regulation in eukaryotes. _Nat. Rev. Mol. Cell Biol._ 25, 168–186 (2024). Article CAS PubMed Google Scholar * Leppek, K., Das, R. & Barna, M. Functional 5′ UTR mRNA structures in
eukaryotic translation regulation and how to find them. _Nat. Rev. Mol. Cell Biol._ 19, 158–174 (2018). Article CAS PubMed Google Scholar * Spitale, R. C. et al. Structural imprints in
vivo decode RNA regulatory mechanisms. _Nature_ 519, 486–490 (2015). Article CAS PubMed PubMed Central Google Scholar * Waldron, J. A. et al. mRNA structural elements immediately
upstream of the start codon dictate dependence upon eIF4A helicase activity. _Genome Biol._ 20, 300 (2019). Article CAS PubMed PubMed Central Google Scholar * Wang, J. et al. Rapid 40S
scanning and its regulation by mRNA structure during eukaryotic translation initiation. _Cell_ 185, 4474–4487.e17 (2022). Article CAS PubMed PubMed Central Google Scholar * Zhang, H.,
Wang, Y. & Lu, J. Function and evolution of upstream ORFs in eukaryotes. _Trends Biochem. Sci._ 44, 782–794 (2019). Article CAS PubMed Google Scholar * Corley, M. et al. An RNA
structure-mediated, posttranscriptional model of human ɑ-1-antitrypsin expression. _Proc. Natl Acad. Sci. USA_ 114, E10244–E10253 (2017). Article CAS PubMed PubMed Central Google Scholar
* Jankowsky, E. & Guenther, U. P. A helicase links upstream ORFs and RNA structure. _Curr. Genet._ 65, 453–456 (2019). Article CAS PubMed Google Scholar * Lyu, K. et al. An RNA
G-quadruplex structure within the ADAR 5′UTR interacts with DHX36 helicase to regulate translation. _Angew. Chem. Int. Ed. Engl._ 61, e202203553 (2022). Article CAS PubMed Google Scholar
* Kwok, C. K., Ding, Y., Shahid, S., Assmann, S. M. & Bevilacqua, P. C. A stable RNA G-quadruplex within the 5′-UTR of _Arabidopsis thaliana_ ATR mRNA inhibits translation. _Biochem.
J._ 467, 91–102 (2015). Article CAS PubMed Google Scholar * Cho, H. et al. Translational control of phloem development by RNA G-quadruplex-JULGI determines plant sink strength. _Nat.
Plants_ 4, 376–390 (2018). Article CAS PubMed Google Scholar * Kikinis, Z., Eisenstein, R. S., Bettany, A. J. & Munro, H. N. Role of RNA secondary structure of the iron-responsive
element in translational regulation of ferritin synthesis. _Nucleic Acids Res._ 23, 4190–4195 (1995). Article CAS PubMed PubMed Central Google Scholar * Zhou, Z. D. & Tan, E. K.
Iron regulatory protein (IRP)–iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. _Mol. Neurodegener._ 12, 75 (2017). Article PubMed PubMed Central Google
Scholar * Pestova, T. V., Shatsky, I. N., Fletcher, S. P., Jackson, R. J. & Hellen, C. U. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon
during internal translation initiation of hepatitis C and classical swine fever virus RNAs. _Genes Dev._ 12, 67–83 (1998). Article CAS PubMed PubMed Central Google Scholar * Kieft, J.
S., Zhou, K., Jubin, R. & Doudna, J. A. Mechanism of ribosome recruitment by hepatitis C IRES RNA. _RNA_ 7, 194–206 (2001). Article CAS PubMed PubMed Central Google Scholar * Otto,
G. A. & Puglisi, J. D. The pathway of HCV IRES-mediated translation initiation. _Cell_ 119, 369–380 (2004). Article CAS PubMed Google Scholar * Weingarten-Gabbay, S. et al.
Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. _Science_ 351, aad4939 (2016). Article PubMed Google Scholar * Beaudoin, J.
D. et al. Analyses of mRNA structure dynamics identify embryonic gene regulatory programs. _Nat. Struct. Mol. Biol._ 25, 677–686 (2018). Article CAS PubMed PubMed Central Google Scholar
* Mustoe, A. M. et al. Pervasive regulatory functions of mRNA structure revealed by high-resolution SHAPE probing. _Cell_ 173, 181–195.e18 (2018). Article CAS PubMed PubMed Central
Google Scholar * Farabaugh, P. J. Programmed translational frameshifting. _Microbiol. Rev._ 60, 103–134 (1996). Article CAS PubMed PubMed Central Google Scholar * Kudla, G., Murray, A.
W., Tollervey, D. & Plotkin, J. B. Coding-sequence determinants of gene expression in _Escherichia coli_. _Science_ 324, 255–258 (2009). Article CAS PubMed PubMed Central Google
Scholar * Goodman, D. B., Church, G. M. & Kosuri, S. Causes and effects of N-terminal codon bias in bacterial genes. _Science_ 342, 475–479 (2013). Article CAS PubMed Google Scholar
* Caliskan, N., Katunin, V. I., Belardinelli, R., Peske, F. & Rodnina, M. V. Programmed –1 frameshifting by kinetic partitioning during impeded translocation. _Cell_ 157, 1619–1631
(2014). Article CAS PubMed PubMed Central Google Scholar * Caliskan, N., Peske, F. & Rodnina, M. V. Changed in translation: mRNA recoding by −1 programmed ribosomal frameshifting.
_Trends Biochem. Sci._ 40, 265–274 (2015). Article CAS PubMed PubMed Central Google Scholar * Jungfleisch, J. et al. A novel translational control mechanism involving RNA structures
within coding sequences. _Genome Res._ 27, 95–106 (2017). Article CAS PubMed PubMed Central Google Scholar * Mao, Y. et al. m6A in mRNA coding regions promotes translation via the RNA
helicase-containing YTHDC2. _Nat. Commun._ 10, 5332 (2019). Article PubMed PubMed Central Google Scholar * Yang, X. et al. RNA G-quadruplex structures exist and function in vivo in
plants. _Genome Biol._ 21, 226 (2020). Article CAS PubMed PubMed Central Google Scholar * Arif, A. et al. The GAIT translational control system. _WIREs RNA_ 9, e1441 (2018). Article
PubMed Google Scholar * Chaudhury, A. et al. TGF-β-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. _Nat. Cell Biol._ 12,
286–293 (2010). Article CAS PubMed PubMed Central Google Scholar * Hussey, GeorgeS. et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation
step. _Mol. Cell_ 41, 419–431 (2011). Article CAS PubMed PubMed Central Google Scholar * Brown, J. A. et al. Structural insights into the stabilization of MALAT1 noncoding RNA by a
bipartite triple helix. _Nat. Struct. Mol. Biol._ 21, 633–640 (2014). Article CAS PubMed PubMed Central Google Scholar * Brown, J. A. Unraveling the structure and biological functions
of RNA triple helices. _Wiley Interdiscip. Rev. RNA_ 11, e1598 (2020). Article CAS PubMed PubMed Central Google Scholar * Wan, Y. et al. Genome-wide measurement of RNA folding energies.
_Mol. Cell_ 48, 169–181 (2012). Article PubMed PubMed Central Google Scholar * Yang, X. et al. RNA G-quadruplex structure contributes to cold adaptation in plants. _Nat. Commun._ 13,
6224 (2022). Article CAS PubMed PubMed Central Google Scholar * Kharel, P. et al. Stress promotes RNA G-quadruplex folding in human cells. _Nat. Commun._ 14, 205 (2023). Article CAS
PubMed PubMed Central Google Scholar * Marzluff, W. F., Wagner, E. J. & Duronio, R. J. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. _Nat. Rev.
Genet._ 9, 843–854 (2008). Article CAS PubMed PubMed Central Google Scholar * Fischer, J. W., Busa, V. F., Shao, Y. & Leung, A. K. L. Structure-mediated RNA decay by UPF1 and G3BP1.
_Mol. Cell_ 78, 70–84.e6 (2020). Article CAS PubMed PubMed Central Google Scholar * Meisner, N.-C. et al. mRNA openers and closers: modulating AU-rich element-controlled mRNA stability
by a molecular switch in mRNA secondary structure. _ChemBioChem_ 5, 1432–1447 (2004). Article CAS PubMed Google Scholar * Carthew, R. W. & Sontheimer, E. J. Origins and mechanisms
of miRNAs and siRNAs. _Cell_ 136, 642–655 (2009). Article CAS PubMed PubMed Central Google Scholar * Yadav, D. K. et al. Staufen1 reads out structure and sequence features in ARF1 dsRNA
for target recognition. _Nucleic Acids Res._ 48, 2091–2106 (2019). Article PubMed Central Google Scholar * Mino, T. et al. Regnase-1 and roquin regulate a common element in inflammatory
mRNAs by spatiotemporally distinct mechanisms. _Cell_ 161, 1058–1073 (2015). Article CAS PubMed Google Scholar * Leppek, K. et al. Roquin promotes constitutive mRNA decay via a conserved
class of stem-loop recognition motifs. _Cell_ 153, 869–881 (2013). Article CAS PubMed Google Scholar * Binas, O. et al. Structural basis for the recognition of transiently structured
AU-rich elements by Roquin. _Nucleic Acids Res._ 48, 7385–7403 (2020). CAS PubMed PubMed Central Google Scholar * Shi, B. et al. RNA structural dynamics regulate early embryogenesis
through controlling transcriptome fate and function. _Genome Biol._ 21, 120 (2020). Article CAS PubMed PubMed Central Google Scholar * Mauger, D. M. et al. mRNA structure regulates
protein expression through changes in functional half-life. _Proc. Natl Acad. Sci. USA_ 116, 24075–24083 (2019). Article CAS PubMed PubMed Central Google Scholar * Gonzalez, I.,
Buonomo, S. B., Nasmyth, K. & von Ahsen, U. ASH1 mRNA localization in yeast involves multiple secondary structural elements and Ash1 protein translation. _Curr. Biol._ 9, 337–340 (1999).
Article CAS PubMed Google Scholar * Macdonald, P. M., Kerr, K., Smith, J. L. & Leask, A. RNA regulatory element BLE1 directs the early steps of bicoid mRNA localization.
_Development_ 118, 1233–1243 (1993). Article CAS PubMed Google Scholar * St Johnston, D., Beuchle, D. & Nusslein-Volhard, C. Staufen, a gene required to localize maternal RNAs in the
_Drosophila_ egg. _Cell_ 66, 51–63 (1991). Article Google Scholar * Ferrandon, D., Elphick, L., Nusslein-Volhard, C. & St Johnston, D. Staufen protein associates with the 3′UTR of
bicoid mRNA to form particles that move in a microtubule-dependent manner. _Cell_ 79, 1221–1232 (1994). Article CAS PubMed Google Scholar * Bergsten, S. E., Huang, T., Chatterjee, S.
& Gavis, E. R. Recognition and long-range interactions of a minimal nanos RNA localization signal element. _Development_ 128, 427–435 (2001). Article CAS PubMed Google Scholar *
Kim-Ha, J., Webster, P. J., Smith, J. L. & Macdonald, P. M. Multiple RNA regulatory elements mediate distinct steps in localization of oskar mRNA. _Development_ 119, 169–178 (1993).
Article CAS PubMed Google Scholar * Van De Bor, V., Hartswood, E., Jones, C., Finnegan, D. & Davis, I. gurken and the I factor retrotransposon RNAs share common localization signals
and machinery. _Dev. Cell_ 9, 51–62 (2005). Article PubMed Google Scholar * Bullock, S. L., Ringel, I., Ish-Horowicz, D. & Lukavsky, P. J. A′-form RNA helices are required for
cytoplasmic mRNA transport in _Drosophila_. _Nat. Struct. Mol. Biol._ 17, 703–709 (2010). Article CAS PubMed PubMed Central Google Scholar * Chao, J. A. et al. ZBP1 recognition of
β-actin zipcode induces RNA looping. _Genes Dev._ 24, 148–158 (2010). Article CAS PubMed PubMed Central Google Scholar * Patel, V. L. et al. Spatial arrangement of an RNA zipcode
identifies mRNAs under post-transcriptional control. _Genes Dev._ 26, 43–53 (2012). Article CAS PubMed PubMed Central Google Scholar * Fernandez-Moya, S. M. et al. _RGS4_ RNA secondary
structure mediates Staufen2 RNP assembly in neurons. _Int. J. Mol. Sci._ 22, 13021 (2021). Article CAS PubMed PubMed Central Google Scholar * Wang, T. et al. RNA motifs and modification
involve in RNA long-distance transport in plants. _Front. Cell Dev. Biol._ 9, 651278 (2021). Article PubMed PubMed Central Google Scholar * Zhang, W. et al. tRNA-related sequences
trigger systemic mRNA transport in plants. _Plant Cell_ 28, 1237–1249 (2016). Article CAS PubMed PubMed Central Google Scholar * Fernandes, J., Jayaraman, B. & Frankel, A. The HIV-1
Rev response element: an RNA scaffold that directs the cooperative assembly of a homo-oligomeric ribonucleoprotein complex. _RNA Biol._ 9, 6–11 (2012). Article CAS PubMed PubMed Central
Google Scholar * Malim, M. H., Hauber, J., Le, S. Y., Maizel, J. V. & Cullen, B. R. The HIV-1 rev _trans_-activator acts through a structured target sequence to activate nuclear
export of unspliced viral mRNA. _Nature_ 338, 254–257 (1989). Article CAS PubMed Google Scholar * Pasquinelli, A. E. et al. The constitutive transport element (CTE) of Mason–Pfizer
monkey virus (MPMV) accesses a cellular mRNA export pathway. _EMBO J._ 16, 7500–7510 (1997). Article CAS PubMed PubMed Central Google Scholar * Gruter, P. et al. TAP, the human homolog
of Mex67p, mediates CTE-dependent RNA export from the nucleus. _Mol. Cell_ 1, 649–659 (1998). Article CAS PubMed Google Scholar * Aibara, S., Katahira, J., Valkov, E. & Stewart, M.
The principal mRNA nuclear export factor NXF1:NXT1 forms a symmetric binding platform that facilitates export of retroviral CTE-RNA. _Nucleic Acids Res._ 43, 1883–1893 (2015). Article CAS
PubMed PubMed Central Google Scholar * Van Treeck, B. et al. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. _Proc. Natl Acad.
Sci. USA_ 115, 2734–2739 (2018). Article PubMed PubMed Central Google Scholar * Poudyal, R. R., Sieg, J. P., Portz, B., Keating, C. D. & Bevilacqua, P. C. RNA sequence and structure
control assembly and function of RNA condensates. _RNA_ 27, 1589–1601 (2021). Article CAS PubMed PubMed Central Google Scholar * Jain, A. & Vale, R. D. RNA phase transitions in
repeat expansion disorders. _Nature_ 546, 243–247 (2017). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y. et al. G-quadruplex structures trigger RNA phase separation.
_Nucleic Acids Res._ 47, 11746–11754 (2019). CAS PubMed PubMed Central Google Scholar * Langdon, E. M. & Gladfelter, A. S. A new lens for RNA localization: liquid–liquid phase
separation. _Annu. Rev. Microbiol._ 72, 255–271 (2018). Article CAS PubMed Google Scholar * Roden, ChristineA. et al. Double-stranded RNA drives SARS-CoV-2 nucleocapsid protein to
undergo phase separation at specific temperatures. _Nucleic Acids Res._ 50, 8168–8192 (2022). Article CAS PubMed PubMed Central Google Scholar * Clemson, C. M. et al. An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. _Mol. Cell_ 33, 717–726 (2009). Article CAS PubMed PubMed Central Google Scholar * Yamazaki,
T. et al. Functional domains of NEAT1 architectural lncRNA induce paraspeckle assembly through phase separation. _Mol. Cell_ 70, 1038–1053.e7 (2018). Article CAS PubMed Google Scholar *
Asamitsu, S. et al. RNA G-quadruplex organizes stress granule assembly through DNAPTP6 in neurons. _Sci. Adv._ 9, eade2035 (2023). Article CAS PubMed PubMed Central Google Scholar *
Mimura, M. et al. Quadruplex folding promotes the condensation of linker histones and DNAs via liquid–liquid phase separation. _J. Am. Chem. Soc._ 143, 9849–9857 (2021). Article CAS PubMed
Google Scholar * Warner, K. D., Hajdin, C. E. & Weeks, K. M. Principles for targeting RNA with drug-like small molecules. _Nat. Rev. Drug Discov._ 17, 547–558 (2018). Article CAS
PubMed PubMed Central Google Scholar * Childs-Disney, J. L. et al. Targeting RNA structures with small molecules. _Nat. Rev. Drug Discov._ 21, 736–762 (2022). Article CAS PubMed PubMed
Central Google Scholar * Abulwerdi, F. A. et al. Development of small molecules with a noncanonical binding mode to HIV-1 trans activation response (TAR) RNA. _J. Med. Chem._ 59,
11148–11160 (2016). Article CAS PubMed PubMed Central Google Scholar * Prado, S. et al. A small-molecule inhibitor of HIV-1 Rev function detected by a diversity screen based on RRE–Rev
interference. _Biochem. Pharmacol._ 156, 68–77 (2018). Article CAS PubMed Google Scholar * Howe, J. A. et al. Selective small-molecule inhibition of an RNA structural element. _Nature_
526, 672–677 (2015). Article CAS PubMed Google Scholar * Blount Kenneth, F. et al. Novel riboswitch-binding flavin analog that protects mice against _Clostridium difficile_ infection
without inhibiting cecal flora. _Antimicrob. Agents Chemother._ 59, 5736–5746 (2015). Article CAS PubMed PubMed Central Google Scholar * Balaratnam, S. et al. Investigating the NRAS 5′
UTR as a target for small molecules. _Cell Chem. Biol._ 30, 643–657.e8 (2023). Article CAS PubMed Google Scholar * Aguilar, R. et al. Targeting Xist with compounds that disrupt RNA
structure and X inactivation. _Nature_ 604, 160–166 (2022). Article CAS PubMed Google Scholar * Dhillon, S. Risdiplam: first approval. _Drugs_ 80, 1853–1858 (2020). Article CAS PubMed
Google Scholar * Naryshkin, N. A. et al. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. _Science_ 345, 688–693 (2014). Article CAS
PubMed Google Scholar * Ratni, H. et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA).
_J. Med. Chem._ 61, 6501–6517 (2018). Article CAS PubMed Google Scholar * Sivaramakrishnan, M. et al. Binding to SMN2 pre-mRNA–protein complex elicits specificity for small molecule
splicing modifiers. _Nat. Commun._ 8, 1476 (2017). Article PubMed PubMed Central Google Scholar * Campagne, S. et al. Structural basis of a small molecule targeting RNA for a specific
splicing correction. _Nat. Chem. Biol._ 15, 1191–1198 (2019). Article CAS PubMed Google Scholar * Wang, J., Schultz, P. G. & Johnson, K. A. Mechanistic studies of a small-molecule
modulator of SMN2 splicing. _Proc. Natl Acad. Sci. USA_ 115, E4604–E4612 (2018). CAS PubMed PubMed Central Google Scholar * Velagapudi, S. P., Gallo, S. M. & Disney, M. D.
Sequence-based design of bioactive small molecules that target precursor microRNAs. _Nat. Chem. Biol._ 10, 291–297 (2014). Article CAS PubMed PubMed Central Google Scholar * Costales,
M. G. et al. A designed small molecule inhibitor of a non-coding RNA sensitizes HER2 negative cancers to herceptin. _J. Am. Chem. Soc._ 141, 2960–2974 (2019). Article CAS PubMed PubMed
Central Google Scholar * Fang, L. et al. Pervasive transcriptome interactions of protein-targeted drugs. _Nat. Chem._ 15, 1374–1383 (2023). Article CAS PubMed Google Scholar *
Costales, M. G., Matsumoto, Y., Velagapudi, S. P. & Disney, M. D. Small molecule targeted recruitment of a nuclease to RNA. _J. Am. Chem. Soc._ 140, 6741–6744 (2018). Article CAS
PubMed PubMed Central Google Scholar * Costales, M. G. et al. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. _Proc.
Natl Acad. Sci. USA_ 117, 2406–2411 (2020). Article CAS PubMed PubMed Central Google Scholar * Tong, Y. et al. Programming inactive RNA-binding small molecules into bioactive degraders.
_Nature_ 618, 169–179 (2023). Article CAS PubMed PubMed Central Google Scholar * McCown, P. J., Corbino, K. A., Stav, S., Sherlock, M. E. & Breaker, R. R. Riboswitch diversity and
distribution. _RNA_ 23, 995–1011 (2017). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the UK Biotechnology and
Biological Sciences Research Council (BBSRC) (BB/X01102X/1) and European Research Council (ERC) (selected by the ERC, funded by BBSRC Horizon Europe Guarantee (EP/Y009886/1)) (Y.D. and
Y.Z.). Y.W. and X.C. are supported by funding from A*STAR, the National Research Foundation of Singapore, the EMBO Young Investigator Programme and a CIFAR Azrieli global scholar fellowship.
AUTHOR INFORMATION Author notes * These authors contributed equally: Xinang Cao, Yueying Zhang. AUTHORS AND AFFILIATIONS * Stem Cell and Regenerative Biology, Genome Institute of Singapore,
Singapore, Singapore Xinang Cao & Yue Wan * Department of Cell and Developmental Biology, John Innes Centre, Norwich, UK Yueying Zhang & Yiliang Ding * Department of Biochemistry,
Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore Yue Wan Authors * Xinang Cao View author publications You can also search for this author inPubMed
Google Scholar * Yueying Zhang View author publications You can also search for this author inPubMed Google Scholar * Yiliang Ding View author publications You can also search for this
author inPubMed Google Scholar * Yue Wan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The authors contributed equally to all aspects of
the article. CORRESPONDING AUTHORS Correspondence to Yiliang Ding or Yue Wan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW
INFORMATION _Nature Reviews Molecular Cell Biology_ thanks Tetsuro Hirose, Yuanchao Xue and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION GLOSSARY * Aptamer domain An RNA structure in a riboswitch that binds to small molecules. * Enzymatic structure probing Refers to the use of nucleases that cleave
RNA selectively at single-stranded or double-stranded regions (for example, RNase T1 and RNase V1, respectively); the resulting digestion footprints of the RNA can chart its structure. *
i-Motifs Cytosine-rich DNAs that form quadruplex structures; also known as intercalated-motif DNAs. * Riboswitches Highly folded segments of (mostly bacterial) mRNAs that, when bound by
environmental small molecules, induce structure changes that regulate the transcription or translation of the mRNA. * R-loop structure A three-stranded nucleic acid structure composed of a
DNA–RNA hybrid and a displaced single strand of DNA. * Small nucleolar RNAs A class of small non-coding RNAs (ncRNAs) that mostly reside in nucleoli, which guide chemical modifications of
other RNA species such as ribosomal RNAs. * Stress granules Dynamic cytoplasmic bodies formed in response to cellular stress, comprising RNA molecules and various proteins; they have a role
in RNA metabolism and are associated with responses to environmental stresses. * Upstream open reading frames (uORFs). Open reading frames (ORFs) located upstream of a main open reading
frame, that is, within the 5′ untranslated region (UTR) of the mRNA. uORFs can encode small peptides, and can regulate the translation of the main ORF by competing for the translation
machinery. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or
other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cao, X., Zhang, Y., Ding, Y. _et al._ Identification of RNA structures and their roles in RNA functions. _Nat Rev Mol Cell Biol_ 25, 784–801
(2024). https://doi.org/10.1038/s41580-024-00748-6 Download citation * Accepted: 28 May 2024 * Published: 26 June 2024 * Issue Date: October 2024 * DOI:
https://doi.org/10.1038/s41580-024-00748-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
