Tau-targeting therapies for alzheimer disease
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

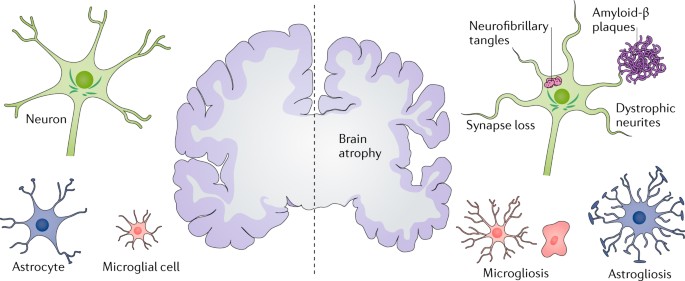
ABSTRACT Alzheimer disease (AD) is the most common form of dementia. Pathologically, AD is characterized by amyloid plaques and neurofibrillary tangles in the brain, with associated loss of
synapses and neurons, resulting in cognitive deficits and eventually dementia. Amyloid-β (Aβ) peptide and tau protein are the primary components of the plaques and tangles, respectively. In
the decades since Aβ and tau were identified, development of therapies for AD has primarily focused on Aβ, but tau has received more attention in recent years, in part because of the failure
of various Aβ-targeting treatments in clinical trials. In this article, we review the current status of tau-targeting therapies for AD. Initially, potential anti-tau therapies were based
mainly on inhibition of kinases or tau aggregation, or on stabilization of microtubules, but most of these approaches have been discontinued because of toxicity and/or lack of efficacy.
Currently, the majority of tau-targeting therapies in clinical trials are immunotherapies, which have shown promise in numerous preclinical studies. Given that tau pathology correlates
better with cognitive impairments than do Aβ lesions, targeting of tau is expected to be more effective than Aβ clearance once the clinical symptoms are evident. With future improvements in
diagnostics, these two hallmarks of the disease might be targeted prophylactically. KEY POINTS * Therapies for Alzheimer disease in clinical trials are gradually shifting from amyloid-β
(Aβ)-targeting to tau-targeting approaches. * Early anti-tau therapies were based mainly on inhibition of kinases or tau aggregation, or on stabilization of microtubules, but most of these
approaches have been discontinued because of toxicity and/or lack of efficacy. * Most of the tau-targeting approaches that are currently in clinical trials are immunotherapies. * Tau is
likely to be a better target than Aβ once cognitive deficits manifest because the tau burden correlates better with clinical impairments than does the Aβ burden. * Eventually, with continued
improvements in diagnostics, both Aβ and tau are likely to be targeted prophylactically for clearance. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TAU-TARGETING THERAPIES FOR ALZHEIMER DISEASE: CURRENT STATUS AND
FUTURE DIRECTIONS Article 24 October 2023 THE AMYLOID HYPOTHESIS IN ALZHEIMER DISEASE: NEW INSIGHTS FROM NEW THERAPEUTICS Article 17 February 2022 AMYLOID Β-BASED THERAPY FOR ALZHEIMER’S
DISEASE: CHALLENGES, SUCCESSES AND FUTURE Article Open access 30 June 2023 REFERENCES * Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. _Alzheimers Dement._ 12, 459–509
(2016). Article Google Scholar * Dixit, R., Ross, J. L., Goldman, Y. E. & Holzbaur, E. L. Differential regulation of dynein and kinesin motor proteins by tau. _Science_ 319, 1086–1089
(2008). Article PubMed PubMed Central CAS Google Scholar * Vershinin, M., Carter, B. C., Razafsky, D. S., King, S. J. & Gross, S. P. Multiple-motor based transport and its
regulation by Tau. _Proc. Natl Acad. Sci. USA_ 104, 87–92 (2007). Article PubMed CAS Google Scholar * Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H. & Del Tredici, K.
Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. _Acta Neuropathol._ 112, 389–404 (2006). Article PubMed PubMed Central
Google Scholar * Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. _J. Neuropathol.
Exp. Neurol._ 70, 960–969 (2011). Article PubMed CAS Google Scholar * Wharton, S. B. et al. Epidemiological pathology of Tau in the ageing brain: application of staging for neuropil
threads (BrainNet Europe protocol) to the MRC cognitive function and ageing brain study. _Acta Neuropathol. Commun._ 4, 11 (2016). Article PubMed PubMed Central CAS Google Scholar *
Grundke-Iqbal, I. et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. _Proc. Natl Acad. Sci. USA_ 83, 4913–4917 (1986).
Article PubMed PubMed Central CAS Google Scholar * Min, S. W. et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. _Neuron_ 67, 953–966 (2010). Article
PubMed PubMed Central CAS Google Scholar * Wang, J. Z., Grundke-Iqbal, I. & Iqbal, K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification
in Alzheimer’s disease. _Nat. Med._ 2, 871–875 (1996). Article PubMed CAS Google Scholar * Mena, R., Edwards, P. C., Harrington, C. R., Mukaetova-Ladinska, E. B. & Wischik, C. M.
Staging the pathological assembly of truncated tau protein into paired helical filaments in Alzheimer’s disease. _Acta Neuropathol._ 91, 633–641 (1996). Article PubMed CAS Google Scholar
* Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. _Acta Neuropathol._ 82, 239–259 (1991). Article PubMed CAS Google Scholar * Braak, H. & Braak,
E. Frequency of stages of Alzheimer-related lesions in different age categories. _Neurobiol. Aging_ 18, 351–357 (1997). Article PubMed CAS Google Scholar * Murray, M. E. et al.
Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. _Lancet Neurol._ 10, 785–796 (2011). Article PubMed PubMed
Central Google Scholar * Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. _Acta Neuropathol._ 128, 755–766 (2014). Article PubMed
PubMed Central CAS Google Scholar * Grundke-Iqbal, I. et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. _J. Biol. Chem._ 261, 6084–6089
(1986). PubMed CAS Google Scholar * Kidd, M. Paired helical filaments in electron microscopy of Alzheimer’s disease. _Nature_ 197, 192–193 (1963). Article PubMed CAS Google Scholar *
Meraz-Rios, M. A., Lira-De Leon, K. I., Campos-Pena, V., De Anda-Hernandez, M. A. & Mena-Lopez, R. Tau oligomers and aggregation in Alzheimer’s disease. _J. Neurochem._ 112, 1353–1367
(2010). Article PubMed CAS Google Scholar * Arima, K. Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in
tauopathies. _Neuropathology_ 26, 475–483 (2006). Article PubMed Google Scholar * Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. Multiple isoforms of
human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. _Neuron_ 3, 519–526 (1989). Article PubMed CAS Google Scholar *
Himmler, A., Drechsel, D., Kirschner, M. W. & Martin, D. W. Jr. Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains.
_Mol. Cell. Biol._ 9, 1381–1388 (1989). Article PubMed PubMed Central CAS Google Scholar * Noble, W., Hanger, D. P., Miller, C. C. & Lovestone, S. The importance of tau
phosphorylation for neurodegenerative diseases. _Front. Neurol._ 4, 83 (2013). Article PubMed PubMed Central CAS Google Scholar * Lindwall, G. & Cole, R. D. Phosphorylation affects
the ability of tau protein to promote microtubule assembly. _J. Biol. Chem._ 259, 5301–5305 (1984). PubMed CAS Google Scholar * Luna-Muñoz, J., Chávez-Macías, L., García-Sierra, F. &
Mena, R. Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. _J. Alzheimers Dis._ 12,
365–375 (2007). Article PubMed Google Scholar * Augustinack, J. C., Schneider, A., Mandelkow, E. M. & Hyman, B. T. Specific tau phosphorylation sites correlate with severity of
neuronal cytopathology in Alzheimer’s disease. _Acta Neuropathol._ 103, 26–35 (2002). Article PubMed CAS Google Scholar * Hanger, D. P. & Wray, S. Tau cleavage and tau aggregation in
neurodegenerative disease. _Biochem. Soc. Trans._ 38, 1016–1020 (2010). Article PubMed CAS Google Scholar * Kimura, T. et al. Sequential changes of tau-site-specific phosphorylation
during development of paired helical filaments. _Dementia_ 7, 177–181 (1996). PubMed CAS Google Scholar * Yoshida, H. & Goedert, M. Sequential phosphorylation of tau protein by
cAMP-dependent protein kinase and SAPK4/p38δ or JNK2 in the presence of heparin generates the AT100 epitope. _J. Neurochem._ 99, 154–164 (2006). Article PubMed CAS Google Scholar *
Shukla, V., Skuntz, S. & Pant, H. C. Deregulated Cdk5 activity is involved in inducing Alzheimer’s disease. _Arch. Med. Res._ 43, 655–662 (2012). Article PubMed PubMed Central CAS
Google Scholar * Tell, V. & Hilgeroth, A. Recent developments of protein kinase inhibitors as potential AD therapeutics. _Front. Cell. Neurosci._ 7, 189 (2013). Article PubMed PubMed
Central CAS Google Scholar * Yarza, R., Vela, S., Solas, M. & Ramirez, M. J. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. _Front.
Pharmacol._ 6, 321 (2015). PubMed Google Scholar * Liu, S. L. et al. The role of Cdk5 in Alzheimer’s disease. _Mol. Neurobiol._ 53, 4328–4342 (2016). Article PubMed CAS Google Scholar
* Wilkaniec, A., Czapski, G. A. & Adamczyk, A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. _J. Neurochem._ 136, 222–233 (2016).
Article PubMed CAS Google Scholar * Liu, F., Grundke-Iqbal, I., Iqbal, K. & Gong, C. X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau
phosphorylation. _Eur. J. Neurosci._ 22, 1942–1950 (2005). Article PubMed Google Scholar * Sontag, J. M. & Sontag, E. Protein phosphatase 2A dysfunction in Alzheimer’s disease.
_Front. Mol. Neurosci._ 7, 16 (2014). Article PubMed PubMed Central CAS Google Scholar * Wang, Y. & Mandelkow, E. Tau in physiology and pathology. _Nat. Rev. Neurosci._ 17, 5–21
(2016). Article PubMed CAS Google Scholar * Cotman, C. W., Poon, W. W., Rissman, R. A. & Blurton-Jones, M. The role of caspase cleavage of tau in Alzheimer disease neuropathology.
_J. Neuropathol. Exp. Neurol._ 64, 104–112 (2005). Article PubMed CAS Google Scholar * Guo, T., Noble, W. & Hanger, D. P. Roles of tau protein in health and disease. _Acta
Neuropathol._ 133, 665–704 (2017). Article PubMed PubMed Central CAS Google Scholar * Zhao, X. et al. Caspase-2 cleavage of tau reversibly impairs memory. _Nat. Med._ 22, 1268–1276
(2016). Article PubMed CAS Google Scholar * Jadhav, S. et al. Truncated tau deregulates synaptic markers in rat model for human tauopathy. _Front. Cell. Neurosci._ 9, 24 (2015). Article
PubMed PubMed Central CAS Google Scholar * Liu, F. et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. _Brain_ 132, 1820–1832
(2009). Article PubMed PubMed Central Google Scholar * Cash, A. D. et al. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. _Am. J.
Pathol._ 162, 1623–1627 (2003). Article PubMed PubMed Central CAS Google Scholar * Hempen, B. & Brion, J. P. Reduction of acetylated alpha-tubulin immunoreactivity in
neurofibrillary tangle-bearing neurons in Alzheimer’s disease. _J. Neuropathol. Exp. Neurol._ 55, 964–972 (1996). Article PubMed CAS Google Scholar * Zhang, F. et al. Posttranslational
modifications of α-tubulin in Alzheimer disease. _Transl Neurodegener._ 4, 9 (2015). Article PubMed PubMed Central CAS Google Scholar * Stokin, G. B. et al. Axonopathy and transport
deficits early in the pathogenesis of Alzheimer’s disease. _Science_ 307, 1282–1288 (2005). Article PubMed CAS Google Scholar * Stamer, K., Vogel, R., Thies, E., Mandelkow, E. &
Mandelkow, E. M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. _J. Cell Biol._ 156, 1051–1063 (2002). Article PubMed PubMed
Central CAS Google Scholar * Rapoport, M., Dawson, H. N., Binder, L. I., Vitek, M. P. & Ferreira, A. Tau is essential to beta -amyloid-induced neurotoxicity. _Proc. Natl Acad. Sci.
USA_ 99, 6364–6369 (2002). Article PubMed PubMed Central CAS Google Scholar * Vossel, K. A. et al. Tau reduction prevents Aβ-induced defects in axonal transport. _Science_ 330, 198
(2010). Article PubMed PubMed Central CAS Google Scholar * Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model.
_Science_ 316, 750–754 (2007). Article PubMed CAS Google Scholar * Nelson, P. T. et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic
plaques and neurofibrillary tangles “do count” when staging disease severity. _J. Neuropathol. Exp. Neurol._ 66, 1136–1146 (2007). Article PubMed PubMed Central Google Scholar * Vazquez,
A. Metabolic states following accumulation of intracellular aggregates: implications for neurodegenerative diseases. _PLOS ONE_ 8, e63822 (2013). Article PubMed PubMed Central CAS
Google Scholar * Mandelkow, E. M., Stamer, K., Vogel, R., Thies, E. & Mandelkow, E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. _Neurobiol. Aging_
24, 1079–1085 (2003). Article PubMed CAS Google Scholar * Callahan, L. M., Vaules, W. A. & Coleman, P. D. Quantitative decrease in synaptophysin message expression and increase in
cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. _J. Neuropathol. Exp. Neurol._ 58, 275–287 (1999). Article PubMed CAS Google Scholar *
Ginsberg, S. D., Hemby, S. E., Lee, V. M., Eberwine, J. H. & Trojanowski, J. Q. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. _Ann. Neurol._ 48,
77–87 (2000). Article PubMed CAS Google Scholar * Shafiei, S. S., Guerrero-Munoz, M. J. & Castillo-Carranza, D. L. Tau oligomers: cytotoxicity, propagation, and mitochondrial damage.
_Front. Aging Neurosci._ 9, 83 (2017). Article PubMed PubMed Central CAS Google Scholar * Cárdenas-Aguayo Mdel, C., Gómez-Virgilio, L., DeRosa, S. & Meraz-Ríos, M. A. The role of
tau oligomers in the onset of Alzheimer’s disease neuropathology. _ACS Chem. Neurosci._ 5, 1178–1191 (2014). Article PubMed CAS Google Scholar * Fagan, A. M. et al. Longitudinal change
in CSF biomarkers in autosomal-dominant Alzheimer’s disease. _Sci. Transl Med._ 6, 226ra30 (2014). Article PubMed PubMed Central CAS Google Scholar * Hall, S. et al. Accuracy of a panel
of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. _Arch. Neurol._ 69, 1445–1452 (2012). Article PubMed Google
Scholar * Hu, W. T., Trojanowski, J. Q. & Shaw, L. M. Biomarkers in frontotemporal lobar degenerations — progress and challenges. _Prog. Neurobiol._ 95, 636–648 (2011). Article PubMed
PubMed Central CAS Google Scholar * Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. _Lancet Neurol._ 15,
673–684 (2016). Article PubMed CAS Google Scholar * Wagshal, D. et al. Divergent CSF tau alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy.
_J. Neurol. Neurosurg. Psychiatry_ 86, 244–250 (2015). Article PubMed Google Scholar * Mufson, E. J., Ward, S. & Binder, L. Prefibrillar tau oligomers in mild cognitive impairment and
Alzheimer’s disease. _Neurodegener. Dis._ 13, 151–153 (2014). Article PubMed CAS Google Scholar * Flach, K. et al. Tau oligomers impair artificial membrane integrity and cellular
viability. _J. Biol. Chem._ 287, 43223–43233 (2012). Article PubMed PubMed Central CAS Google Scholar * Lasagna-Reeves, C. A. et al. The formation of tau pore-like structures is
prevalent and cell specific: possible implications for the disease phenotypes. _Acta Neuropathol. Commun._ 2, 56 (2014). Article PubMed PubMed Central Google Scholar * Goedert, M. &
Spillantini, M. G. Propagation of Tau aggregates. _Mol. Brain_ 10, 18 (2017). Article PubMed PubMed Central Google Scholar * Usenovic, M. et al. Internalized tau oligomers cause
neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. _J. Neurosci._ 35, 14234–14250 (2015). Article PubMed CAS Google
Scholar * Whyte, L. S., Lau, A. A., Hemsley, K. M., Hopwood, J. J. & Sargeant, T. J. Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer’s disease? _J. Neurochem._
140, 703–717 (2017). Article PubMed CAS Google Scholar * Zare-Shahabadi, A., Masliah, E., Johnson, G. V. & Rezaei, N. Autophagy in Alzheimer’s disease. _Rev. Neurosci._ 26, 385–395
(2015). Article PubMed PubMed Central Google Scholar * Nixon, R. A. The role of autophagy in neurodegenerative disease. _Nat. Med._ 19, 983–997 (2013). Article PubMed CAS Google
Scholar * Tramutola, A. et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild
cognitive impairment and late-stage AD. _J. Neurochem._ 133, 739–749 (2015). Article PubMed CAS Google Scholar * Li, X., Alafuzoff, I., Soininen, H., Winblad, B. & Pei, J. J. Levels
of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. _FEBS J._ 272, 4211–4220 (2005). Article PubMed CAS Google Scholar
* Lafay-Chebassier, C. et al. mTOR/p70S6k signalling alteration by Aβ exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. _J. Neurochem._ 94, 215–225
(2005). Article PubMed CAS Google Scholar * Pickford, F. et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β
accumulation in mice. _J. Clin. Invest._ 118, 2190–2199 (2008). PubMed PubMed Central CAS Google Scholar * Boland, B. et al. Autophagy induction and autophagosome clearance in neurons:
relationship to autophagic pathology in Alzheimer’s disease. _J. Neurosci._ 28, 6926–6937 (2008). Article PubMed PubMed Central CAS Google Scholar * Sanchez-Varo, R. et al. Abnormal
accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. _Acta Neuropathol._ 123, 53–70 (2012). Article PubMed Google
Scholar * Bordi, M. et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. _Autophagy_ 12, 2467–2483
(2016). Article PubMed PubMed Central CAS Google Scholar * Piras, A., Collin, L., Gruninger, F., Graff, C. & Ronnback, A. Autophagic and lysosomal defects in human tauopathies:
analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. _Acta Neuropathol. Commun._ 4, 22 (2016). Article
PubMed PubMed Central CAS Google Scholar * Yu, W. H. et al. Macroautophagy — a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. _J. Cell Biol._ 171, 87–98
(2005). Article PubMed PubMed Central CAS Google Scholar * Lin, C. L. et al. Amyloid-β suppresses AMP-activated protein kinase (AMPK) signaling and contributes to α-synuclein-induced
cytotoxicity. _Exp. Neurol._ 275, 84–98 (2016). Article PubMed CAS Google Scholar * Park, H. et al. Neuropathogenic role of adenylate kinase-1 in Aβ-mediated tau phosphorylation via AMPK
and GSK3β. _Hum. Mol. Genet._ 21, 2725–2737 (2012). Article PubMed CAS Google Scholar * Wang, Y. & Mandelkow, E. Degradation of tau protein by autophagy and proteasomal pathways.
_Biochem. Soc. Trans._ 40, 644–652 (2012). Article PubMed CAS Google Scholar * Mori, H., Kondo, J. & Ihara, Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s
disease. _Science_ 235, 1641–1644 (1987). Article PubMed CAS Google Scholar * Perry, G., Friedman, R., Shaw, G. & Chau, V. Ubiquitin is detected in neurofibrillary tangles and senile
plaque neurites of Alzheimer disease brains. _Proc. Natl Acad. Sci. USA_ 84, 3033–3036 (1987). Article PubMed PubMed Central CAS Google Scholar * Gong, B., Radulovic, M.,
Figueiredo-Pereira, M. E. & Cardozo, C. The ubiquitin–proteasome system: potential therapeutic targets for Alzheimer’s disease and spinal cord injury. _Front. Mol. Neurosci._ 9, 4
(2016). Article PubMed PubMed Central CAS Google Scholar * Morawe, T., Hiebel, C., Kern, A. & Behl, C. Protein homeostasis, aging and Alzheimer’s disease. _Mol. Neurobiol._ 46,
41–54 (2012). Article PubMed PubMed Central CAS Google Scholar * Gurney, M. E., D’Amato, E. C. & Burgin, A. B. Phosphodiesterase-4 (PDE4) molecular pharmacology and Alzheimer’s
disease. _Neurotherapeutics_ 12, 49–56 (2015). Article PubMed CAS Google Scholar * Sweeney, P. et al. Protein misfolding in neurodegenerative diseases: implications and strategies.
_Transl Neurodegener._ 6, 6 (2017). Article PubMed PubMed Central CAS Google Scholar * Deger, J. M., Gerson, J. E. & Kayed, R. The interrelationship of proteasome impairment and
oligomeric intermediates in neurodegeneration. _Aging Cell_ 14, 715–724 (2015). Article PubMed PubMed Central CAS Google Scholar * Ittner, L. M. & Götz, J. Amyloid-β and tau — a
toxic pas de deux in Alzheimer’s disease. _Nat. Rev. Neurosci._ 12, 65–72 (2011). Article PubMed CAS Google Scholar * Ke, Y. D. et al. Lessons from tau-deficient mice. _Int. J.
Alzheimers Dis._ 2012, 873270 (2012). PubMed PubMed Central Google Scholar * Wittrup, A. & Lieberman, J. Knocking down disease: a progress report on siRNA therapeutics. _Nat. Rev.
Genet._ 16, 543–552 (2015). Article PubMed PubMed Central CAS Google Scholar * Zuckerman, J. E. & Davis, M. E. Clinical experiences with systemically administered siRNA-based
therapeutics in cancer. _Nat. Rev. Drug Discov._ 14, 843–856 (2015). Article PubMed CAS Google Scholar * Corey, D. R. Nusinersen, an antisense oligonucleotide drug for spinal muscular
atrophy. _Nat. Neurosci._ 20, 497–499 (2017). Article PubMed CAS Google Scholar * Finkel, R. S. et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2,
open-label, dose-escalation study. _Lancet_ 388, 3017–3026 (2016). Article PubMed CAS Google Scholar * Bormann, J. Memantine is a potent blocker of _N_-methyl-D-aspartate (NMDA) receptor
channels. _Eur. J. Pharmacol._ 166, 591–592 (1989). Article PubMed CAS Google Scholar * Kornhuber, J., Bormann, J., Retz, W., Hubers, M. & Riederer, P. Memantine displaces
[3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. _Eur. J. Pharmacol._ 166, 589–590 (1989). Article PubMed CAS Google Scholar * Chohan, M. O., Khatoon, S.,
Iqbal, I. G. & Iqbal, K. Involvement of I2 PP2A in the abnormal hyperphosphorylation of tau and its reversal by memantine. _FEBS Lett._ 580, 3973–3979 (2004). Article CAS Google
Scholar * Miltner, F. O. Use of symptomatic therapy with memantine in cerebral coma. II. Development of stretch synergisms in coma with brain stem symptoms. _Arzneimittelforschung_ 32,
1271–1273 (1982). PubMed CAS Google Scholar * Miltner, F. O. Use of symptomatic therapy with memantine in cerebral coma. I. Correlation of coma stages and EEG spectral paters.
_Arzneimittelforschung_ 32, 1268–1270 (1982). PubMed CAS Google Scholar * Jiang, J. & Jiang, H. Efficacy and adverse effects of memantine treatment for Alzheimer’s disease from
randomized controlled trials. _Neurol. Sci._ 36, 1633–1641 (2015). Article PubMed Google Scholar * Matsunaga, S., Kishi, T. & Iwata, N. Memantine monotherapy for Alzheimer’s disease:
a systematic review and meta-analysis. _PLoS ONE_ 10, e0123289 (2015). Article PubMed PubMed Central CAS Google Scholar * Kishi, T., Matsunaga, S. & Iwata, N. Memantine for the
treatment of frontotemporal dementia: a meta-analysis. _Neuropsychiatr. Dis. Treat._ 11, 2883–2885 (2015). Article PubMed PubMed Central CAS Google Scholar * Tsoi, K. K. et al.
Combination therapy showed limited superiority over monotherapy for Alzheimer disease: a meta-analysis of 14 randomized trials. _J. Am. Med. Dir. Assoc._ 17, 863.e1–863.e8 (2016). Article
Google Scholar * Corcoran, N. M. et al. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. _J. Clin.
Neurosci._ 17, 1025–1033 (2010). Article PubMed CAS Google Scholar * Rueli, R. H. et al. Selenprotein S reduces endoplasmic reticulum stress-induced phosphorylation of tau: potential
selenate mitigation of tau pathology. _J. Alzheimers Dis._ 55, 749–762 (2017). Article PubMed PubMed Central CAS Google Scholar * van Eersel, J. et al. Sodium selenate mitigates tau
pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. _Proc. Natl Acad. Sci. USA_ 107, 13888–13893 (2010). Article PubMed PubMed Central Google Scholar *
Jones, N. C. et al. Targeting hyperphosphorylated tau with sodium selenate suppresses seizures in rodent models. _Neurobiol. Dis._ 45, 897–901 (2012). Article PubMed CAS Google Scholar *
Liu, S. J. et al. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. _Brain_ 139, 1919–1938 (2016).
Article PubMed Google Scholar * Shultz, S. R. et al. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. _Brain_ 138, 1297–1313 (2015).
Article PubMed PubMed Central Google Scholar * Malpas, C. B. et al. A phase IIa randomized control trial of VEL (sodium selenate) in mild–moderate Alzheimer’s disease. _J. Alzheimers
Dis._ 54, 223–232 (2016). Article PubMed CAS Google Scholar * Carlson, B. A., Dubay, M. M., Sausville, E. A., Brizuela, L. & Worland, P. J. Flavopiridol induces G1 arrest with
inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. _Cancer Res._ 56, 2973–2978 (1996). PubMed CAS Google Scholar * Meijer, L. et al. Biochemical and
cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. _Eur. J. Biochem._ 243, 527–536 (1997). Article PubMed CAS Google
Scholar * Mapelli, M. et al. Mechanism of CDK5/p25 binding by CDK inhibitors. _J. Med. Chem._ 48, 671–679 (2005). Article PubMed CAS Google Scholar * Wu, J., Stoica, B. A. & Faden,
A. I. Cell cycle activation and spinal cord injury. _Neurotherapeutics_ 8, 221–228 (2011). Article PubMed PubMed Central CAS Google Scholar * Khalil, H. S., Mitev, V., Vlaykova, T.,
Cavicchi, L. & Zhelev, N. Discovery and development of Seliciclib. How systems biology approaches can lead to better drug performance. _J. Biotechnol._ 202, 40–49 (2015). Article PubMed
CAS Google Scholar * Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. _Nat. Rev. Drug
Discov._ 14, 130–146 (2015). Article PubMed PubMed Central CAS Google Scholar * Dominguez, J. M. et al. Evidence for irreversible inhibition of glycogen synthase kinase-3β by
tideglusib. _J. Biol. Chem._ 287, 893–904 (2012). Article PubMed CAS Google Scholar * Martinez, A., Alonso, M., Castro, A., Perez, C. & Moreno, F. J. First non-ATP competitive
glycogen synthase kinase 3 β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. _J. Med. Chem._ 45, 1292–1299 (2002). Article PubMed
CAS Google Scholar * DaRocha-Souto, B. et al. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. _Neurobiol. Dis._ 45,
425–437 (2012). Article PubMed CAS Google Scholar * Sereno, L. et al. A novel GSK-3β inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. _Neurobiol. Dis_ 35,
359–367 (2009). Article PubMed CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT00948259 (2009). * del Ser, T. et al.
Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. _J. Alzheimers Dis._ 33, 205–215 (2013). PubMed Google Scholar * Lovestone, S. et al. A phase II trial
of tideglusib in Alzheimer’s disease. _J. Alzheimers Dis._ 45, 75–88 (2015). Article PubMed CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT01049399 (2012). * Tolosa, E. et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. _Mov. Disord._ 29, 470–478
(2014). Article PubMed CAS Google Scholar * Stambolic, V., Ruel, L. & Woodgett, J. R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact
cells. _Curr. Biol._ 6, 1664–1668 (1996). Article PubMed CAS Google Scholar * Klein, P. S. & Melton, D. A. A molecular mechanism for the effect of lithium on development. _Proc. Natl
Acad. Sci. USA_ 93, 8455–8459 (1996). Article PubMed PubMed Central CAS Google Scholar * Forlenza, O. V., De-Paula, V. J. & Diniz, B. S. Neuroprotective effects of lithium:
implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. _ACS Chem. Neurosci._ 5, 443–450 (2014). Article PubMed PubMed Central CAS Google Scholar
* Forlenza, O. V. et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. _Br. J. Psychiatry_ 198, 351–356
(2011). Article PubMed Google Scholar * Nunes, M. A., Viel, T. A. & Buck, H. S. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease.
_Curr. Alzheimer Res._ 10, 104–107 (2013). PubMed CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02601859 (2015). * US
National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02862210 (2017). * Min, S. W. et al. Critical role of acetylation in tau-mediated neurodegeneration and
cognitive deficits. _Nat. Med._ 21, 1154–1162 (2015). Article PubMed PubMed Central CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT02422485 (2017). * Sandhu, P. et al. Pharmacokinetics and pharmacodynamics to support clinical studies of MK-8719: an _O_-GlcNAcase inhibitor for
progressive supranuclear palsy [abstract]. _Alzheimers Dement._ 12 (Suppl.), P4–036 (2016). Google Scholar * [No authors listed.] Alectos Therapeutics announces FDA orphan drug designation
for MK-8719: an investigational small-molecule OGA inhibitor for treatment of progressive supranuclear palsy. _Alectos_
http://alectos.com/content/alectos-therapeutics-announces-fda-orphan-drug-designation-mk-8719-investigational-small-molecule-oga-inhibitor-treatment-progressive-supranuclear-palsy/ (2016). *
Rohn, T. T. The role of caspases in Alzheimer’s disease; potential novel therapeutic opportunities. _Apoptosis_ 15, 1403–1409 (2010). Article PubMed CAS Google Scholar * Panza, F. et
al. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. _Biomed. Res. Int._ 2016, 3245935 (2016). Article PubMed PubMed Central CAS Google
Scholar * Wischik, C. M., Edwards, P. C., Lai, R. Y., Roth, M. & Harrington, C. R. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. _Proc. Natl Acad.
Sci. USA_ 93, 11213–11218 (1996). Article PubMed PubMed Central CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01253122
(2010). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT00515333 (2008). * Wischik, C. M. et al. Tau aggregation inhibitor therapy: an exploratory
phase 2 study in mild or moderate Alzheimer’s disease. _J. Alzheimers Dis._ 44, 705–720 (2015). Article PubMed CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT00684944 (2012). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01689233 (2018). * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01689246 (2018). * Gauthier, S. et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or
moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. _Lancet_ 388, 2873–2884 (2016). Article PubMed PubMed Central CAS Google Scholar *
Fagan, T. In first phase 3 trial, the tau drug LMTM did not work. Period. _Alzforum_
http://www.alzforum.org/news/conference-coverage/first-phase-3-trial-tau-drug-lmtm-did-not-work-period#show-more (2016). * Fagan, T. Tau inhibitor fails again — subgroup analysis irks
clinicians at CTAD. _Alzforum_ http://www.alzforum.org/news/conference-coverage/tau-inhibitor-fails-again-subgroup-analysis-irks-clinicians-ctad (2016). * US National Library of Medicine.
_ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02245568 (2018). * Hu, S. et al. Clinical development of curcumin in neurodegenerative disease. _Expert Rev. Neurother._ 15, 629–637
(2015). Article PubMed CAS Google Scholar * Hamaguchi, T., Ono, K. & Yamada, M. Review: Curcumin and Alzheimer’s disease. _CNS Neurosci. Ther._ 16, 285–297 (2010). Article PubMed
CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT00164749 (2008). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT00099710 (2009). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT00595582 (2012). * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01383161 (2017). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01811381 (2018).
* Bollag, D. M. et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. _Cancer Res._ 55, 2325–2333 (1995). PubMed CAS Google Scholar *
Brunden, K. R. et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. _J. Neurosci._ 30, 13861–13866 (2010). Article
PubMed PubMed Central CAS Google Scholar * Zhang, B. et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and
Alzheimer-like pathology in an interventional study with aged tau transgenic mice. _J. Neurosci._ 32, 3601–3611 (2012). Article PubMed PubMed Central CAS Google Scholar * Barten, D. M.
et al. Hyperdynamic microtubules, cognitive deficits, and pathology are improved in tau transgenic mice with low doses of the microtubule-stabilizing agent BMS-241027. _J. Neurosci._ 32,
7137–7145 (2012). Article PubMed CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01492374 (2014). * Magen, I. & Gozes,
I. Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: focus on davunetide (NAP). _Neuropeptides_ 47, 489–495 (2013). Article PubMed CAS
Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01110720 (2013). * Boxer, A. L. et al. Davunetide in patients with progressive
supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. _Lancet Neurol._ 13, 676–685 (2014). Article PubMed PubMed Central CAS Google Scholar * US National
Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01966666 (2013). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT02133846 (2016). * Fitzgerald, D. P. et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. _Mol.
Cancer Ther._ 11, 1959–1967 (2012). Article PubMed CAS Google Scholar * McQuade, J. L. et al. A phase I study of TPI 287 in combination with temozolomide for patients with metastatic
melanoma. _Melanoma Res._ 26, 604–608 (2016). Article PubMed PubMed Central CAS Google Scholar * Mitchell, D. et al. A phase 1 trial of TPI 287 as a single agent and in combination with
temozolomide in patients with refractory or recurrent neuroblastoma or medulloblastoma. _Pediatr. Blood Cancer_ 63, 39–46 (2016). Article PubMed CAS Google Scholar * Wachtel, H.
Potential antidepressant activity of rolipram and other selective cyclic adenosine 3ʹ,5ʹ-monophosphate phosphodiesterase inhibitors. _Neuropharmacology_ 22, 267–272 (1983). Article PubMed
CAS Google Scholar * Myeku, N. et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP–PKA signaling. _Nat. Med._ 22,
46–53 (2016). Article PubMed CAS Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02648672 (2017). * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02840279 (2017). * Lobello, K., Ryan, J. M., Liu, E., Rippon, G. & Black, R. Targeting beta amyloid: a clinical review
of immunotherapeutic approaches in Alzheimer’s disease. _Int. J. Alzheimers Dis._ 2012, 628070 (2012). PubMed PubMed Central Google Scholar * Valera, E., Spencer, B. & Masliah, E.
Immunotherapeutic approaches targeting amyloid-β, α-synuclein, and tau for the treatment of neurodegenerative disorders. _Neurotherapeutics_ 13, 179–189 (2016). Article PubMed CAS Google
Scholar * Abushouk, A. I. et al. Bapineuzumab for mild to moderate Alzheimer’s disease: a meta-analysis of randomized controlled trials. _BMC Neurol._ 17, 66 (2017). Article PubMed PubMed
Central Google Scholar * Penninkilampi, R., Brothers, H. M. & Eslick, G. D. Safety and efficacy of anti-amyloid-β immunotherapy in Alzheimer’s disease: a systematic review and
meta-analysis. _J. Neuroimmune Pharmacol._ 12, 194–203 (2017). Article PubMed Google Scholar * Wisniewski, T. & Drummond, E. Developing therapeutic vaccines against Alzheimer’s
disease. _Expert Rev. Vaccines_ 15, 401–415 (2016). PubMed CAS Google Scholar * Panza, F., Solfrizzi, V., Imbimbo, B. P. & Logroscino, G. Amyloid-directed monoclonal antibodies for
the treatment of Alzheimer’s disease: the point of no return? _Expert Opin. Biol. Ther._ 14, 1465–1476 (2014). Article PubMed CAS Google Scholar * Serrano-Pozo, A. et al. Beneficial
effect of human anti-amyloid-β active immunization on neurite morphology and tau pathology. _Brain_ 133, 1312–1327 (2010). Article PubMed PubMed Central Google Scholar * Boche, D.,
Denham, N., Holmes, C. & Nicoll, J. A. Neuropathology after active Aβ42 immunotherapy: implications for Alzheimer’s disease pathogenesis. _Acta Neuropathol._ 120, 369–384 (2010). Article
PubMed CAS Google Scholar * Boche, D. et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Aβ42 immunisation in Alzheimer’s disease. _Acta
Neuropathol._ 120, 13–20 (2010). Article PubMed CAS Google Scholar * Blennow, K. et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with
mild to moderate Alzheimer disease. _Arch. Neurol._ 69, 1002–1010 (2012). Article PubMed Google Scholar * Salloway, S. et al. A phase 2 multiple ascending dose trial of bapineuzumab in
mild to moderate Alzheimer disease. _Neurology_ 73, 2061–2070 (2009). Article PubMed PubMed Central CAS Google Scholar * Vandenberghe, R. et al. Bapineuzumab for mild to moderate
Alzheimer’s disease in two global, randomized, phase 3 trials. _Alzheimers Res. Ther._ 8, 18 (2016). Article PubMed PubMed Central CAS Google Scholar * Asuni, A. A., Boutajangout, A.,
Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. _J.
Neurosci._ 27, 9115–9129 (2007). Article PubMed CAS Google Scholar * Boutajangout, A., Ingadottir, J., Davies, P. & Sigurdsson, E. M. Passive immunization targeting pathological
phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. _J. Neurochem._ 118, 658–667 (2011). Article PubMed PubMed Central CAS Google
Scholar * Asuni, A. A., Quartermain, D. & Sigurdsson, E. M. Tau-based immunotherapy for dementia [abstract]. _Alzheimers Dement._ 2 (Suppl.), O2-05-04 (2006). Google Scholar *
Boutajangout, A., Ingadottir, J., Davies, P. & Sigurdsson, E. M. Passive tau immunotherapy diminishes functional decline and clears tau aggregates in a mouse model of tauopathy
[abstract]. _Alzheimers Dement._ 6 (Suppl.), P3–427 (2010). Google Scholar * Bi, M., Ittner, A., Ke, Y. D., Gotz, J. & Ittner, L. M. Tau-targeted immunization impedes progression of
neurofibrillary histopathology in aged P301L tau transgenic mice. _PLoS ONE_ 6, e26860 (2011). Article PubMed PubMed Central CAS Google Scholar * Theunis, C. et al. Efficacy and safety
of a liposome-based vaccine against protein tau, assessed in tau. P301L mice that model tauopathy. _PLoS ONE_ 8, e72301 (2013). Article PubMed PubMed Central Google Scholar *
Boutajangout, A., Quartermain, D. & Sigurdsson, E. M. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. _J. Neurosci._ 30, 16559–16566
(2010). Article PubMed PubMed Central CAS Google Scholar * Troquier, L. et al. Targeting phospho-Ser422 by active tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic
approach. _Curr. Alzheimer Res._ 9, 397–405 (2012). Article PubMed PubMed Central Google Scholar * Rajamohamedsait, H., Rasool, S., Rajamohamedsait, W., Lin, Y. & Sigurdsson, E. M.
Prophylactic active tau immunization leads to sustained reduction in both tau and amyloid-β pathologies in 3xTg mice. _Sci. Rep_. 7, 17034 (2017). Article PubMed PubMed Central CAS
Google Scholar * Boimel, M. et al. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. _Exp. Neurol._ 224, 472–485 (2010). Article PubMed
CAS Google Scholar * Davtyan, H. et al. MultiTEP platform-based DNA epitope vaccine targeting N-terminus of tau induces strong immune responses and reduces tau pathology in THY-Tau22 mice.
_Vaccine_ 35, 2015–2024 (2017). Article PubMed PubMed Central CAS Google Scholar * Selenica, M. L. et al. Epitope analysis following active immunization with tau proteins reveals
immunogens implicated in tau pathogenesis. _J. Neuroinflamm._ 11, 152 (2014). Article CAS Google Scholar * Kontsekova, E., Zilka, N., Kovacech, B., Novak, P. & Novak, M. First-in-man
tau vaccine targeting structural determinants essential for pathological tau–tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model.
_Alzheimers Res. Ther._ 6, 44 (2014). Article PubMed PubMed Central CAS Google Scholar * Ando, K. et al. Vaccination with Sarkosyl insoluble PHF-tau decrease neurofibrillary tangles
formation in aged tau transgenic mouse model: a pilot study. _J. Alzheimers Dis._ 40 (Suppl. 1), S135–S145 (2014). Article PubMed CAS Google Scholar * Rosenmann, H. et al. Tauopathy-like
abnormalities and neurologic deficits in mice immunized with neuronal tau protein. _Arch. Neurol._ 63, 1459–1467 (2006). Article PubMed Google Scholar * Rozenstein-Tsalkovich, L. et al.
Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. _Exp. Neurol._ 248, 451–456 (2013). Article PubMed CAS Google Scholar * Chai, X. et al. Passive
immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. _J. Biol. Chem._ 286, 34457–34467 (2011). Article PubMed PubMed
Central CAS Google Scholar * Congdon, E. E., Gu, J., Sait, H. B. & Sigurdsson, E. M. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and
is a prerequisite for acute tau protein clearance. _J. Biol. Chem._ 288, 35452–35465 (2013). Article PubMed PubMed Central CAS Google Scholar * Congdon, E. E. et al. Affinity of tau
antibodies for solubilized pathological tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy. _Mol. Neurodegener_ 11, 62–86 (2016). Article
PubMed PubMed Central CAS Google Scholar * Gu, J., Congdon, E. E. & Sigurdsson, E. M. Two novel tau antibodies targeting the 396/404 region are primarily taken up by neurons and
reduce tau protein pathology. _J. Biol. Chem._ 288, 33081–33095 (2013). Article PubMed PubMed Central CAS Google Scholar * Ittner, A. et al. Tau-targeting passive immunization modulates
aspects of pathology in tau transgenic mice. _J. Neurochem._ 132, 135–145 (2015). Article PubMed CAS Google Scholar * Krishnamurthy, P. K., Deng, Y. & Sigurdsson, E. M. Mechanistic
studies of antibody-mediated clearance of tau aggregates using an ex vivo brain slice model. _Front. Psychiatry_ 2, 59 (2011). Article PubMed PubMed Central CAS Google Scholar * Liu, W.
et al. Vectored intracerebral immunization with the anti-tau monoclonal antibody PHF1 markedly reduces tau pathology in mutant tau transgenic mice. _J. Neurosci._ 36, 12425–12435 (2016).
Article PubMed CAS Google Scholar * Sankaranarayanan, S. et al. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse
tauopathy models. _PLoS ONE_ 10, e0125614 (2015). Article PubMed PubMed Central CAS Google Scholar * Subramanian, S., Savanur, G. & Madhavadas, S. Passive immunization targeting the
N-terminal region of phosphorylated tau (residues 68–71) improves spatial memory in okadaic acid induced tauopathy model rats. _Biochem. Biophys. Res. Commun._ 483, 585–589 (2017). Article
PubMed CAS Google Scholar * Dai, C. L. et al. Passive immunization targeting the N-terminal projection domain of tau decreases tau pathology and improves cognition in a transgenic mouse
model of Alzheimer disease and tauopathies. _J. Neural Transm._ 122, 607–617 (2015). Article PubMed CAS Google Scholar * Yanamandra, K. et al. Anti-tau antibody administration increases
plasma tau in transgenic mice and patients with tauopathy. _Sci. Transl Med._ 9, eaal2029 (2017). Article PubMed PubMed Central Google Scholar * Kfoury, N., Holmes, B. B., Jiang, H.,
Holtzman, D. M. & Diamond, M. I. Trans-cellular propagation of Tau aggregation by fibrillar species. _J. Biol. Chem._ 287, 19440–19451 (2012). Article PubMed PubMed Central CAS
Google Scholar * Yanamandra, K. et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. _Neuron_ 80, 402–414 (2013).
Article PubMed PubMed Central CAS Google Scholar * Dai, C. L., Tung, Y. C., Liu, F., Gong, C. X. & Iqbal, K. Tau passive immunization inhibits not only tau but also Aβ pathology.
_Alzheimers Res. Ther._ 9, 1 (2017). Article PubMed PubMed Central CAS Google Scholar * Bright, J. et al. Human secreted tau increases amyloid-beta production. _Neurobiol. Aging_ 36,
693–709 (2015). Article PubMed CAS Google Scholar * Castillo-Carranza, D. L. et al. Passive immunization with tau oligomer monoclonal antibody reverses tauopathy phenotypes without
affecting hyperphosphorylated neurofibrillary tangles. _J. Neurosci._ 34, 4260–4272 (2014). Article PubMed CAS Google Scholar * Castillo-Carranza, D. L. et al. Specific targeting of tau
oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. _J. Alzheimers Dis._ 40 (Suppl. 1), S97–S111 (2014).
Article PubMed CAS Google Scholar * d’Abramo, C., Acker, C. M., Jimenez, H. T. & Davies, P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity.
_PLoS ONE_ 8, e62402 (2013). Article PubMed PubMed Central CAS Google Scholar * Kondo, A. et al. Antibody against early driver of neurodegeneration _cis_ P-tau blocks brain injury and
tauopathy. _Nature_ 523, 431–436 (2015). Article PubMed PubMed Central CAS Google Scholar * d’Abramo, C., Acker, C. M., Jimenez, H. & Davies P. Passive immunization in JNPL3
transgenic mice using an array of phospho-tau specific antibodies. _PLoS ONE_ 10, e0135774 (2015). * Walls, K. C. et al. p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD
mice. _Neurosci. Lett_. 575, 96–100 (2014). Article PubMed PubMed Central CAS Google Scholar * Lee, S. H. et al. Antibody-mediated targeting of tau in vivo does not require effector
function and microglial engagement. _Cell Rep._ 16, 1690–1700 (2016). Article PubMed CAS Google Scholar * Umeda, T. et al. Passive immunotherapy of tauopathy targeting pSer413-tau: a
pilot study in mice. _Ann. Clin. Transl Neurol._ 2, 241–255 (2015). Article PubMed PubMed Central CAS Google Scholar * Collin, L. et al. Neuronal uptake of tau/pS422 antibody and
reduced progression of tau pathology in a mouse model of Alzheimer’s disease. _Brain_ 137, 2834–2846 (2014). Article PubMed Google Scholar * Nisbet, R. M. et al. Combined effects of
scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. _Brain_ 140, 1220–1330 (2017). Article PubMed PubMed Central Google Scholar * Ising, C. et
al. AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. _J. Exp. Med._ 214, 1227–1238 (2017). Article PubMed PubMed Central CAS Google
Scholar * Pedersen, J. T. & Sigurdsson, E. M. Tau immunotherapy for Alzheimer’s disease. _Trends Mol. Med._ 21, 394–402 (2015). Article PubMed CAS Google Scholar * Walker, L. C.,
Diamond, M. I., Duff, K. E. & Hyman, B. T. Mechanisms of protein seeding in neurodegenerative diseases. _JAMA Neurol._ 70, 304–310 (2013). Article PubMed Google Scholar * Sigurdsson,
E. M. Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. _J. Alzheimers Dis._ 15, 157–168 (2008). Article PubMed PubMed Central CAS Google
Scholar * Krishnaswamy, S. et al. Antibody-derived in vivo imaging of tau pathology. _J. Neurosci._ 34, 16835–16850 (2014). Article PubMed PubMed Central CAS Google Scholar * Fuller,
J. P., Stavenhagen, J. B. & Teeling, J. L. New roles for Fc receptors in neurodegeneration — the impact on immunotherapy for Alzheimer’s disease. _Front. Neurosci._ 8, 235 (2014). PubMed
PubMed Central Google Scholar * van der Kleij, H. et al. Evidence for neuronal expression of functional Fc (ε and γ) receptors. _J. Allergy Clin. Immunol._ 125, 757–760 (2010). Article
PubMed PubMed Central CAS Google Scholar * McEwan, W. A. et al. Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. _Proc. Natl Acad. Sci. USA_ 114, 574–579 (2017). Article
PubMed PubMed Central CAS Google Scholar * Medina, M. & Avila, J. The role of extracellular tau in the spreading of neurofibrillary pathology. _Front. Cell. Neurosci._ 8, 113 (2014).
PubMed PubMed Central Google Scholar * Sigurdsson, E. M., Wisniewski, T. & Frangione, B. Infectivity of amyloid diseases. _Trends Mol. Med._ 8, 411–413 (2002). Article PubMed CAS
Google Scholar * Herukka, S. K. et al. Amyloid-β and tau dynamics in human brain interstitial fluid in patients with suspected normal pressure hydrocephalus. _J. Alzheimers Dis._ 46,
261–269 (2015). Article PubMed CAS Google Scholar * Croft, C. L. et al. Membrane association and release of wild-type and pathological tau from organotypic brain slice cultures. _Cell
Death Dis._ 8, e2671 (2017). Article PubMed PubMed Central CAS Google Scholar * Sigurdsson, E. M. Tau immunotherapy. _Neurodegener. Dis._ 16, 34–38 (2016). Article PubMed CAS Google
Scholar * Yanamandra, K. et al. Anti-tau antibody reduces insoluble tau and decreases brain atrophy. _Ann. Clin. Transl Neurol._ 2, 278–288 (2015). Article PubMed PubMed Central CAS
Google Scholar * Kontsekova, E., Zilka, N., Kovacech, B., Skrabana, R. & Novak, M. Identification of structural determinants on tau protein essential for its pathological function:
novel therapeutic target for tau immunotherapy in Alzheimer’s disease. _Alzheimers Res. Ther._ 6, 45 (2014). Article PubMed PubMed Central CAS Google Scholar * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT01850238 (2015). * Novak, P. et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s
disease: a randomised, double-blind, placebo-controlled, phase 1 trial. _Lancet Neurol._ 16, 123–134 (2017). Article PubMed CAS Google Scholar * US National Library of Medicine.
_ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02031198 (2017). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02579252 (2017). * Ondrus,
M. & Novak, P. Design of the phase II clinical study of the tau vaccine AADvac1 in patients with mild Alzheimer’s disease. _Neurobiol. Aging_ 39 (Suppl. 1), S26 (2016). Article Google
Scholar * International Clincal Trials Registry Platform. _ISRCTN.com_ http://www.isrctn.com/ISRCTN13033912 (2013). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT02281786 (2016). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02294851 (2014). * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02460094 (2017). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02658916 (2018).
* [No authors listed.] Biogen licenses phase 2 anti-tau antibody from Bristol-Myers Squibb. _Biogen_
http://media.biogen.com/press-release/corporate/biogen-licenses-phase-2-anti-tau-antibody-bristol-myers-squibb (2017). * West, T. et al. Safety, tolerability and pharmacokinetics of
ABBV-8E12, a humanized anti-tau monoclonal antibody, in a Phase I, single ascending dose, placebo-controlled study in subjects with progressive supranuclear palsy. _J. Prev. Alzheimers Dis._
3, 285 (2016). Google Scholar * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02985879 (2018). * US National Library of Medicine.
_ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02880956 (2018). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT02820896 (2017). * Rogers,
M. B. Treating tau: finally, clinical candidates are stepping into the ring. _Alzforum_
http://www.alzforum.org/news/conference-coverage/treating-tau-finally-clinical-candidates-are-stepping-ring (2017). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/show/NCT02754830 (2018). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/show/NCT03019536 (2018). * Hayashi, M. L., Lu, J.,
Driver, D. & Alvarado, A. Antibodies to tau and uses thereof. US Patent US20160251420 A1 (2016). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/ct2/show/NCT03375697 (2018). * Rogers, M. B. To block tau’s proteopathic spread, antibody must attack its mid-region. _Alzforum_
https://www.alzforum.org/news/conference-coverage/block-taus-proteopathic-spread-antibody-must-attack-its-mid-region (2018). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/ct2/show/NCT03464227 (2018). * Congdon, E. E., Krishnaswamy, S. & Sigurdsson, E. M. Harnessing the immune system for treatment and detection of tau pathology.
_J. Alzheimers Dis._ 40 (Suppl.1), S113–S121 (2014). Article PubMed PubMed Central CAS Google Scholar * Barthelemy, N. R. et al. differential mass spectrometry profiles of tau protein
in the cerebrospinal fluid of patients with Alzheimer’s disease, progressive supranuclear palsy, and dementia with lewy bodies. _J. Alzheimers Dis._ 51, 1033–1043 (2016). Article PubMed
CAS Google Scholar * Barthelemy, N. R. et al. Tau protein quantification in human cerebrospinal fluid by targeted mass spectrometry at high sequence coverage provides insights into its
primary structure heterogeneity. _J. Proteome Res._ 15, 667–676 (2016). Article PubMed CAS Google Scholar * Giacobini, E. & Gold, G. Alzheimer disease therapy — moving from amyloid-β
to tau. _Nat. Rev. Neurol._ 9, 677–686 (2013). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS E.M.S. was supported by NIH grants R01 NS077239 and R01 AG032611
and a pilot grant from the Michael J. Fox Foundation. E.E.C. was supported by a grant from the Alzheimer’s Association. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Neuroscience and Physiology, New York University School of Medicine, New York, NY, USA Erin E. Congdon & Einar M. Sigurdsson * Department of Psychiatry, New York University School of
Medicine, New York, NY, USA Einar M. Sigurdsson Authors * Erin E. Congdon View author publications You can also search for this author inPubMed Google Scholar * Einar M. Sigurdsson View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Both authors researched data for the article, made substantial contributions to discussion of
the content, wrote the article, developed the figures and tables and reviewed and edited the manuscript before submission. CORRESPONDING AUTHOR Correspondence to Einar M. Sigurdsson. ETHICS
DECLARATIONS COMPETING INTERESTS E.M.S. is an inventor on various patents on immunotherapies and related diagnostics that are assigned to New York University. Some of those focusing on the
tau protein are licensed to and are being co-developed with H. Lundbeck A/S. E.E.C. declares no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Congdon, E.E.,
Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. _Nat Rev Neurol_ 14, 399–415 (2018). https://doi.org/10.1038/s41582-018-0013-z Download citation * Published: 12 June 2018 *
Issue Date: July 2018 * DOI: https://doi.org/10.1038/s41582-018-0013-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
