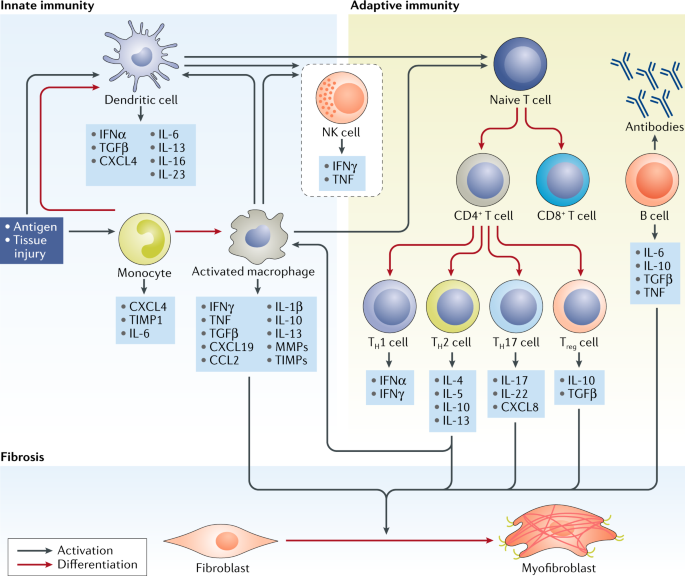
Involvement of the myeloid cell compartment in fibrogenesis and systemic sclerosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Systemic sclerosis (SSc) is an autoimmune fibrotic disease of unknown aetiology that is characterized by vascular changes in the skin and visceral organs. Autologous haematopoietic
stem cell transplantation can improve skin and organ fibrosis in patients with progressive disease and a high risk of organ failure, indicating that cells originating in the bone marrow are
important contributors to the pathogenesis of SSc. Animal studies also indicate a pivotal function of myeloid cells in the development of fibrosis leading to changes in the tissue
architecture and dysfunction in multiple organs such as the heart, lungs, liver and kidney. In this Review, we summarize current knowledge about the function of myeloid cells in fibrogenesis
that occurs in patients with SSc. Targeted therapies currently in clinical studies for SSc might affect myeloid cell-related pathways. Therefore, myeloid cells might be used as cellular
biomarkers of disease through the application of high-dimensional techniques such as mass cytometry and single-cell RNA sequencing. KEY POINTS * Chronic tissue injury, inflammation and
prolonged fibroblast activation lead to fibrosis and organ dysfunction in systemic sclerosis (SSc). * Myeloid cells are crucial antigen-presenting cells, regulators of inflammatory responses
and producers of cytokines, processes that are implicated in fibrogenesis. * Despite comprehensive investigation of myeloid cells, the function of these cells in SSc is not fully
understood. * High-dimensional analysis of myeloid cells might identify a biomarker of SSc onset and progression. * Strategies that target myeloid cell-related pathways might prevent or
reverse tissue fibrosis. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS IMMUNE CELL DYSREGULATION AS A MEDIATOR OF FIBROSIS IN SYSTEMIC SCLEROSIS Article 09 November 2022 AN INTERNATIONAL PERSPECTIVE ON THE FUTURE OF
SYSTEMIC SCLEROSIS RESEARCH Article 14 February 2025 IMMUNOLOGY OF HUMAN FIBROSIS Article 20 July 2023 REFERENCES * Jordan, S., Chung, J. & Distler, O. Preclinical and translational
research to discover potentially effective antifibrotic therapies in systemic sclerosis. _Curr. Opin. Rheumatol._ 25, 679–685 (2013). Article CAS PubMed Google Scholar * Mayes, M. D. et
al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. _Arthritis Rheum._ 48, 2246–2255 (2003). Article PubMed Google Scholar *
Peoples, C. et al. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. _J. Scleroderma Relat. Disord._ 1, 177–240 (2016). Article
PubMed Google Scholar * Allanore, Y. et al. Systemic sclerosis. _Nat. Rev. Dis. Primers_ 1, 15002 (2015). Article PubMed Google Scholar * Bujak, M. & Frangogiannis, N. G. The role
of TGF-beta signaling in myocardial infarction and cardiac remodeling. _Cardiovasc. Res._ 74, 184–195 (2007). Article CAS PubMed Google Scholar * Lech, M. & Anders, H. J. Macrophages
and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. _Biochim. Biophys. Acta_ 1832, 989–997 (2013). Article CAS PubMed
Google Scholar * Chizzolini, C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. _Curr. Opin. Rheumatol._ 20, 707–712 (2008). Article
CAS PubMed Google Scholar * O’Reilly, S., Hugle, T. & van Laar, J. M. T cells in systemic sclerosis: a reappraisal. _Rheumatology_ 51, 1540–1549 (2012). Article CAS Google Scholar
* Sakkas, L. I. & Platsoucas, C. D. Is systemic sclerosis an antigen-driven T cell disease? _Arthritis Rheum._ 50, 1721–1733 (2004). Article CAS PubMed Google Scholar * Guilliams,
M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. _Nat. Rev. Immunol._ 14, 571–578 (2014). Article CAS PubMed PubMed Central Google Scholar
* Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. _Nat. Rev. Immunol._ 6, 823–835 (2006). Article CAS PubMed PubMed Central Google Scholar * Waldner, H. The
role of innate immune responses in autoimmune disease development. _Autoimmun. Rev._ 8, 400–404 (2009). Article CAS PubMed Google Scholar * Ginhoux, F. & Jung, S. Monocytes and
macrophages: developmental pathways and tissue homeostasis. _Nat. Rev. Immunol._ 14, 392–404 (2014). Article CAS PubMed Google Scholar * Ginhoux, F. et al. Fate mapping analysis reveals
that adult microglia derive from primitive macrophages. _Science_ 330, 841–845 (2010). Article CAS PubMed PubMed Central Google Scholar * Epelman, S., Lavine, K. J. & Randolph, G.
J. Origin and functions of tissue macrophages. _Immunity_ 41, 21–35 (2014). Article CAS PubMed PubMed Central Google Scholar * Guilliams, M. et al. Alveolar macrophages develop from
fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. _J. Exp. Med._ 210, 1977–1992 (2013). Article CAS PubMed PubMed Central Google Scholar *
Hoeffel, G. et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. _Immunity_ 42, 665–678 (2015). Article CAS PubMed PubMed
Central Google Scholar * Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. _J. Exp. Med._ 211, 2151–2158 (2014). Article CAS PubMed PubMed
Central Google Scholar * Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. _Immunity_ 39,
925–938 (2013). Article CAS PubMed Google Scholar * Ginhoux, F. & Guilliams, M. Tissue-resident macrophage ontogeny and homeostasis. _Immunity_ 44, 439–449 (2016). Article CAS
PubMed Google Scholar * Satoh, T. et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. _Nature_ 541, 96–101 (2017). Article CAS PubMed Google
Scholar * Shi, C. & Pamer, E. G. Monocyte recruitment during infection and inflammation. _Nat. Rev. Immunol._ 11, 762–774 (2011). Article CAS PubMed PubMed Central Google Scholar *
Passlick, B., Flieger, D. & Ziegler-Heitbrock, H. W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. _Blood_ 74, 2527–2534 (1989).
Article CAS PubMed Google Scholar * Wong, K. L. et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets.
_Blood_ 118, e16–31 (2011). Article CAS PubMed Google Scholar * Higashi-Kuwata, N. et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from
patients with systemic sclerosis. _Arthritis Res. Ther._ 12, R128 (2010). Article PubMed PubMed Central CAS Google Scholar * Lescoat, A. et al. CD16-positive circulating monocytes and
fibrotic manifestations of systemic sclerosis. _Clin. Rheumatol._ 36, 1649–1654 (2017). Article PubMed Google Scholar * Ong, S. M. et al. The pro-inflammatory phenotype of the human
non-classical monocyte subset is attributed to senescence. _Cell Death Dis._ 9, 266 (2018). Article PubMed PubMed Central CAS Google Scholar * Mathai, S. K. et al. Circulating monocytes
from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. _Lab Invest._ 90, 812–823 (2010). Article CAS PubMed PubMed Central Google
Scholar * Soldano, S. P. et al. Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. _Ann. Rheum. Dis._ 77, 1842–1845 (2018).
Article CAS PubMed Google Scholar * Chia, J. J. & Lu, T. T. Update on macrophages and innate immunity in scleroderma. _Curr. Opin. Rheumatol._ 27, 530–536 (2015). Article CAS
PubMed PubMed Central Google Scholar * Misharin, A. V. et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. _J. Exp. Med._ 214,
2387–2404 (2017). Article CAS PubMed PubMed Central Google Scholar * Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. _Immunity_ 32,
593–604 (2010). Article CAS PubMed Google Scholar * Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. _Nat. Rev. Immunol._ 8, 958–969 (2008). CAS
PubMed PubMed Central Google Scholar * Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. _Immunity_ 41, 14–20 (2014). Article CAS
PubMed PubMed Central Google Scholar * Stifano, G. & Christmann, R. B. Macrophage involvement in systemic sclerosis: do we need more evidence? _Curr. Rheumatol. Rep._ 18, 2 (2016).
Article PubMed CAS Google Scholar * Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. _F1000Prime Rep._ 6, 13 (2014). Article
PubMed PubMed Central Google Scholar * Schlitzer, A. & Ginhoux, F. Organization of the mouse and human DC network. _Curr. Opin. Immunol._ 26, 90–99 (2014). Article CAS PubMed
Google Scholar * Satpathy, A. T. et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. _J. Exp. Med._ 209, 1135–1152
(2012). Article CAS PubMed PubMed Central Google Scholar * Haniffa, M. et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+
nonlymphoid dendritic cells. _Immunity_ 37, 60–73 (2012). Article CAS PubMed PubMed Central Google Scholar * Schlitzer, A., Zhang, W., Song, M. & Ma, X. Recent advances in
understanding dendritic cell development, classification, and phenotype. _F1000Res._ 7, 1558 (2018). Article CAS Google Scholar * Segura, E. & Amigorena, S. Inflammatory dendritic
cells in mice and humans. _Trends Immunol._ 34, 440–445 (2013). Article CAS PubMed Google Scholar * Valladeau, J. et al. Langerin, a novel C-type lectin specific to Langerhans cells, is
an endocytic receptor that induces the formation of Birbeck granules. _Immunity_ 12, 71–81 (2000). Article CAS PubMed Google Scholar * Affandi, A. J., Carvalheiro, T., Radstake, T. &
Marut, W. Dendritic cells in systemic sclerosis: advances from human and mice studies. _Immunol. Lett._ 195, 18–29 (2018). Article CAS PubMed Google Scholar * Veglia, F. &
Gabrilovich, D. I. Dendritic cells in cancer: the role revisited. _Curr. Opin. Immunol._ 45, 43–51 (2017). Article CAS PubMed PubMed Central Google Scholar * Binai, N., O’Reilly, S.,
Griffiths, B., van Laar, J. M. & Hugle, T. Differentiation potential of CD14+ monocytes into myofibroblasts in patients with systemic sclerosis. _PLOS ONE_ 7, e33508 (2012). Article CAS
PubMed PubMed Central Google Scholar * Dantas, A. T. et al. Reassessing the role of the active TGF-beta1 as a biomarker in systemic sclerosis: association of serum levels with clinical
manifestations. _Dis. Markers_ 2016, 6064830 (2016). Article PubMed PubMed Central CAS Google Scholar * Hasegawa, M., Sato, S. & Takehara, K. Augmented production of transforming
growth factor-beta by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. _Arch. Dermatol. Res._ 296, 89–93 (2004). Article CAS PubMed Google Scholar *
Reese, C. et al. Caveolin-1 deficiency may predispose African Americans to systemic sclerosis-related interstitial lung disease. _Arthritis Rheumatol._ 66, 1909–1919 (2014). Article CAS
PubMed PubMed Central Google Scholar * Tourkina, E. et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1
scaffolding domain peptide. _Fibrogenesis Tissue Repair_ 4, 15 (2011). Article CAS PubMed PubMed Central Google Scholar * Lee, R. et al. Enhanced chemokine-receptor expression,
function, and signaling in healthy African American and scleroderma-patient monocytes are regulated by caveolin-1. _Fibrogenesis Tissue Repair_ 8, 11 (2015). Article PubMed PubMed Central
CAS Google Scholar * Ciechomska, M. et al. Toll-like receptor-mediated, enhanced production of profibrotic TIMP-1 in monocytes from patients with systemic sclerosis: role of serum
factors. _Ann. Rheum. Dis._ 72, 1382–1389 (2013). Article CAS PubMed Google Scholar * Visse, R. & Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. _Circ. Res._ 92, 827–839 (2003). Article CAS PubMed Google Scholar * Ciechomska, M. et al. Histone demethylation and Toll-like receptor 8-dependent
cross-talk in monocytes promotes transdifferentiation of fibroblasts in systemic sclerosis via Fra-2. _Arthritis Rheumatol._ 68, 1493–1504 (2016). Article CAS PubMed Google Scholar *
Maurer, B. et al. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. _Ann. Rheum. Dis._ 71, 1382–1387 (2012). Article CAS PubMed Google
Scholar * Maurer, B. et al. Transcription factor fos-related antigen-2 induces progressive peripheral vasculopathy in mice closely resembling human systemic sclerosis. _Circulation_ 120,
2367–2376 (2009). Article CAS PubMed Google Scholar * Reich, N. et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. _Arthritis
Rheum._ 62, 280–290 (2010). Article CAS PubMed Google Scholar * Bohgaki, T. et al. Up regulated expression of tumour necrosis factor {alpha} converting enzyme in peripheral monocytes of
patients with early systemic sclerosis. _Ann. Rheum. Dis._ 64, 1165–1173 (2005). Article CAS PubMed PubMed Central Google Scholar * Moss, M. L. & Minond, D. Recent advances in
ADAM17 research: a promising target for cancer and inflammation. _Mediators Inflamm._ 2017, 9673537 (2017). Article PubMed PubMed Central CAS Google Scholar * Schumacher, N. et al.
Shedding of endogenous interleukin-6 receptor (IL-6R) is governed by a disintegrin and metalloproteinase (ADAM) proteases while a full-length IL-6R isoform localizes to circulating
microvesicles. _J. Biol. Chem._ 290, 26059–26071 (2015). Article CAS PubMed PubMed Central Google Scholar * Duffy, M. J., Crown, J. & Mullooly, M. ADAM10 and ADAM17: new players in
trastuzumab tesistance. _Oncotarget_ 5, 10963–10964 (2014). Article PubMed PubMed Central Google Scholar * Mahoney, J. M. et al. Systems level analysis of systemic sclerosis shows a
network of immune and profibrotic pathways connected with genetic polymorphisms. _PLOS Comput. Biol._ 11, e1004005 (2015). Article PubMed PubMed Central CAS Google Scholar * Heinrich,
P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. _Biochem. J._ 374, 1–20 (2003). CAS PubMed PubMed Central Google Scholar * O’Reilly, S., Cant,
R., Ciechomska, M. & van Laar, J. M. Interleukin-6: a new therapeutic target in systemic sclerosis? _Clin. Transl Immunol._ 2, e4 (2013). Article CAS Google Scholar * Khanna, D. et
al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. _Lancet_ 387, 2630–2640 (2016). Article CAS
PubMed Google Scholar * Sahin, U. et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. _J. Cell Biol._ 164, 769–779 (2004). Article CAS PubMed PubMed
Central Google Scholar * Midgley, A. C. et al. Transforming growth factor-beta1 (TGF-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan
(HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. _J. Biol. Chem._ 288, 14824–14838 (2013). Article CAS PubMed PubMed Central Google
Scholar * Tan, F. K. et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients.
_Rheumatology_ 45, 694–702 (2006). Article CAS Google Scholar * Farina, G. A. et al. Poly(I:C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered
gene expression in systemic sclerosis. _J. Invest. Dermatol._ 130, 2583–2593 (2010). Article CAS PubMed PubMed Central Google Scholar * Christmann, R. B. et al. Interferon and
alternative activation of monocyte/macrophages in systemic sclerosis-associated pulmonary arterial hypertension. _Arthritis Rheum._ 63, 1718–1728 (2011). Article CAS PubMed PubMed Central
Google Scholar * York, M. R. et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and
toll-like receptor agonists. _Arthritis Rheum._ 56, 1010–1020 (2007). Article CAS PubMed Google Scholar * Moreno-Moral, A. et al. Changes in macrophage transcriptome associate with
systemic sclerosis and mediate GSDMA contribution to disease risk. _Ann. Rheum. Dis._ 77, 596–601 (2018). Article CAS PubMed Google Scholar * Manetti, M. Deciphering the alternatively
activated (M2) phenotype of macrophages in scleroderma. _Exp. Dermatol._ 24, 576–578 (2015). Article CAS PubMed Google Scholar * Del Galdo, F., Maul, G. G., Jimenez, S. A. & Artlett,
C. M. Expression of allograft inflammatory factor 1 in tissues from patients with systemic sclerosis and in vitro differential expression of its isoforms in response to transforming growth
factor beta. _Arthritis Rheum._ 54, 2616–2625 (2006). Article PubMed CAS Google Scholar * Utans, U., Arceci, R. J., Yamashita, Y. & Russell, M. E. Cloning and characterization of
allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. _J. Clin. Invest._ 95, 2954–2962 (1995). Article CAS PubMed PubMed
Central Google Scholar * Otieno, F. G., Lopez, A. M., Jimenez, S. A., Gentiletti, J. & Artlett, C. M. Allograft inflammatory factor-1 and tumor necrosis factor single nucleotide
polymorphisms in systemic sclerosis. _Tissue Antigens_ 69, 583–591 (2007). Article CAS PubMed Google Scholar * Yamamoto, A. et al. Allograft inflammatory factor-1 is overexpressed and
induces fibroblast chemotaxis in the skin of sclerodermatous GVHD in a murine model. _Immunol. Lett._ 135, 144–150 (2011). Article CAS PubMed Google Scholar * Rabquer, B. J. et al.
Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. _Arthritis Res. Ther._ 13, R18 (2011). Article CAS PubMed PubMed Central Google
Scholar * Distler, O. et al. Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet-derived growth factor and effects on monocyte chemotaxis and
collagen synthesis. _Arthritis Rheum._ 44, 2665–2678 (2001). Article CAS PubMed Google Scholar * Galindo, M. et al. Chemokine expression by systemic sclerosis fibroblasts: abnormal
regulation of monocyte chemoattractant protein 1 expression. _Arthritis Rheum._ 44, 1382–1386 (2001). Article CAS PubMed Google Scholar * Yamamoto, T., Eckes, B., Hartmann, K. &
Krieg, T. Expression of monocyte chemoattractant protein-1 in the lesional skin of systemic sclerosis. _J. Dermatol. Sci._ 26, 133–139 (2001). Article CAS PubMed Google Scholar * Ong, V.
H. et al. Monocyte chemoattractant protein 3 as a mediator of fibrosis: Overexpression in systemic sclerosis and the type 1 tight-skin mouse. _Arthritis Rheum._ 48, 1979–1991 (2003).
Article CAS PubMed Google Scholar * Bandinelli, F. et al. CCL2, CCL3 and CCL5 chemokines in systemic sclerosis: the correlation with SSc clinical features and the effect of prostaglandin
E1 treatment. _Clin. Exp. Rheumatol._ 30, S44–S49 (2012). PubMed Google Scholar * Assassi, S. et al. Skin gene expression correlates of severity of interstitial lung disease in systemic
sclerosis. _Arthritis Rheum._ 65, 2917–2927 (2013). Article CAS PubMed PubMed Central Google Scholar * Carulli, M. T. et al. Chemokine receptor CCR2 expression by systemic sclerosis
fibroblasts: evidence for autocrine regulation of myofibroblast differentiation. _Arthritis Rheum._ 52, 3772–3782 (2005). Article CAS PubMed Google Scholar * Abraham, D. J., Krieg, T.,
Distler, J. & Distler, O. Overview of pathogenesis of systemic sclerosis. _Rheumatology_ 48 (Suppl. 3), iii3–iii7 (2009). CAS Google Scholar * Distler, J. H. et al. Monocyte
chemoattractant protein 1 released from glycosaminoglycans mediates its profibrotic effects in systemic sclerosis via the release of interleukin-4 from T cells. _Arthritis Rheum._ 54,
214–225 (2006). Article CAS PubMed Google Scholar * Zhang, K., Gharaee-Kermani, M., Jones, M. L., Warren, J. S. & Phan, S. H. Lung monocyte chemoattractant protein-1 gene expression
in bleomycin-induced pulmonary fibrosis. _J. Immunol._ 153, 4733–4741 (1994). CAS PubMed Google Scholar * Ferreira, A. M. et al. Diminished induction of skin fibrosis in mice with MCP-1
deficiency. _J. Invest. Dermatol._ 126, 1900–1908 (2006). Article CAS PubMed Google Scholar * Distler, J. H., Akhmetshina, A., Schett, G. & Distler, O. Monocyte chemoattractant
proteins in the pathogenesis of systemic sclerosis. _Rheumatology_ 48, 98–103 (2009). Article CAS Google Scholar * Beyer, C., Schett, G., Distler, O. & Distler, J. H. Animal models of
systemic sclerosis: prospects and limitations. _Arthritis Rheum._ 62, 2831–2844 (2010). Article CAS PubMed Google Scholar * Vergunst, C. E. et al. Modulation of CCR2 in rheumatoid
arthritis: a double-blind, randomized, placebo-controlled clinical trial. _Arthritis Rheum._ 58, 1931–1939 (2008). Article CAS PubMed Google Scholar * Mathes, A. L. et al. Global
chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skin. _Ann. Rheum. Dis._ 73, 1864–1872 (2014). Article CAS PubMed Google
Scholar * Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. _Immunity_ 38, 1013–1024 (2013). Article
CAS PubMed PubMed Central Google Scholar * Fasnacht, N. et al. Specific fibroblastic niches in secondary lymphoid organs orchestrate distinct Notch-regulated immune responses. _J. Exp.
Med._ 211, 2265–2279 (2014). Article PubMed PubMed Central Google Scholar * Wu, M. et al. Identification of cadherin 11 as a mediator of dermal fibrosis and possible role in systemic
sclerosis. _Arthritis Rheumatol._ 66, 1010–1021 (2014). Article CAS PubMed PubMed Central Google Scholar * Kato, A. et al. Oligosaccharide modification by
N-acetylglucosaminyltransferase-V in macrophages are involved in pathogenesis of bleomycin-induced scleroderma. _Exp. Dermatol._ 24, 585–590 (2015). Article CAS PubMed Google Scholar *
Tian, H. et al. The implication of N-acetylglucosaminyltransferase V expression in gastric cancer. _Pathobiology_ 75, 288–294 (2008). Article CAS PubMed Google Scholar * Demetriou, M.,
Granovsky, M., Quaggin, S. & Dennis, J. W. Negative regulation of T cell activation and autoimmunity by Mgat5 N-glycosylation. _Nature_ 409, 733–739 (2001). Article CAS PubMed Google
Scholar * Morgan, R. et al. N-Acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. _J. Immunol._ 173, 7200–7208 (2004).
Article CAS PubMed Google Scholar * Knipper, J. A. et al. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. _Immunity_ 43, 803–816
(2015). Article CAS PubMed PubMed Central Google Scholar * Martins, V. et al. FIZZ1-induced myofibroblast transdifferentiation from adipocytes and its potential role in dermal fibrosis
and lipoatrophy. _Am. J. Pathol._ 185, 2768–2776 (2015). Article CAS PubMed PubMed Central Google Scholar * Wu, M. et al. Rosiglitazone abrogates bleomycin-induced scleroderma and
blocks profibrotic responses through peroxisome proliferator-activated receptor-gamma. _Am. J. Pathol._ 174, 519–533 (2009). Article CAS PubMed PubMed Central Google Scholar *
Kruglikov, I. L. Interfacial adipose tissue in systemic sclerosis. _Curr. Rheumatol. Rep._ 19, 4 (2017). Article PubMed PubMed Central CAS Google Scholar * Khanna, D. et al. Systemic
sclerosis-associated interstitial lung disease: lessons from clinical trials, outcome measures, and future study design. _Curr. Rheumatol. Rev._ 6, 138–144 (2010). Article PubMed PubMed
Central Google Scholar * Christmann, R. B. et al. Association of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related
progressive lung fibrosis. _Arthritis Rheumatol._ 66, 714–725 (2014). Article CAS PubMed PubMed Central Google Scholar * Luzina, I. G. et al. Gene expression in bronchoalveolar lavage
cells from scleroderma patients. _Am. J. Respir. Cell. Mol. Biol._ 26, 549–557 (2002). Article CAS PubMed Google Scholar * Schupp, J. et al. Serum CCL18 is predictive for lung disease
progression and mortality in systemic sclerosis. _Eur. Respir. J._ 43, 1530–1532 (2014). Article PubMed Google Scholar * Taroni, J. N. et al. A novel multi-network approach reveals
tissue-specific cellular modulators of fibrosis in systemic sclerosis. _Genome Med._ 9, 27 (2017). Article PubMed PubMed Central CAS Google Scholar * Larson-Casey, J. L., Deshane, J.
S., Ryan, A. J., Thannickal, V. J. & Carter, A. B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. _Immunity_ 44, 582–596 (2016). Article
CAS PubMed PubMed Central Google Scholar * Murray, L. A. et al. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. _Int. J. Biochem. Cell Biol._
40, 2174–2182 (2008). Article CAS PubMed Google Scholar * Ishida, Y. et al. Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of
fibrocyte and M2 macrophage migration. _Sci. Rep._ 7, 16833 (2017). Article PubMed PubMed Central CAS Google Scholar * Ayaub, E. A. et al. Overexpression of OSM and IL-6 impacts the
polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis. _Sci. Rep._ 7, 1328 (2017). Article CAS Google Scholar * Huang, J. et al. Nintedanib
inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. _Ann. Rheum. Dis._ 75, 883–890 (2016). Article CAS PubMed Google Scholar * Huang, J.
et al. Nintedanib macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. _Ann. Rheum. Dis._ 76, 1941–1948 (2017). Article
CAS PubMed Google Scholar * Spina, D. PDE4 inhibitors: current status. _Br. J. Pharmacol._ 155, 308–315 (2008). Article CAS PubMed PubMed Central Google Scholar * Essayan, D. M.
Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. _Biochem. Pharmacol._ 57, 965–973 (1999). Article CAS PubMed Google Scholar * Maier, C. et al. Inhibition of
phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. _Ann. Rheum. Dis._ 76, 1133–1141 (2017). Article CAS PubMed Google
Scholar * Schneider, D. J. et al. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. _FASEB J._ 26, 503–512
(2012). Article CAS PubMed PubMed Central Google Scholar * Nagahara, H. et al. Allograft inflammatory factor-1 in the pathogenesis of bleomycin-induced acute lung injury. _Biosci.
Trends_ 10, 47–53 (2016). Article CAS PubMed Google Scholar * Nagahara, H. et al. Role of allograft inflammatory factor-1 in bleomycin-induced lung fibrosis. _Biochem. Biophys. Res.
Commun._ 495, 1901–1907 (2018). Article CAS PubMed Google Scholar * Christmann, R. B. et al. miR-155 in the progression of lung fibrosis in systemic sclerosis. _Arthritis Res. Ther._ 18,
155 (2016). Article PubMed PubMed Central CAS Google Scholar * Gunawan, M., Jardine, L. & Haniffa, M. Isolation of human skin dendritic cell subsets. _Methods Mol. Biol._ 1423,
119–128 (2016). Article CAS PubMed Google Scholar * Hall, J. C. & Rosen, A. Type I interferons: crucial participants in disease amplification in autoimmunity. _Nat. Rev. Rheumatol._
6, 40–49 (2010). Article CAS PubMed PubMed Central Google Scholar * Ueno, H., Schmitt, N., Palucka, A. K. & Banchereau, J. Dendritic cells and humoral immunity in humans. _Immunol.
Cell Biol._ 88, 376–380 (2010). Article PubMed PubMed Central Google Scholar * Qi, H., Egen, J. G., Huang, A. Y. & Germain, R. N. Extrafollicular activation of lymph node B cells by
antigen-bearing dendritic cells. _Science_ 312, 1672–1676 (2006). Article CAS PubMed Google Scholar * Ganguly, D., Haak, S., Sisirak, V. & Reizis, B. The role of dendritic cells in
autoimmunity. _Nat. Rev. Immunol._ 13, 566–577 (2013). Article CAS PubMed PubMed Central Google Scholar * Boltjes, A. & van Wijk, F. Human dendritic cell functional specialization
in steady-state and inflammation. _Front. Immunol._ 5, 131 (2014). Article PubMed PubMed Central CAS Google Scholar * Poulin, L. F. et al. Characterization of human DNGR-1+ BDCA3+
leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. _J. Exp. Med._ 207, 1261–1271 (2010). Article CAS PubMed PubMed Central Google Scholar * Lauterbach, H. et al.
Mouse CD8alpha+ DCs and human BDCA3+DCs are major producers of IFN-lambda in response to poly IC. _J. Exp. Med._ 207, 2703–2717 (2010). Article CAS PubMed PubMed Central Google Scholar
* Chia, J. J. et al. Dendritic cells maintain dermal adipose-derived stromal cells in skin fibrosis. _J. Clin. Invest._ 126, 4331–4345 (2016). Article PubMed PubMed Central Google Scholar
* Fleischmajer, R., Damiano, V. & Nedwich, A. Scleroderma and the subcutaneous tissue. _Science_ 171, 1019–1021 (1971). Article CAS PubMed Google Scholar * Siegal, F. P. et al. The
nature of the principal type 1 interferon-producing cells in human blood. _Science_ 284, 1835–1837 (1999). Article CAS PubMed Google Scholar * Guiducci, C. et al. Autoimmune skin
inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. _J. Exp. Med._ 207, 2931–2942 (2010). Article CAS PubMed PubMed Central Google
Scholar * Swiecki, M. & Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. _Nat. Rev. Immunol._ 15, 471–485 (2015). Article CAS PubMed PubMed Central Google
Scholar * Ah Kioon, M. D. et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. _Sci. Transl Med._ 10, eaam8458 (2018). Article PubMed CAS Google
Scholar * Cipriani, P. et al. Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: pathogenetic
implications. _Arthritis Rheum._ 54, 3022–3033 (2006). Article CAS PubMed Google Scholar * Zabel, B. A., Silverio, A. M. & Butcher, E. C. Chemokine-like receptor 1 expression and
chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. _J. Immunol._ 174, 244–251 (2005). Article CAS PubMed Google Scholar * Akamata, K. et
al. Increased expression of chemerin in endothelial cells due to Fli1 deficiency may contribute to the development of digital ulcers in systemic sclerosis. _Rheumatology_ 54, 1308–1316
(2015). Article CAS Google Scholar * van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. _N. Engl. J. Med._ 370, 433–443 (2014). Article PubMed CAS
Google Scholar * Duan, H. et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. _Arthritis Rheum._ 58,
1465–1474 (2008). Article CAS PubMed Google Scholar * Rossato, M. et al. Association of MicroRNA-618 expression with altered frequency and activation of plasmacytoid dendritic cells in
patients with systemic sclerosis. _Arthritis Rheumatol._ 69, 1891–1902 (2017). Article CAS PubMed Google Scholar * Takahashi, T. et al. A potential contribution of antimicrobial peptide
LL-37 to tissue fibrosis and vasculopathy in systemic sclerosis. _Br. J. Dermatol._ 175, 1195–1203 (2016). Article CAS PubMed Google Scholar * Kafaja, S. et al. pDCs in lung and skin
fibrosis in a bleomycin-induced model and patients with systemic sclerosis. _JCI Insight_ 3, 98380 (2018). Article PubMed Google Scholar * McKenna, K., Beignon, A. S. & Bhardwaj, N.
Plasmacytoid dendritic cells: linking innate and adaptive immunity. _J. Virol._ 79, 17–27 (2005). Article CAS PubMed PubMed Central Google Scholar * Yanaba, K., Yoshizaki, A., Asano,
Y., Kadono, T. & Sato, S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: association with lower frequency and severity of pulmonary fibrosis. _J.
Rheumatol._ 38, 2193–2197 (2011). Article CAS PubMed Google Scholar * Lafyatis, R. Transforming growth factor beta — at the centre of systemic sclerosis. _Nat. Rev. Rheumatol._ 10,
706–719 (2014). Article CAS PubMed Google Scholar * Usategui, A. et al. A profibrotic role for thymic stromal lymphopoietin in systemic sclerosis. _Ann. Rheum. Dis._ 72, 2018–2023
(2013). Article CAS PubMed Google Scholar * Guggino, G. et al. Interleukin-9 over-expression and T helper 9 polarization in systemic sclerosis patients. _Clin. Exp. Immunol._ 190,
208–216 (2017). Article CAS PubMed PubMed Central Google Scholar * Chakraborty, K., Chatterjee, S. & Bhattacharyya, A. Modulation of CD11c + lung dendritic cells in respect to
TGF-beta in experimental pulmonary fibrosis. _Cell Biol. Int._ 41, 991–1000 (2017). Article CAS PubMed Google Scholar * Gerber, E. E. et al. Integrin-modulating therapy prevents fibrosis
and autoimmunity in mouse models of scleroderma. _Nature_ 503, 126–130 (2013). Article CAS PubMed PubMed Central Google Scholar * Siracusa, L. D. et al. A tandem duplication within the
fibrillin 1 gene is associated with the mouse tight skin mutation. _Genome Res._ 6, 300–313 (1996). Article CAS PubMed Google Scholar * Varghese, M. V. et al. Oxidative stress induced
by the chemotherapeutic agent arsenic trioxide. _3 Biotech._ 4, 425–430 (2014). Article PubMed Google Scholar * Kavian, N. et al. Arsenic trioxide prevents murine sclerodermatous
graft-versus-host disease. _J. Immunol._ 188, 5142–5149 (2012). Article CAS PubMed Google Scholar * Delaney, T. A. et al. Type I IFNs regulate inflammation, vasculopathy, and fibrosis in
chronic cutaneous graft-versus-host disease. _J. Immunol._ 197, 42–50 (2016). Article CAS PubMed Google Scholar * Municio, C. et al. Methotrexate limits inflammation through an
A20-dependent cross-tolerance mechanism. _Ann. Rheum. Dis._ 77, 752–759 (2018). Article CAS PubMed Google Scholar * Manno, R. & Boin, F. Immunotherapy of systemic sclerosis.
_Immunotherapy_ 2, 863–878 (2010). Article CAS PubMed Google Scholar * Fraticelli, P. et al. Low-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease
unresponsive to cyclophosphamide: a phase II pilot study. _Arthritis Res. Ther._ 16, R144 (2014). Article PubMed PubMed Central CAS Google Scholar * Kowal-Bielecka, O. et al. Update of
EULAR recommendations for the treatment of systemic sclerosis. _Ann. Rheum. Dis._ 76, 1327–1339 (2017). Article PubMed Google Scholar * Smith, V. et al. Systemic sclerosis: state of the
art on clinical practice guidelines. _RMD Open_ 4, e000782 (2018). Article PubMed PubMed Central Google Scholar * van Rhijn-Brouwer, F. C. C., Spierings, J. & van Laar, J. M.
Autologous hematopoietic stem cell transplantation in systemic sclerosis: a reset to tolerance? _Immunol. Lett._ 195, 88–96 (2018). Article PubMed CAS Google Scholar * van Laar, J. M.,
Naraghi, K. & Tyndall, A. Haematopoietic stem cell transplantation for poor-prognosis systemic sclerosis. _Rheumatology_ 54, 2126–2133 (2015). Article CAS Google Scholar * Sullivan,
K. M., Shah, A., Sarantopoulos, S. & Furst, D. E. Review: hematopoietic stem cell transplantation for scleroderma: effective immunomodulatory therapy for patients with pulmonary
involvement. _Arthritis Rheumatol._ 68, 2361–2371 (2016). Article PubMed PubMed Central Google Scholar * Tsukamoto, H. et al. Analysis of immune reconstitution after autologous
CD34+stem/progenitor cell transplantation for systemic sclerosis: predominant reconstitution of Th1 CD4+ T cells. _Rheumatology_ 50, 944–952 (2011). Article CAS Google Scholar * Michel,
L. et al. Evolution of serum cytokine profile after hematopoietic stem cell transplantation in systemic sclerosis patients. _Bone Marrow Transplant._ 51, 1146–1149 (2016). Article CAS
PubMed Google Scholar * Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. _J. Clin. Invest._ 125, 2795–2807
(2015). Article PubMed PubMed Central Google Scholar * Distler, O. et al. Design of a randomised, placebo-controlled clinical trial of nintedanib in patients with systemic
sclerosis-associated interstitial lung disease (SENSCIS). _Clin. Exp. Rheumatol._ 35 (Suppl. 106), 75–81 (2017). PubMed Google Scholar * Huang, J. et al. Nintedanib inhibits macrophage
activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. _Ann. Rheum. Dis._ 76, 1941–1948 (2017). Article CAS PubMed Google Scholar
* Kania, G. et al. Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental
autoimmune myocarditis. _Circ. Res._ 105, 462–470 (2009). Article CAS PubMed Google Scholar * Grimminger, F. et al. First acute haemodynamic study of soluble guanylate cyclase stimulator
riociguat in pulmonary hypertension. _Eur. Respir. J._ 33, 785–792 (2009). Article CAS PubMed Google Scholar * Beyer, C. et al. Stimulation of soluble guanylate cyclase reduces
experimental dermal fibrosis. _Ann. Rheum. Dis._ 71, 1019–1026 (2012). Article CAS PubMed Google Scholar * Beyer, C. et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits
fibrosis by blocking non-canonical TGFbeta signalling. _Ann. Rheum. Dis._ 74, 1408–1416 (2015). Article CAS PubMed Google Scholar * Dees, C. et al. Stimulators of soluble guanylate
cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. _Ann. Rheum. Dis._ 74, 1621–1625 (2015). Article CAS PubMed Google Scholar * Shaw, C. A., Webb, D. J., Rossi,
A. G. & Megson, I. L. Cyclic GMP protects human macrophages against peroxynitrite-induced apoptosis. _J. Inflamm._ 6, 14 (2009). Article CAS Google Scholar * Mitani, H. et al.
Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. _Br. J. Haematol._ 109, 288–295 (2000). Article CAS PubMed Google Scholar * Howlett, A.
C. The cannabinoid receptors. _Prostaglandins Other Lipid Mediat._ 68–69, 619–631 (2002). Article PubMed Google Scholar * Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C.
& Bonner, T. I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. _Nature_ 346, 561–564 (1990). Article CAS PubMed Google Scholar * Pacher, P. &
Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? _Prog. Lipid Res._ 50, 193–211 (2011). Article CAS PubMed PubMed Central Google Scholar *
Taylor, L. et al. Primary macrophage chemotaxis induced by cannabinoid receptor 2 agonists occurs independently of the CB2 receptor. _Sci. Rep._ 5, 10682 (2015). Article CAS PubMed PubMed
Central Google Scholar * Marchalant, Y., Cerbai, F., Brothers, H. M. & Wenk, G. L. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. _Neurobiol.
Aging_ 29, 1894–1901 (2008). Article CAS PubMed Google Scholar * Burstein, S. H. Ajulemic acid: potential treatment for chronic inflammation. _Pharmacol. Res. Perspect._ 6, e00394
(2018). Article PubMed PubMed Central CAS Google Scholar * Jin, Z. et al. Single-cell gene expression patterns in lupus monocytes independently indicate disease activity, interferon and
therapy. _Lupus Sci. Med._ 4, e000202 (2017). Article PubMed PubMed Central Google Scholar * Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in
rheumatoid arthritis. _Nature_ 542, 110–114 (2017). Article CAS PubMed PubMed Central Google Scholar * Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular
resolution by mass cytometry. _Nat. Methods_ 11, 417–422 (2014). Article CAS PubMed Google Scholar * Krieg, C. et al. High-dimensional single-cell analysis predicts response to anti-PD-1
immunotherapy. _Nat. Med._ 24, 144–153 (2018). Article CAS PubMed Google Scholar * Blyszczuk, P., Behnke, S., Luscher, T. F., Eriksson, U. & Kania, G. GM-CSF promotes inflammatory
dendritic cell formation but does not contribute to disease progression in experimental autoimmune myocarditis. _Biochim. Biophys. Acta_ 1833, 934–944 (2013). Article CAS PubMed Google
Scholar * Blyszczuk, P. et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy.
_Circ. Res._ 105, 912–920 (2009). Article CAS PubMed Google Scholar * Kania, G., Blyszczuk, P. & Eriksson, U. Mechanisms of cardiac fibrosis in inflammatory heart disease. _Trends
Cardiovasc. Med._ 19, 247–252 (2009). Article CAS PubMed Google Scholar * Nie, Y. et al. AKT2 regulates pulmonary inflammation and fibrosis via modulating macrophage activation. _J.
Immunol._ 198, 4470–4480 (2017). Article CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Rheumatology, Center of Experimental
Rheumatology, University Hospital Zurich, Zurich, Switzerland Gabriela Kania, Michal Rudnik & Oliver Distler Authors * Gabriela Kania View author publications You can also search for
this author inPubMed Google Scholar * Michal Rudnik View author publications You can also search for this author inPubMed Google Scholar * Oliver Distler View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS The authors contributed equally to all aspects of the article. CORRESPONDING AUTHOR Correspondence to Oliver Distler. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kania, G., Rudnik, M. & Distler, O. Involvement of the
myeloid cell compartment in fibrogenesis and systemic sclerosis. _Nat Rev Rheumatol_ 15, 288–302 (2019). https://doi.org/10.1038/s41584-019-0212-z Download citation * Published: 05 April
2019 * Issue Date: May 2019 * DOI: https://doi.org/10.1038/s41584-019-0212-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
