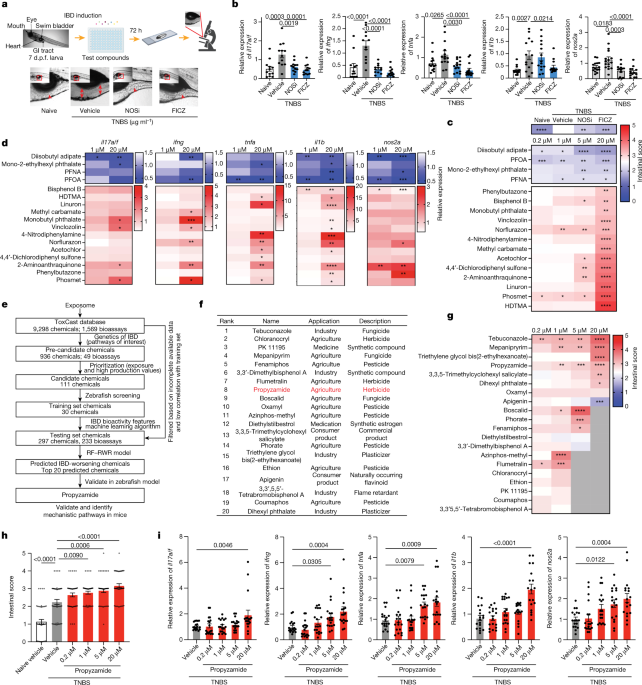
Identification of environmental factors that promote intestinal inflammation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Genome-wide association studies have identified risk loci linked to inflammatory bowel disease (IBD)1—a complex chronic inflammatory disorder of the gastrointestinal tract. The
increasing prevalence of IBD in industrialized countries and the augmented disease risk observed in migrants who move into areas of higher disease prevalence suggest that environmental
factors are also important determinants of IBD susceptibility and severity2. However, the identification of environmental factors relevant to IBD and the mechanisms by which they influence
disease has been hampered by the lack of platforms for their systematic investigation. Here we describe an integrated systems approach, combining publicly available databases, zebrafish
chemical screens, machine learning and mouse preclinical models to identify environmental factors that control intestinal inflammation. This approach established that the herbicide
propyzamide increases inflammation in the small and large intestine. Moreover, we show that an AHR–NF-κB–C/EBPβ signalling axis operates in T cells and dendritic cells to promote intestinal
inflammation, and is targeted by propyzamide. In conclusion, we developed a pipeline for the identification of environmental factors and mechanisms of pathogenesis in IBD and, potentially,
other inflammatory diseases. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may
be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS INFLAMMATION STATUS MODULATES THE EFFECT OF HOST GENETIC VARIATION ON INTESTINAL GENE EXPRESSION IN INFLAMMATORY BOWEL DISEASE Article Open access 18
February 2021 MULTIOMICS TO ELUCIDATE INFLAMMATORY BOWEL DISEASE RISK FACTORS AND PATHWAYS Article 17 March 2022 IDENTIFYING HIGH-IMPACT VARIANTS AND GENES IN EXOMES OF ASHKENAZI JEWISH
INFLAMMATORY BOWEL DISEASE PATIENTS Article Open access 20 April 2023 DATA AVAILABILITY RNA-seq and scRNA-seq data have been deposited at the GEO database under the following accession
number GSE194412 and GSE175766. 16S rRNA-sequencing data have been submitted to the NCBI sequence-read archive under BioProject number PRJNA804134. The machine learning codes used for this
study can be accessed at https://github.com/QuintanaLab/IBD_function_public. Source data are provided with this paper. REFERENCES * Huang, H. et al. Fine-mapping inflammatory bowel disease
loci to single-variant resolution. _Nature_ 547, 173–178 (2017). ADS CAS PubMed PubMed Central Google Scholar * Kamm, M. A. Rapid changes in epidemiology of inflammatory bowel disease.
_Lancet_ 390, 2741–2742 (2018). Google Scholar * Covacu, R. et al. System-wide analysis of the T cell response. _Cell Rep._ 14, 2733–2744 (2016). CAS PubMed PubMed Central Google Scholar
* Quintana, F. J. et al. Adaptive autoimmunity and Foxp3-based immunoregulation in zebrafish. _PLoS ONE_ 5, e9478 (2010). ADS PubMed PubMed Central Google Scholar * Wheeler, M. A. et
al. Environmental control of astrocyte pathogenic activities in CNS inflammation. _Cell_ 176, 581–596 (2019). CAS PubMed PubMed Central Google Scholar * White, R. M. et al. DHODH
modulates transcriptional elongation in the neural crest and melanoma. _Nature_ 471, 518–522 (2011). ADS CAS PubMed PubMed Central Google Scholar * Scott, B. M. et al. Self-tunable
engineered yeast probiotics for the treatment of inflammatory bowel disease. _Nat. Med._ 27, 1212–1222 (2021). CAS PubMed Google Scholar * Fleming, A., Jankowski, J. & Goldsmith, P.
In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: a feasibility study. _Inflamm. Bowel Dis._ 16, 1162–1172 (2010). PubMed
Google Scholar * Goettel, J. A. et al. AHR activation is protective against colitis driven by T cells in humanized mice. _Cell Rep._ 17, 1318–1329 (2016). CAS PubMed PubMed Central
Google Scholar * Richard, A. M. et al. ToxCast chemical landscape: paving the road to 21st century toxicology. _Chem. Res. Toxicol._ 29, 1225–1251 (2016). CAS PubMed Google Scholar *
Gut, P. et al. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. _Nat. Chem. Biol._ 9, 97–104 (2013). CAS PubMed Google Scholar * North, T. E. et
al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. _Nature_ 447, 1007–1011 (2007). ADS CAS PubMed PubMed Central Google Scholar * Richter, S., Schulze, U.,
Tomancak, P. & Oates, A. C. Small molecule screen in embryonic zebrafish using modular variations to target segmentation. _Nat. Commun._ 8, 1901 (2017). ADS PubMed PubMed Central
Google Scholar * Keiser, M. J. et al. Predicting new molecular targets for known drugs. _Nature_ 462, 175–181 (2009). ADS CAS PubMed PubMed Central Google Scholar * Chassaing, B. et
al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. _Nature_ 519, 92–96 (2015). ADS CAS PubMed PubMed Central Google Scholar * Kaakoush, N.
O. Sutterella species, IgA-degrading bacteria in ulcerative colitis. _Trends Microbiol._ 28, 519–522 (2020). CAS PubMed Google Scholar * Sanmarco, L. M. et al. Gut-licensed IFNγ+ NK
cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. _Nature_ 590, 473–479 (2021). ADS CAS PubMed PubMed Central Google Scholar * Schiering, C. et al. Feedback control of AHR
signalling regulates intestinal immunity. _Nature_ 542, 242–245 (2017). ADS CAS PubMed PubMed Central Google Scholar * Rothhammer, V. et al. Microglial control of astrocytes in response
to microbial metabolites. _Nature_ 557, 724–728 (2018). ADS CAS PubMed PubMed Central Google Scholar * Okey, A. B., Vella, L. M. & Harper, P. A. Detection and characterization of a
low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1-450 by 3-methylcholanthrene. _Mol. Pharmacol._ 35, 823–830 (1989). CAS PubMed
Google Scholar * Akashi, T. I., Nagano, K., Enomoto, E., Mizuno, M. & Shibaok, K. Effects of propyzamide on tobacco cell microtubules in vivo and in vitro. _Plant Cell Physiol._ 29,
1053–1062 (1988). CAS Google Scholar * Jackman, R. W., Rhoads, M. G., Cornwell, E. & Kandarian, S. C. Microtubule-mediated NF-κB activation in the TNF-α signaling pathway. _Exp. Cell.
Res._ 315, 3242–3249 (2009). CAS PubMed PubMed Central Google Scholar * Garber, M. et al. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene
regulation in mammals. _Mol. Cell_ 47, 810–822 (2012). CAS PubMed Google Scholar * Satpathy, A. T. et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to
attaching-and-effacing bacterial pathogens. _Nat. Immunol._ 14, 937–948 (2013). CAS PubMed PubMed Central Google Scholar * Meredith, M. M. et al. Expression of the zinc finger
transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. _J. Exp. Med._ 209, 1153–1165 (2012). CAS PubMed PubMed Central Google Scholar * Elmentaite, R. et
al. Single-cell sequencing of developing human gut reveals transcriptional links to childhood Crohn’s disease. _Dev. Cell_ 55, 771–783 (2020). CAS PubMed PubMed Central Google Scholar *
Martin, J. C. et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. _Cell_ 178, 1493–1508 (2019). CAS
PubMed PubMed Central Google Scholar * Boland, B. S. et al. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. _Sci.
Immunol._ 5, eabb4432 (2020). CAS PubMed PubMed Central Google Scholar * Cybulsky, M. I. et al. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the
human _VCAM1_ gene. _Proc. Natl Acad. Sci. USA_ 88, 7859–7863 (1991). ADS CAS PubMed PubMed Central Google Scholar * Oh, H. & Ghosh, S. NF-κB: roles and regulation in different CD4+
T-cell subsets. _Immunol. Rev._ 252, 41–51 (2013). PubMed PubMed Central Google Scholar * Chu, H. et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel
disease. _Science_ 352, 1116–1120 (2016). ADS CAS PubMed PubMed Central Google Scholar * Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into
aryl hydrocarbon receptor ligands. _Nat. Med._ 22, 598–605 (2016). CAS PubMed PubMed Central Google Scholar * Rothschild, D. et al. Environment dominates over host genetics in shaping
human gut microbiota. _Nature_ 555, 210–215 (2018). ADS CAS PubMed Google Scholar * Iyer, S. S. et al. Dietary and microbial oxazoles induce intestinal inflammation by modulating aryl
hydrocarbon receptor responses. _Cell_ 173, 1123–1134 (2018). CAS PubMed PubMed Central Google Scholar * Cole, D. J. _Metabolic Pathways of Agrochemicals. Part One—Herbicides and Plant
Growth Regulators_ (eds Roberts, T. et al.) (Royal Society of Chemistry, 1998). * _Propyzamide; Pesticide Tolerances;_
https://www.federalregister.gov/documents/2016/01/13/2016-00534/propyzamide-pesticide-tolerances (US Government, 2016). * Chaiklieng, S., Suggaravetsiri, P. & Autrup, H. Risk assessment
on benzene exposure among gasoline station workers. _Int. J. Environ. Res. Publ. Health_ 16, 2545 (2019). CAS Google Scholar * Ott, M. G., Diller, W. F. & Jolly, A. T. Respiratory
effects of toluene diisocyanate in the workplace: a discussion of exposure-response relationships. _Crit. Rev. Toxicol._ 33, 1–59 (2003). CAS PubMed Google Scholar * Cuenca, L. et al.
Environmentally-relevant exposure to diethylhexyl phthalate (DEHP) alters regulation of double-strand break formation and crossover designation leading to germline dysfunction in
_Caenorhabditis elegans_. _PLoS Genet._ 16, e1008529 (2020). CAS PubMed PubMed Central Google Scholar * World Health Organization. _Guidelines for Drinking-Water Quality_ Vol. 2, Ch.
14.11, 461–467 (1996). * _Toxicological Profile for Toluene Diisocyanate and Methylenediphenyl Diisocyanate_ (US Department of Health and Human Services, 2018). * World Health Organization.
_Guidelines for Drinking-Water Quality_ Vol. 2, Ch. 14.21, 530–540 (1996). * Sorg, O. AhR signalling and dioxin toxicity. _Toxicol. Lett._ 230, 225–233 (2014). CAS PubMed Google Scholar *
Muku, G. E., Murray, I. A., Espín, J. C. & Perdew, G. H. Urolithin A is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. _Metabolites_ 8, 86 (2018). PubMed
PubMed Central Google Scholar * Gerondakis, S., Fulford, T. S., Messina, N. L. & Grumont, R. J. NF-κB control of T cell development. _Nat. Immunol._ 15, 15–25 (2014). CAS PubMed
Google Scholar * Balasubramani, A. et al. Modular utilization of distal _cis_-regulatory elements controls _Ifng_ gene expression in T cells activated by distinct stimuli. _Immunity_ 33,
35–47 (2010). CAS PubMed PubMed Central Google Scholar * Ruan, Q. et al. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. _J. Exp. Med._ 208, 2321–2333
(2011). CAS PubMed PubMed Central Google Scholar * Yosef, N. et al. Dynamic regulatory network controlling TH17 cell differentiation. _Nature_ 496, 461–468 (2013). ADS CAS PubMed
PubMed Central Google Scholar * Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. _Nature_ 491, 119–124 (2012). CAS PubMed
PubMed Central Google Scholar * Satoh, T. et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. _Nature_ 541, 96–101 (2017). ADS CAS PubMed Google
Scholar * Jaronen, M., Wheeler, M. A. & Quintana, F. J. Protocol for inducing inflammation and acute myelin degeneration in larval zebrafish. _STAR Protoc._ 3, 101134 (2022). CAS
PubMed PubMed Central Google Scholar * Nüsslein-Volhard, C. & Dahm, R. _Zebrafish: A Practical Approach_ 1st edn (Oxford Univ. Press, 2002). * Cusick, M. F., Libbey, J. E., Trede, N.
S., Eckels, D. D. & Fujinami, R. S. Human T cell expansion and experimental autoimmune encephalomyelitis inhibited by Lenaldekar, a small molecule discovered in a zebrafish screen. _J.
Neuroimmunol._ 244, 35–44 (2012). CAS PubMed Google Scholar * Ridges, S. et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. _Blood_ 119, 5621–5631
(2012). CAS PubMed PubMed Central Google Scholar * _ToxCast & Tox21 Summary Files from invitrodb_v3_; https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data (US
EPA, accessed 28 October 2018). * Ruder, B., Atreya, R. & Becker, C. Tumour necrosis factor alpha in intestinal homeostasis and gut related diseases. _Int. J. Mol. Sci._ 20, 1887 (2019).
CAS PubMed PubMed Central Google Scholar * Andreou, N. P., Legaki, E. & Gazouli, M. Inflammatory bowel disease pathobiology: the role of the interferon signature. _Ann.
Gastroenterol._ 33, 125–133 (2020). PubMed PubMed Central Google Scholar * McEntee, C. P., Finlay, C. M. & Lavelle, E. C. Divergent roles for the IL-1 family in gastrointestinal
homeostasis and inflammation. _Front. Immunol._ 10, 1266 (2019). CAS PubMed PubMed Central Google Scholar * Salas, A. et al. JAK-STAT pathway targeting for the treatment of inflammatory
bowel disease. _Nat. Rev. Gastroenterol. Hepatol._ 17, 323–337 (2020). PubMed Google Scholar * Decara, J. et al. Peroxisome proliferator-activated receptors: experimental targeting for the
treatment of inflammatory bowel diseases. _Front. Pharmacol._ 11, 730 (2020). CAS PubMed PubMed Central Google Scholar * Pernomian, L., Duarte-Silva, M. & de Barros Cardoso, C. R.
The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. _Clin. Rev. Allergy Immunol._ 59,
382–390 (2020). CAS PubMed Google Scholar * Langfelder, P. & Horvath, S. Eigengene networks for studying the relationships between co-expression modules. _BMC Syst. Biol._ 1, 54
(2007). PubMed PubMed Central Google Scholar * Martínez-Camblor, P., Pérez-Fernández, S. & Díaz-Coto, S. The role of the _p_-value in the multitesting problem. _J. Appl. Stat._ 47,
1529–1542 (2020). MathSciNet PubMed MATH Google Scholar * Breiman, L. Random forests. _Mach. Learn._ 45, 5–32 (2001). MATH Google Scholar * Tong, H., Faloutsos, C. & Pan, J. Fast
random walk with restart and its applications. In _Proc. Sixth International Conference on Data Mining (ICDM’06)_ 613–622 (IEEE, 2006). * Kohler, S., Bauer, S., Horn, D. & Robinson, P.
N. Walking the interactome for prioritization of candidate disease genes. _Am. J. Hum. Genet._ 82, 949–958 (2008). PubMed PubMed Central Google Scholar * Zhang, B. & Horvath, S. A
general framework for weighted gene co-expression network analysis. _Stat. Appl. Genet. Mol. Biol._ 4, 17 (2005). MathSciNet MATH Google Scholar * Zhou, G. et al. NetworkAnalyst 3.0: a
visual analytics platform for comprehensive gene expression profiling and meta-analysis. _Nucleic Acids Res._ 47, W234–W241 (2019). CAS PubMed PubMed Central Google Scholar * Neurath, M.
F., Fuss, I., Kelsall, B. L., Stuber, E. & Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. _J. Exp. Med._ 182, 1281–1290 (1995). CAS PubMed
Google Scholar * Soumillon, M., Cacchiarelli, D., Semrau, S., van Oudenaarden, A. & Mikkelsen, T. S. Characterization of directed differentiation by high-throughput single-cell
RNA-Seq. Preprint at _bioRxiv_ https://doi.org/10.1101/003236 (2014). * Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). CAS PubMed Google
Scholar * Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. _BMC Bioinform._ 12, 323 (2011). CAS Google Scholar *
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. _Nat. Biotechnol._ 34, 525–527 (2016). CAS PubMed Google Scholar * Love, M. S.,
Soneson, C. & Robinson, M. D. Importing transcript abundance datasets with tximport. _Bioconductor_
https://bioconductor.org/packages/devel/bioc/vignettes/tximport/inst/doc/tximport.html (2017). * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion
for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). PubMed PubMed Central Google Scholar * Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence
count data: removing the noise and preserving large differences. _Bioinformatics_ 35, 2084–2092 (2019). CAS PubMed Google Scholar * Zheng, G. X. et al. Massively parallel digital
transcriptional profiling of single cells. _Nat. Commun._ 8, 14049 (2017). ADS CAS PubMed PubMed Central Google Scholar * Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet:
computational identification of cell doublets in single-cell transcriptomic data. _Cell Syst._ 8, 281–291 (2019). CAS PubMed PubMed Central Google Scholar * Hafemeister, C. & Satija,
R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. _Genome Biol._ 20, 296 (2019). CAS PubMed PubMed Central Google
Scholar * Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. _Nat.
Biotechnol._ 36, 411–420 (2018). CAS PubMed PubMed Central Google Scholar * Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. _Nat. Methods_
16, 1289–1296 (2019). CAS PubMed PubMed Central Google Scholar * Mi, H., Muruganujan, A., Ebert, D., Huang, X. & Thomas, P. D. PANTHER version 14: more genomes, a new PANTHER
GO-slim and improvements in enrichment analysis tools. _Nucleic Acids Res._ 47, D419–D426 (2019). CAS PubMed Google Scholar * Finak, G. et al. MAST: a flexible statistical framework for
assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. _Genome Biol._ 16, 278 (2015). PubMed PubMed Central Google Scholar * Caporaso, J.
G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. _ISME J._ 6, 1621–1624 (2012). CAS PubMed PubMed Central Google Scholar * Walters,
W. et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. _mSystems_ 1, e00009-15 (2016). PubMed
Google Scholar * Cox, L. M. et al. Calorie restriction slows age-related microbiota changes in an Alzheimer’s disease model in female mice. _Sci. Rep._ 9, 17904 (2019). ADS PubMed PubMed
Central Google Scholar * Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. _Nat. Methods_ 7, 335–336 (2010). CAS PubMed PubMed Central Google
Scholar * Yoon, S. H. et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. _Int. J. Syst. Evol. Microbiol._ 67, 1613–1617
(2017). CAS PubMed PubMed Central Google Scholar * Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J. & Knight, R. UniFrac: an effective distance metric for microbial community
comparison. _ISME J._ 5, 169–172 (2011). PubMed Google Scholar * Chu, C. et al. The microbiota regulate neuronal function and fear extinction learning. _Nature_ 574, 543–548 (2019). ADS
CAS PubMed PubMed Central Google Scholar * Yeste, A. et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. _Sci. Signal._ 9, ra61 (2016). PubMed Google
Scholar * Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon
receptor. _Nat. Med._ 22, 586–597 (2016). CAS PubMed PubMed Central Google Scholar * Burbach, K. M., Poland, A. & Bradfield, C. A. Cloning of the Ah receptor cDNA reveals a
distinctive ligand-activated transcription factor. _Proc. Natl Acad. Sci. USA_ 89, 8185–8189 (1992). ADS CAS PubMed PubMed Central Google Scholar * Dolwick, K. M., Schmidt, J. V.,
Carver, L. A., Swanson, H. I. & Bradfield, C. A. Cloning and expression of a human Ah receptor cDNA. _Mol. Pharmacol._ 44, 911–917 (1993). CAS PubMed Google Scholar * Lowe, M. M. et
al. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. _PLoS ONE_ 9, e87877 (2014). ADS PubMed PubMed Central Google
Scholar * Song, J. et al. A ligand for the aryl hydrocarbon receptor isolated from lung. _Proc. Natl Acad. Sci. USA_ 99, 14694–14699 (2002). ADS CAS PubMed PubMed Central Google Scholar
* Parks, A. J. et al. In silico identification of an aryl hydrocarbon receptor (AHR) antagonist with biological activity in vitro and in vivo. _Mol. Pharmacol._ 86, 593–608 (2014). PubMed
PubMed Central Google Scholar * Mascanfroni, I. D. et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39.
_Nat. Immunol._ 14, 1054–1063 (2013). CAS PubMed PubMed Central Google Scholar * Joung, J. et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. _Nat.
Protoc._ 12, 828–863 (2017). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank all of the members of the Quintana laboratory for advice and
discussions; R. Krishnan for technical assistance with flow cytometry studies; E. Buys and the BWH aquatics facility for assistance with breeding and maintaining the zebrafish; the staff at
the Tufts and Harvard histology core facilities for providing histopathology services; the staff at the NeuroTechnology Studio at Brigham and Women’s Hospital for providing instrument
access; L. Zon and G. Stirtz at Boston Children’s Hospital for providing zebrafish lines and advice; D. Rojas Marquez (@darwid_illustration) for help with the model figure. All of the other
illustrations were created using BioRender. This work was supported by grants NS087867, ES025530, ES032323, AI126880 and AI093903 from the National Institutes of Health. C.-C.C. received
support (104-2917-I-564 −024) from the Ministry of Science and Technology, Taiwan. Y.-C.W. received support by grants and 109-2221-E-010-013-MY3 and 107-2221-E-010-019-MY3 from the Ministry
of Science and Technology, Taiwan. C.M.P. was supported by a fellowship from the FAPESP BEPE (2019/13731-0). C.G.-V. was supported by an Alfonso Martín Escudero Foundation postdoctoral
fellowship and by a postdoctoral fellowship from the European Molecular Biology Organization (ALTF 610-2017). G.P. is a trainee in the Medical Scientist Training Program funded by NIH T32
GM007356. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical
Science or NIH. H.-G.L. was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A3A14039088).
M.J. was supported by a post-doctoral fellowship from Sigrid Juselius, personal post-doctoral grants from Saastamoinen Foundation, Paulo Foundation, The Finnish MS-Foundation, Orion Farmos
Research Foundation and Maud Kuistila Memory Foundation. M.A.W. was supported by the NIH (1K99NS114111, F32NS101790), a training grant from the NIH and Dana-Farber Cancer Institute
(T32CA207201), a travelling neuroscience fellowship from the Program in Interdisciplinary Neuroscience at the Brigham and Women’s Hospital and the Women’s Brain Initiative at the Brigham and
Women’s Hospital. V.R. received support from an educational grant from Mallinkrodt Pharmaceuticals (A219074) and by a fellowship from the German Research Foundation (DFG RO4866 1/1). R.C.
received support by a postdoctoral fellowship from the Swedish Research Council. B.M.A. received support from K12CA090354 from the NIH. AUTHOR INFORMATION Author notes * These authors
contributed equally: Liliana M. Sanmarco, Chun-Cheih Chao, Yu-Chao Wang, Jessica E. Kenison AUTHORS AND AFFILIATIONS * Ann Romney Center for Neurologic Diseases, Brigham and Women’s
Hospital, Harvard Medical School, Boston, MA, USA Liliana M. Sanmarco, Chun-Cheih Chao, Jessica E. Kenison, Zhaorong Li, Joseph M. Rone, Claudia M. Rejano-Gordillo, Carolina M. Polonio,
Cristina Gutierrez-Vazquez, Gavin Piester, Agustin Plasencia, Lucinda Li, Federico Giovannoni, Hong-Gyun Lee, Camilo Faust Akl, Michael A. Wheeler, Ivan Mascanfroni, Merja Jaronen, Moneera
Alsuwailm, Patrick Hewson, Ada Yeste, Brian M. Andersen, Millicent Ekwudo, Emily C. Tjon, Veit Rothhammer, Maisa Takenaka, Kalil Alves de Lima, Mathias Linnerbauer, Lydia Guo, Ruxandra
Covacu, Hugo Queva, Pedro Henrique Fonseca-Castro, Maha Al Bladi, Laura M. Cox, Kevin J. Hodgetts & Francisco J. Quintana * Institute of Biomedical Informatics, National Yang Ming Chiao
Tung University, Taipei, Taiwan Yu-Chao Wang & Chien-Jung Huang * Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, NY, USA Gavin
Piester * The Broad Institute of Harvard and MIT, Cambridge, MA, USA Michael A. Wheeler & Francisco J. Quintana * Center for Neuro-Oncology, Dana-Farber Cancer Institute, Harvard Medical
School, Boston, MA, USA Brian M. Andersen * Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA, USA Diana G. Franks & Mark E. Hahn * Max-Delbrück-Center for
Molecular Medicine (MDC), Berlin, Germany Alexander Mildner * Department of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Joshua Korzenik * Harvard T. H. Chan School of Public Health, Boston, MA, USA Russ Hauser * Department of Pediatrics, Division of Gastroenterology, Hepatology and Nutrition, Boston
Children’s Hospital, Boston, MA, USA Scott B. Snapper * Department of Medicine, Harvard Medical School, Boston, MA, USA Scott B. Snapper Authors * Liliana M. Sanmarco View author
publications You can also search for this author inPubMed Google Scholar * Chun-Cheih Chao View author publications You can also search for this author inPubMed Google Scholar * Yu-Chao Wang
View author publications You can also search for this author inPubMed Google Scholar * Jessica E. Kenison View author publications You can also search for this author inPubMed Google
Scholar * Zhaorong Li View author publications You can also search for this author inPubMed Google Scholar * Joseph M. Rone View author publications You can also search for this author
inPubMed Google Scholar * Claudia M. Rejano-Gordillo View author publications You can also search for this author inPubMed Google Scholar * Carolina M. Polonio View author publications You
can also search for this author inPubMed Google Scholar * Cristina Gutierrez-Vazquez View author publications You can also search for this author inPubMed Google Scholar * Gavin Piester View
author publications You can also search for this author inPubMed Google Scholar * Agustin Plasencia View author publications You can also search for this author inPubMed Google Scholar *
Lucinda Li View author publications You can also search for this author inPubMed Google Scholar * Federico Giovannoni View author publications You can also search for this author inPubMed
Google Scholar * Hong-Gyun Lee View author publications You can also search for this author inPubMed Google Scholar * Camilo Faust Akl View author publications You can also search for this
author inPubMed Google Scholar * Michael A. Wheeler View author publications You can also search for this author inPubMed Google Scholar * Ivan Mascanfroni View author publications You can
also search for this author inPubMed Google Scholar * Merja Jaronen View author publications You can also search for this author inPubMed Google Scholar * Moneera Alsuwailm View author
publications You can also search for this author inPubMed Google Scholar * Patrick Hewson View author publications You can also search for this author inPubMed Google Scholar * Ada Yeste
View author publications You can also search for this author inPubMed Google Scholar * Brian M. Andersen View author publications You can also search for this author inPubMed Google Scholar
* Diana G. Franks View author publications You can also search for this author inPubMed Google Scholar * Chien-Jung Huang View author publications You can also search for this author
inPubMed Google Scholar * Millicent Ekwudo View author publications You can also search for this author inPubMed Google Scholar * Emily C. Tjon View author publications You can also search
for this author inPubMed Google Scholar * Veit Rothhammer View author publications You can also search for this author inPubMed Google Scholar * Maisa Takenaka View author publications You
can also search for this author inPubMed Google Scholar * Kalil Alves de Lima View author publications You can also search for this author inPubMed Google Scholar * Mathias Linnerbauer View
author publications You can also search for this author inPubMed Google Scholar * Lydia Guo View author publications You can also search for this author inPubMed Google Scholar * Ruxandra
Covacu View author publications You can also search for this author inPubMed Google Scholar * Hugo Queva View author publications You can also search for this author inPubMed Google Scholar
* Pedro Henrique Fonseca-Castro View author publications You can also search for this author inPubMed Google Scholar * Maha Al Bladi View author publications You can also search for this
author inPubMed Google Scholar * Laura M. Cox View author publications You can also search for this author inPubMed Google Scholar * Kevin J. Hodgetts View author publications You can also
search for this author inPubMed Google Scholar * Mark E. Hahn View author publications You can also search for this author inPubMed Google Scholar * Alexander Mildner View author
publications You can also search for this author inPubMed Google Scholar * Joshua Korzenik View author publications You can also search for this author inPubMed Google Scholar * Russ Hauser
View author publications You can also search for this author inPubMed Google Scholar * Scott B. Snapper View author publications You can also search for this author inPubMed Google Scholar *
Francisco J. Quintana View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.M.S., C.-C.C., J.E.K. and F.J.Q. designed research. L.M.S.,
C.-C.C., J.E.K., J.M.R., C.M.R.-G., C.M.P., C.G.-V., G.P., A.P., L.L., F.G., H.-G.L., C.F.A., M.A.W., I.M., M.J., M.A., P.H., A.Y., B.M.A., D.G.F., M.E., V.R., M.T., K.A.d.L., M.L., L.G.,
R.C., H.Q., P.H.F.-C. and M.A.B. performed experiments. L.M.S., C.-C.C., J.E.K., Z.L., E.C.T., V.R., L.M.C., K.J.H, M.E.H., J.K., R.H., S.B.S. and F.J.Q. analysed data. A.M. contributed mice
and reagents. Y.-C.W., E.C.T. and C.-J.H. performed machine learning. L.M.S., C.-C.C., J.E.K. and F.J.Q. wrote the paper with input from all of the authors. F.J.Q. directed and supervised
the study. CORRESPONDING AUTHOR Correspondence to Francisco J. Quintana. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW
INFORMATION _Nature_ thanks Judy Cho, Mark Sundrud and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 TNBS-INDUCED
INTESTINAL INFLAMMATION IN ZEBRAFISH. (A) Zebrafish survival when treated with 25, 50, or 75 μg ml−1 TNBS for 24, 48, 72, or 96 h. Data shown as mean percent survival ±SEM of 3 independent
experiments with n = 20 fish per group per experiment. (B) Intestinal scores of naive 10 d.p.f. zebrafish, or zebrafish exposed for 24 or 72 h starting at 7 d.p.f. to TNBS (25 μg ml−1). (n =
24 per group). (C) Intestinal scores of zebrafish exposed for 72 h starting at 7 d.p.f. to TNBS plus vehicle, NOS inhibitor (NOSi, 10 μM), or FICZ (10 μM). (n = 24 per group). (D–F)
Intestinal scores (D) and _lck_ (E), _il17a/f_, _tnfa_, _ifng_, _il1b_ and _nos2a_ (F) expression in 10 d.p.f. naive, or TNBS-exposed (25 μg ml−1, 72 h) vehicle- or lenaldekar-treated (LDK,
5 μM) zebrafish (n = 24 per group for intestinal scores, n = 6 for _lck_ naive vehicle, n = 11 otherwise). (G) Intestinal scores in 10 d.p.f. naive vehicle or TNBS-exposed (25 μg ml−1, 72 h)
WT, _rag1-_ and _rag2_-deficient zebrafish. (n = 24 for WT naive and TNBS, n = 44 for _rag1_ and _rag2_ KO groups). (H–I) _lck_ (H), _il17a/f_, _tnfa_, _ifng_, _il1b_ and _nos2a_ (I)
expression in 10 d.p.f. TNBS-exposed (25 μg ml−1, 72 h) WT or _rag2_-deficient zebrafish. (n = 18 per group). (J) T cells expressing GFP under the control of the _lck_ promoter in the
intestine of naive 10 d.p.f. lck:gfp zebrafish, or lck:gfp zebrafish exposed for 72 h starting at 7 d.p.f. to TNBS (25 μg ml−1) alone or in combination with lenaldekar (LDK, 5 μM) or
propyzamide (20 μM). Top panels are brightfield, middle panels are _lck:gfp_ expression, bottom panels are a composite of both. (K) Quantification of intestinal GFP-positive T cells in
lck:gfp zebrafish shown in (J). (n = 12 per group). (L) Activity of candidate chemicals in 49 ToxCast bioassays targeting genes linked to IBD; shown as active (red), inactive (blue), or no
data (grey). (M) Principal component analysis based on ToxCast bioassays in chemicals found to ameliorate, promote, or have no effect on intestinal inflammation in the TNBS-induced zebrafish
model. (N) Intestinal scores in zebrafish exposed for 72 h to TNBS and chemicals randomly selected from the ToxCast database at 0.2, 1, 5, or 20 μM. Each panel represents the average of n =
12 zebrafish per group. Grey panels indicate concentrations lethal to zebrafish larvae. (O,P) Intestinal scores (O) and _il17a/f_, _tnfa_, _ifng_, _il1b_ and _nos2a_ expression (P) in naive
zebrafish treated with vehicle or propyzamide (0.2, 1, 5, or 20 μM) (n = 24 per group for intestinal scores, n = 9 per group for gene expression). (Q) Intestinal scores of TNBS-exposed (25
μg ml−1, 72 h) zebrafish treated with vehicle, NOS inhibitor (10 μM), FICZ (10 μM), or propyzamide (20 μM), or a combination as indicated. (n = 36 for TNBS+vehicle and TNBS+propyzamide
groups, n = 24 otherwise). Two-way ANOVA followed by Šídák’s multiple comparisons test for B. One-way ANOVA followed by Šídák’s or Dunnett’s multiple comparisons test for C–G,K,N,O,Q.
Unpaired student’s T test for H,I. Data shown as mean±SEM. Source Data EXTENDED DATA FIG. 2 PROPYZAMIDE BOOSTS TNBS-INDUCED COLITIS IN MICE. (A) Gating strategy used to analyse CD4+ T cells.
(B) CD3+ lymphocytes in colon normalized by tissue length from vehicle- or propyzamide-treated (100 mg kg−1) mice during TNBS-induced colitis (n = 5 mice per group). (C) Representative dot
plots of IFNγ and IL17 expression in CD4+ T cells. (D) Representative dot plots of IL-17 and RORγt expression in CD4 T cells and number of IL17+RORγt+ CD4 T cells in vehicle- or
propyzamide-treated TNBS mice (n = 4 for vehicle, n = 3 for propyzamide). Unpaired student’s T test. (E) _Ifng_ and _Il17_ expression determined by qPCR in lamina propria mononuclear cells
(LPMC) from naive mice (n = 5) and vehicle- or propyzamide-treated TNBS mice (n = 4 mice per group). (F) IL17+γδ+ or CD8+ T cells isolated from colons of vehicle- (n = 7) or
propyzamide-treated (n = 9) mice during TNBS-induced colitis. (G,H) Weight change (g) and colon length (h) of mice treated with vehicle or propyzamide (100 mg kg−1) for 10 days (n = 20 mice
per group). (I) Total CD4+ (n = 10 propyzamide, n = 9 vehicle), IFNγ+ CD4+ (n = 17 per group) and IL-17+ CD4+ (n = 9 per group) T cells in colons of vehicle- or propyzamide-treated mice. (J)
CD8+and IFNγ+ CD8+ T cells in colons of vehicle- (n = 9 for CD8+, n = 10 for IFNγ+ CD8+) or propyzamide-treated (n = 8 per group) mice. (K) γδ+T and IL-17+ γδ+T cells in colons of vehicle-
or propyzamide-treated mice (n = 10 per group). (L) CD127+ ILCs (n = 10 vehicle, n = 7 propyzamide) and IL-17+ILC3s (n = 6 per group) in colons of vehicle- or propyzamide-treated mice. (M)
_Rela_ and _Cebpb_ expression determined by qPCR from colonic CD45+ cells isolated from vehicle- or propyzamide-treated mice (n = 4 for propyzamide _Rela_, n = 5 otherwise). (N) Propyzamide
concentrations in plasma (n = 6 per timepoint), faeces (n = 1) and urine (n = 1) after propyzamide administration (100 mg kg−1). (O) Propyzamide levels in plasma, faeces and urine collected
from naive or TNBS-induced colitis mice (n = 3 per group). One-way ANOVA followed by Holm-Šídák’s or Tukey’s of multiple comparisons test for B and E. Data shown as mean±SEM. Source Data
EXTENDED DATA FIG. 3 EFFECTS OF PROPYZAMIDE ON THE GUT MICROBIOME. (A) α-diversity of the faecal microbiome (n = 6 for Jejunum vehicle, n = 7 for Ileum, Caecum and Colon vehicle, n = 8 for
Ileum Propyzamide, n = 9 for Jejunum, Caecum and Colon propyzamide). Kruskal–Wallis nonparametric ANOVA test. (B) β-diversity shown as Principal-coordinate analysis (PCoA) based on
unweighted UniFrac metrics. (C) Relative abundance of bacteria classified at a family-level taxonomy. (D) Relative abundance of the _Suterellaceae_ family (n = 7 for TNBS vehicle, n = 9 for
TNBS propyzamide, n = 10 for naive vehicle and propyzamide) Kruskal–Wallis nonparametric ANOVA test. (E) Schematic of faecal microbiota transplant (FMT) to germ free mice. This schematic was
created using BioRender. (F) 16S quantification by qPCR after FMT (n = 4 control, n = 20 before reconstitution, n = 8 for FMT from vehicle- and propyzamide-treated mice). (G,H,I) Weight
loss (g) (n = 10 vehicle, n = 6 propyzamide), colon length (h) (n = 10 vehicle, n = 7 propyzamide)and representative hematoxylin & eosin staining in colons (i) from germ free mice after
FMT from propyzamide- or vehicle-treated mice (n = 8 vehicle, n = 5 propyzamide for quantification). (J) CD4+, IFNγ+ CD4+ and IL-17+CD4+ T cells in colons of germ free mice after FMT from
propyzamide- or vehicle-treated mice. (n = 10 mice for vehicle CD4+ and IFNγ+ CD4+, n = 9 for vehicle IL-17+CD4+, n = 8 mice for all propyzamide groups). Data shown as mean±SEM. ***p <
0.001, ** p < 0.01, *p < 0.05. Source Data EXTENDED DATA FIG. 4 TRANSCRIPTIONAL ANALYSIS OF COLONIC T CELLS AND DCS. (A) _Tnf, Il23, Il1b_ and _Il6_ expression determined by qPCR in
LPMC from naive mice (n = 7) and vehicle- or propyzamide-treated mice during TNBS-induced colitis (n = 6 mice per group). (B) _Tgfb and Il10_ expression determined by qPCR in LPMC from naive
mice (n = 7) and vehicle- or propyzamide-treated mice during TNBS-induced colitis (n = 6 mice per group). Data shown as mean±SEM. (C) Dot plot visualization of features that define cell
clusters in Fig. 2i. (D) UMAP plots of colonic cells from naive or TNBS-induced colitis mice treated with vehicle or propyzamide (100 mg kg−1). (E) Cluster distribution per replicates of
colonic cells from naive or TNBS-induced colitis mice treated with vehicle or propyzamide (n = 5 mice per group). (F) Heatmap of differentially expressed genes that cluster colonic DC
populations from scRNAseq analysis. (G) UMAP plots of DCs from colons from naive or TNBS-induced colitis mice treated with vehicle or propyzamide (100 mg kg−1). (H) GSEA analysis showing
pathways activated in DCs from propyzamide-treated mice during TNBS-colitis. (I) Percentage of each DC subpopulation from vehicle- or propyzamide-treated naive mice. (J) mRNA expression
determined by bulk RNA-seq in colon samples from vehicle- or propyzamide-treated mice 24 h after anti-CD3 administration (n = 4 mice per group). (K) _Cyp1a1_ and _Cyp1b1_ expression in
colonic CD45+ cells from vehicle- or propyzamide-treated mice 24 h after anti-CD3 injection (n = 6 mice per group). (L) IPA showing pathways significantly upregulated in propyzamide-treated
mice analysed by bulk-RNA-seq. (M,N) _Rela_ and _Cebpb_ (m) and _Ifng, Il17, Rorc_ and _Il12rb1_ (n) expression in colonic CD45+ cells from vehicle- and propyzamide-treated mice 24 h after
anti-CD3 administration (n = 10 mice for _Cebpb_ vehicle, n = 8 mice for _Cebpb_ propyzamide, n = 5 for _Ifng_ vehicle, n = 4 for _Ifng_ propyzamide, n = 5 for _Il17_ propyzamide, n = 5 for
_Rorc_ vehicle, n = 5 for _Il12rb1_ vehicle, n = 6 mice otherwise). (O) T cells, IFNγ+ and IL-17+ CD4 T cells and IFNγ+ CD8 T cells in colon from propyzamide- and vehicle-treated mice 24 h
after anti-CD3 injection. Data shown as mean±SEM. ***p < 0.001, ** p < 0.01, *p < 0.05. Source Data EXTENDED DATA FIG. 5 EFFECTS OF PROPYZAMIDE ON THE SMALL INTESTINE. (A) mRNA
expression determined by bulk RNA-seq in jejunal CD45+ cells from vehicle- and propyzamide-treated mice (n = 4 mice per group. (B,C,D,E) CD4, IFNγ+ CD4 and IL-17+ CD4 T cells (b), CD8 and
IFNγ+ CD8 T cells (c), γδ and IL-17+ γδ T cells (d), ILC and IL-17+ILC3 (e) in jejunum from vehicle- or propyzamide-treated mice. (n = 10 mice for IL-17+CD4 T cells vehicle, IFNγ+ CD8 T
cells vehicle, γδ and IL-17+ γδ T cells vehicle, ILC and IL-17+ILC3 vehicle, n = 7 mice for CD8 T cells propyzamide and γδ T cells propyzamide, n = 8 for ILC propyzamide, n = 9 mice
otherwise). (F) Transactivation of the RARα promoter in _RARa_-luciferase transfected HEK293T cells treated with retinoic acid or retinoic acid and propyzamide. (G) Transactivation of the
PPARα promoter in _PPARa_-luciferase transfected HEK293T cells treated with fenofibrate, or propyzamide and fenofibrate for 24 h. (H) _Rorc_ and _Il12rb1_ expression evaluated by qPCR in
colonic CD4 T cells sorted from vehicle- or propyzamide-treated WT or AHRd mice after TNBS-colitis. (I) _Il1b, Tnf_ and _Il23_ expression in sorted DCs. (J) _Rela_ expression in BMDCs (n = 5
per group). (K) C/EBPβ expression, determined by ELISA, following _Rela_ knockdown in BMDCs (n = 3 per group). (L) Relative expression of p65 subunit of NF-κB in primary murine DCs as a
result of the depicted chemical treatment previously identified to be linked to IBD in Fig. 1d. (M) Microtubule destabilization after paclitaxel and/or propyzamide incubation with
fluorescent tubulin. Data shown as mean±SEM. ****p < 0.0001, ***p < 0.001, ** p < 0.01, *p < 0.05. Source Data EXTENDED DATA FIG. 6 C/EBPΒ ACTIVATION IN DCS BOOSTS COLITOGENIC
T-CELL DIFFERENTIATION. (A) _Cebpb_ expression following propyzamide treatment in primary DCs from WT and _Cebpb__−/−_ mice (n = 14 vehicle-treated WT cells, n = 9 propyzamide-treated WT
cells, n = 4 vehicle-treated _Cebpb__−/−_ cells, n = 5 propyzamide-treated _Cebpb__−/−_ cells). One-way ANOVA test followed by Tukey’s post-hoc test. (B) _Il1b, Il23_ and _Tnf_, expression
following _Cebpb_ knockdown in BMDCs. (For _Il1b_ n = 6 siNT vehicle, n = 4 siNT propyzamide, n = 5 siCebpb vehicle and n = 6 siCebpb propyzamide. For _Tnf_, n = 6 siNT vehicle, n = 3 siNT
propyzamide, n = 6 siCebpb vehicle and n = 6 siCebpb propyzamide. For _Il23_, n = 5 siNT vehicle, n = 3 siNT propyzamide, n = 7 siCebpb vehicle and n = 9 siCebpb propyzamide). One-way ANOVA
test followed by Holm-Šidák’s post-hoc test. (C) _Cebpb_ expression following _Cebpb_ silencing in BMDCs (n = 8 siNT vehicle, n = 5 siNT propyzamide, n = 9 siCebpb vehicle and n = 5 siCebpb
propyzamide). (D) Gate strategy used to sort human DCs from PBMCs. (E) _CEBPB_ expression in human DCs treated with propyzamide after knockdown with _CEBPB-_targeting (siCEBPB) or
non-targeting (siNT) siRNAs (n = 6 per group). Two-way ANOVA followed by Tukey’s multiple comparisons post-hoc test. (F) ChIP-seq and ATAC-seq re-analyses from23 of Cebpb binding to _Il1b_,
_Tnf_ and _Il23_ promoter in GM-CSF or Flt3 differentiated DCs. (G) Analysis of splenic DCs from TNBS-induced colitis mice. This schematic was created using BioRender. (H) IFNγ, IL-17 and
TNF representative histograms in T cells co-cultured with splenic DCs from vehicle- or propyzamide-treated TNBS-induced colitis mice. MFI of IL-17, IFNγ and TNF OT-II CD4+ T cells following
co-culture with splenic DCs from propyzamide- or vehicle-treated mice (n = 6 vehicle-treated mice, n = 4 propyzamide-treated mice). Unpaired t-test. (I) _Ifng_ and _Il17_ expression
determined by qPCR in co-cultures from WT and _Cebpb__−/−_ DCs pre-treated with vehicle or propyzamide in presence of OVA peptide and co-culture with OT-II naive CD4 T cells for 48 h (For
_Ifng_, n = 4 WT vehicle, n = 4 WT propyzamide, n = 7 _Cebpb__−/−_ vehicle and n = 6 _Cebpb__−/−_ propyzamide. For _Il17_, n = 7 WT vehicle, n = 4 WT propyzamide, n = 4 _Cebpb__−/−_ vehicle
and n = 6 _Cebpb__−/−_ propyzamide). One-way ANOVA test followed by Holm-Šidák’s multiple comparisons post-hoc test. (J) TNF, IL-17 and IL-6 relative levels determined in co-culture
supernatants of human DCs pre-treated as specified in the figure, and then incubated with allogenic T cells for 48 h. (For _Tnf_, n = 3 per group. For _Il17_, n = 4 per group). One-way ANOVA
test followed by Holm-Šidák’s multiple comparisons post-hoc test. (K–L) _Cebpb_ expression (k) and _Il1b, Il23_ and _Tnf_ expression (l) determined by qPCR in primary splenic DCs
transfected with cumate-inducible _Cebpb_-expressing plasmid after cumate treatment as depicted for 96 h. (For _Cebpb_ (k), n = 5 vehicle-treated and n = 3 for each cumate-treated group. For
_Tnf_ and _Il23_ (l) n = 4 vehicle-treated and n = 3 for each cumate-treated group). One-way ANOVA test followed by Holm-Šidák’s post-hoc test. Data shown as mean±SEM. Source Data EXTENDED
DATA FIG. 7 SCRNASEQ ANALYSIS OF INTESTINAL DCS FROM IBD PATIENTS. (A) UMAP plot and cluster distribution per replicates of colonic leukocytes isolated from _Cebpb__−/−_ (n = 2 mice) and WT
(n = 5 mice) DC chimeras after TNBS-colitis. (B) UMAP plot showing DC clusters analysed in colon from WT and _Cebpb__−/−_ DC chimeras analysed by scRNAseq. (C) Number of cells per DC cluster
and cluster distribution per replicates. (D) _Cebpb_ expression in DCs recovered from WT or _Cebpb__−/−_ DC chimera. (E) UMAP plot of 58 samples from 44 IBD patients and healthy controls.
(F) Dot Plot visualization of features that define DCs and T cells. (G) Differentially regulated pathways in DCs from IBD patients. (H) Violin plot depicting _CEBPB_ expression in DCs from
IBD and healthy controls. (I) UMAP depicting intestinal DCs from IBD and healthy controls analysed by scRNA-seq. (J) _CEBPB_ expression in DC1 and DC2 subsets. (K) Differentially regulated
pathways in _CEBPB_ expressing DCs. EXTENDED DATA FIG. 8 VCAM-1 BLOCKADE AMELIORATES INTESTINAL INFLAMMATION. (A) Network analysis of molecules reported in ToxCast database to be induced by
propyzamide. (B) Effect of propyzamide on _Vcam1_ promoter activity (n = 10 for 0.2 μM propyzamide, n = 7 otherwise). (C) _Vcam1_ expression in colons from naive and TNBS-induced colitis
mice treated with vehicle or propyzamide (100 mg kg−1) (n = 5 mice per group). Unpaired t-test. (D) Effect of propyzamide on _Itga4_ and _Itga1_ expression in T cells (n = 3 for Itga4
propyzamide, n = 4 otherwise). Unpaired t test. (E) Evaluation of VCAM-1 blocking antibodies in TNBS-induced colitis. This schematic was created using BioRender. (F) Effect of VCAM-1
blocking antibodies (100 μg per mouse) or isotype controls on TNBS-induced colitis (Weight: n = 12 for vehicle isotype control, n = 9 for vehicle anti-VCAM1, n = 7 for propyzamide
anti-VCAM1, n = 13 otherwise, Colon length: n = 15 for naive, n = 5 for vehicle isotype control, n = 13 for propyzamide isotype control, n = 7 otherwise). (G,H) Representative hematoxylin
& eosin staining (g) and clinical histomorphology scores (h) (n = 4 mice for antiVCAM1-treated mice, n = 5 mice otherwise). Arrows show leukocyte infiltrates. (I) Effect of VCAM-1
blocking antibodies (100 μg per mouse) or isotype controls on colonic IFNγ+ and IL17+ CD4+ T cells during TNBS-induced colitis determined by flow cytometry (IFNγ: n = 6 mice for vehicle
isotype control, n = 5 mice for propyzamide isotype control, n = 4 otherwise. IL17: n = 4 mice for vehicle and propyzamide anti-VCAM1, n = 5 otherwise). One-way ANOVA followed by post-hoc
tests Tukey’s or Holm-Sidak’s test for selected multiple comparisons for B, F, H and I. Data shown as mean±SEM. Source Data EXTENDED DATA FIG. 9 PROPYZAMIDE BOOSTS COLITOGENIC T-CELL
DIFFERENTIATION. (A) Gate strategy used to study CD4 T cells. (B) Percentage of IFNγ+ CD4+ T cells and _Ifng_ expression of CD4+ T cells activated under Th1-polarizing conditions in the
presence of vehicle or propyzamide (n = 3 per group). Unpaired t-test. (C) Percentage of IL-17+ CD4+ T cells and _Il17_ expression of CD4+ T cells activated under Th17-polarizing conditions
in the presence of vehicle or propyzamide (n = 3 per group). Unpaired t-test. (D) _Tbx21_, _Csf2_ and _Il12rb1_ expression in CD4+ T cells activated under Th1 polarizing conditions in the
presence of vehicle or propyzamide (n = 3 per group, except propyzamide-treated cells expressing _Il12rb1_, n = 4). Unpaired t-test (E) _Csf2, Tnf_ and _Rorc_ expression in CD4+ T cells
activated under Th17 polarizing conditions in the presence of vehicle or propyzamide (For _Csf2_ n = 3per group, for _Rorc_ n = 3 vehicle-treated and n = 4 propyzamide-treated, for _Tnf_ n =
5 per group) Unpaired t-test. (F) Nuclear p65 translocation in splenic CD4+ T cells activated with anti-CD3 and anti-CD28 in the presence of vehicle or propyzamide (n = 3 per group).
Unpaired t-test. (G,H) _Rela_ (g) and _Cebpb_ (h) expression following _Rela_ knockdown in T cells and treated with vehicle or propyzamide (For (g) n = 3 per siNT group and n = 4 per siRela
group, for (h) n = 5 per siNT group and n = 4 per siRela group). One-way ANOVA test followed by Holm-Šidák’s multiple comparisons post-hoc test. (I) _Cebpb_ expression in murine splenic WT
and _Cebpb__−/−_ T cells (n = 9 vehicle-treated WT cells, n = 7 propyzamide-treated WT cells, n = 3 vehicle-treated _Cebpb__−/−_ cells, n = 3 propyzamide-treated _Cebpb__−/−_ cells). One-way
ANOVA test followed by Holm-Šidák’s multiple comparisons post-hoc test. (J) _CEBPB_ expression following CEBPB knockdown in human T cells.(n = 4 per group except n = 3 siCEBPB
propyzamide-treated group). (K) ILCs and CD8 T cells in colons from _Rag1__−/−_ mice reconstituted with WT or _Cebpb__−/−_ T cells. (n = 4 per group except _Cebpb__−/−_ CD8 T cells n = 5).
Data shown as mean±SEM. ***p < 0.001, ** p < 0.01, *p < 0.05. Source Data EXTENDED DATA FIG. 10 SCRNASEQ ANALYSIS OF INTESTINAL T CELLS FROM IBD PATIENTS. (A) UMAP depicting total
intestinal T cells analysed by scRNA-seq. (B) Dot plot visualization of features to identify T-cell subsets. (C) Heat map of differentially expressed genes in T cells from IBD patients and
healthy controls. (D) Upstream analysis of NF-κB-driven _CEBPB_ expression in T cells. (E) _CEBPB_ expressing T cells from IBD and HC samples. (F,G,H) Pathway analysis of differentially
expressed genes in Resident Memory T cells (f), CD8 T cells (g) and ILCs (h) from IBD and healthy control samples. (I) Graphical model of modulation of colitogenic T cell responses by
NF-κB-driven C/EBPβ signalling and propyzamide. SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLE 1 Effect of 936 candidate chemicals in ToxCast assays related to genes
relevant to IBD pathogenesis. Red highlights indicate that the tested chemical is active in the assay, blue highlights indicate that the chemical is inactive and ND indicates that no data
are available in the assay. SUPPLEMENTARY TABLE 2 Descriptions and details of the assays used in Supplementary Table 1, including citations for the assays themselves, and references for the
assay targets’ relevance to IBD. SUPPLEMENTARY TABLE 3 Results of the in vivo zebrafish screen of 111 candidate chemicals on TNBS-induced intestinal inflammation, and predicted exposure
levels for each chemical in zebrafish and humans (based on data from the EPA ToxCast website). SUPPLEMENTARY TABLE 4 Effect of 327 chemicals (including the 30 used as the training set, plus
an additional 297 identified from the ToxCast database) in the 16 ToxCast bioassays defined as an IBD bioactivity feature. Results shown are the AC50 values determined in each bioassay and
publicly available from the ToxCast website. SUPPLEMENTARY TABLE 5 Description of the assays used in Supplementary Table 1, including citations for the assays themselves, SUPPLEMENTARY TABLE
6 Candidate chemicals identified from the ToxCast database predicted to worsen intestinal inflammation by a RF model and ranked using a RWR algorithm. Additional details about the
classification and uses of the top 20 predicted chemicals are provided, along with relevant sources. SUPPLEMENTARY TABLE 7 Statistical analyses of _α_-diversity of the microbiome as a result
of propyzamide treatment in naive and TNBS-colitis mice. SUPPLEMENTARY TABLE 8 Statistical analysis of the _β_-diversity in the gut microbiome after propyzamide administration in naive and
TNBS-colitis mice. SUPPLEMENTARY TABLE 9 Demographic information of the samples analysed by scRNA-seq in Extended Data Figs. 7 and 10. SOURCE DATA SOURCE DATA FIG. 1 SOURCE DATA FIG. 2
SOURCE DATA FIG. 3 SOURCE DATA FIG. 4 SOURCE DATA EXTENDED DATA FIG. 1 SOURCE DATA EXTENDED DATA FIG. 2 SOURCE DATA EXTENDED DATA FIG. 3 SOURCE DATA EXTENDED DATA FIG. 4 SOURCE DATA EXTENDED
DATA FIG. 5 SOURCE DATA EXTENDED DATA FIG. 6 SOURCE DATA EXTENDED DATA FIG. 8 SOURCE DATA EXTENDED DATA FIG. 9 RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or
other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of
this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sanmarco, L.M., Chao, CC., Wang,
YC. _et al._ Identification of environmental factors that promote intestinal inflammation. _Nature_ 611, 801–809 (2022). https://doi.org/10.1038/s41586-022-05308-6 Download citation *
Received: 31 May 2021 * Accepted: 01 September 2022 * Published: 20 October 2022 * Issue Date: 24 November 2022 * DOI: https://doi.org/10.1038/s41586-022-05308-6 SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative
