Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without aura
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Migraine is a complex neurovascular disease with a range of severity and symptoms, yet mostly studied as one phenotype in genome-wide association studies (GWAS). Here we combine
large GWAS datasets from six European populations to study the main migraine subtypes, migraine with aura (MA) and migraine without aura (MO). We identified four new MA-associated variants
(in _PRRT2_, _PALMD_, _ABO_ and _LRRK2_) and classified 13 MO-associated variants. Rare variants with large effects highlight three genes. A rare frameshift variant in brain-expressed
_PRRT2_ confers large risk of MA and epilepsy, but not MO. A burden test of rare loss-of-function variants in _SCN11A_, encoding a neuron-expressed sodium channel with a key role in pain
sensation, shows strong protection against migraine. Finally, a rare variant with _cis_-regulatory effects on _KCNK5_ confers large protection against migraine and brain aneurysms. Our
findings offer new insights with therapeutic potential into the complex biology of migraine and its subtypes. SIMILAR CONTENT BEING VIEWED BY OTHERS GENOME-WIDE ANALYSIS OF 102,084 MIGRAINE
CASES IDENTIFIES 123 RISK LOCI AND SUBTYPE-SPECIFIC RISK ALLELES Article Open access 03 February 2022 COMMON VARIANTS IN _KCNK5_ AND _FHL5_ GENES CONTRIBUTED TO THE SUSCEPTIBILITY OF
MIGRAINE WITHOUT AURA IN HAN CHINESE POPULATION Article Open access 24 March 2021 COMPREHENSIVE ANALYSIS OF GENES ASSOCIATED WITH MIGRAINE IN THE INDIAN POPULATION: A META-ANALYSIS OF
GENETIC ASSOCIATION STUDIES WITH TRIAL SEQUENTIAL ANALYSIS Article Open access 04 November 2023 MAIN Migraine is a complex neurovascular disease characterized by recurrent, disabling
headache attacks that are difficult to treat. It is among the most common pain disorders worldwide, with prevalence of up to 20% in adult populations and affecting three times more females
than males1. Two main subtypes are clinically distinguished, migraine with aura (MA) and migraine without aura (MO)2. MO is characterized by severe headache attacks accompanied by nausea and
hypersensitivity to light and sound, whereas MA is characterized by gradually spreading, fully reversible focal neurological symptoms, collectively called aura, that are usually followed by
headache1. An estimated 30% of migraineurs have MA, and the most frequently experienced aura involves visual disturbances (for example, flashes of bright light and blurred vision)3. During
MA attacks, characteristic regional brain blood flow changes indicate that MA is caused by cortical spreading depression, a transient wave of neuronal depolarization of the cortex4,5. Such
findings are not observed in MO6,7, suggesting divergent pathogenesis of these migraine subtypes. A rare and clinically distinct subtype of MA is familial hemiplegic migraine (FHM)2. Three
genes have been linked to FHM—one encoding a membrane protein involved in maintaining gradients of sodium and potassium ions across plasma membranes (_ATP1A2_), and two genes encoding sodium
and calcium channels expressed in brain (_SCN1A_ and _CACNA1A_, respectively)8. More is known about the genetics and biology of migraine than any other pain disorder, leading to recent
treatment advances such as those targeting the calcitonin gene-related peptide (CGRP) activation of the trigeminovascular system9,10. The largest genome-wide association studies (GWAS)
meta-analysis of migraine to date identified 123 migraine risk loci, among them a locus including genes encoding CGRP (_CALCA_ and _CALCB_)11. However, the pathophysiology of migraine is not
fully understood, and a substantial subset of patients has treatment-resistant migraine12. In the study reporting 123 common (minor allele frequency (MAF) > 2%) migraine variants,
subtype analysis showed that 5 associate specifically with migraine subtypes—3 with MA (in or near _CACNA1A_, _HMOX2_ and _MPPED2_) and 2 with MO (near _SPINK2_ and _FECH_)11,13. These
findings suggest that the genetics of MA and MO should be studied separately and with more emphasis on detecting rare variants. To identify both distinct and common biological underpinnings
of these migraine subtypes, we performed GWAS meta-analyses of clinically defined MA, MO and overall migraine, using six datasets and analyzing variants down to 0.001% in frequency. We used
samples from about 1.3 million individuals, of which 12,000 have MO, 17,000 have MA and 80,000 have migraine. Because migraine and especially its subtypes are considerably underdiagnosed14,
and to obtain measures of specific symptoms and severity, we also assessed self-reported proxy phenotypes representing severe and recurrent migraine headaches (52,000 cases) as well as
migraine’s most distinctive subtype, headaches preceded by visual aura (30,000 cases). Here we report 4 new MA-associated variants and show that 13 known migraine variants associate with MO
over MA. In all, we observed associations with 44 lead variants, 12 of which are new for migraine, and we found functional evidence implicating 22 genes—3 in MA, 3 in MO and the remainder in
overall migraine. Among the findings are rare variants with large effects providing new insights into biological underpinnings of distinct characteristics of migraine, with and without
aura. RESULTS We conducted GWAS meta-analyses of clinically defined migraine, MA and MO, using datasets from Iceland (deCODE Genetics), Denmark (Copenhagen Hospital Biobank (CHB)15 and
Danish Blood Donor Study (DBDS)16), the United Kingdom (UK; UK Biobank17), the United States (US; Intermountain Health18), Norway (the Hordaland Health Study (HUSK)19) and Finland
(FinnGen20). We also performed GWAS meta-analyses of two self-reported proxy phenotypes available in three datasets (Iceland, UK and Denmark)—an MA proxy represented by experiencing visual
disturbances (VD) preceding headaches, and a severe migraine proxy represented by bad and recurrent headaches (BRH). In total, we analyzed data on 1.3 million individuals, including 16,603
with MA, 11,718 with MO, 79,495 with any migraine, 30,297 with VD and 51,803 with BRH (Methods; Supplementary Table 1). We analyzed up to 85 million variants, and using a significance
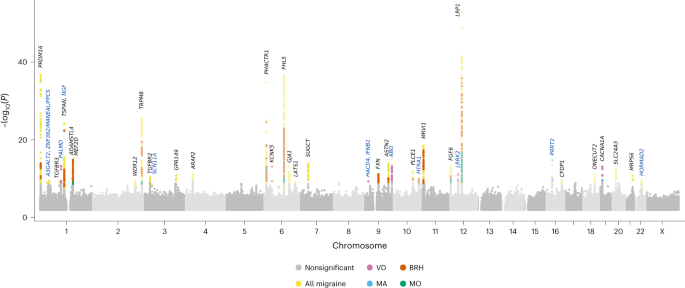
threshold weighted by variant impact21, we found associations with 44 lead variants at 39 loci (Fig. 1, Tables 1 and 2 and Supplementary Tables 2–7). Two variants associate with MA (one
new), five with the MA-proxy VD (four new) and six with MO. The remaining variants associate with overall migraine or BRH. In all, we report 12 new migraine variants (regional plots shown in
Supplementary Figs. 1 and 2). Using cross-trait linkage disequilibrium (LD) score regression22, we calculated genetic correlations in nonoverlapping samples (Methods) showing that VD
correlates genetically with clinically defined MA (_r__g_ = 0.65, _P_ = 4.0 × 10−23) but not MO (_r__g_ = −0.09, _P_ = 0.21), and BRH correlate strongly with clinically defined migraine
(_r__g_ = 0.85, _P_ = 7.4 × 10−91; Supplementary Table 8 and Supplementary Fig. 3). Further supporting VD as an MA proxy, the GWAS meta-analysis of VD reveals an association with a variant
(rs11085837-A) in high LD (_r__2_ = 0.96) with the reported MA variant in _CACNA1A_, rs10405121-A11 (Fig. 1 and Table 1). Its VD effect (odds ratio (OR) = 0.926, _P_ = 8.8 × 10−14) is
consistent with its MA effect (OR = 0.930, _P_ = 1.8 × 10−9), and no association is detected with MO (OR = 0.983, _P_ = 0.22). In Supplementary Table 9, we list associations with all
migraine phenotypes of the current study with the recently published 123 migraine variants11, finding support (_P_ < 0.05) in our data for all but 9 variants (Supplementary Note 1). A
RARE LOSS-OF-FUNCTION _PRRT2_ VARIANT ASSOCIATES WITH MA The top MA association is with a rare insertion in _PRRT2_ leading to frameshift (rs587778771-GCC, p.Arg217ProfsTer8; OR = 5.446, _P_
= 5.6 × 10−16). This variant also associates with VD (OR = 3.634, _P_ = 0.0037) but not MO (_P_ = 0.97; Table 3). It is detected in only three cohorts, with a founder effect observed in
Iceland (frequency = 0.117%), compared to UK and US (frequency = 0.013% and 0.0051%, respectively). It is detected at even lower frequencies in samples from Denmark, with no carriers
detected in Norway or Finland. This variant has been reported in case studies of rare neurological disorders, including benign infantile seizures and paroxysmal kinesigenic dyskinesia
(PKD)23. In a few carriers, FHM has also been detected8. Among six Danish heterozygous carriers identified, five are in the same family, of which three have FHM. The p.Arg217ProfsTer8
insertion is located in an unstable DNA site24,25 where we find another rarer (0.024%) deletion (p.Arg217GlufsTer12) that also leads to premature PRRT2 truncation25. This variant also shows
a founder effect in Iceland, being tenfold more frequent than in the UK (frequency of 0.0025%), and not detected in other cohorts. It was previously reported in a single case study of a
homozygous carrier with severe PKD that responded to carbamazepine, an epilepsy drug that reduces the generation of rapid action potentials in the brain26 and is also used to treat migraine.
We found p.Arg217GlufsTer12 in 38 heterozygous carriers in Iceland, mainly in two families where it segregates with migraine and epilepsy. Of 38 carriers, 11 (29%) are diagnosed with
migraine (without subtype), six (16%) with epilepsy and one with MA and epilepsy. For these rare variants, we looked for associations with other phenotypes. Apart from the MA and migraine
associations, p.Arg217ProfsTer8 associates only with epilepsy (OR = 7.077, _P_ = 1.9 × 10−35; Table 3 and Supplementary Table 10). We find epilepsy moderately genetically correlated with
migraine (_r__g_ = 0.28, _P_ = 9.4 × 10−6) and VD (_r__g_ = 0.28, _P_ = 2.8 × 10−4), but not with MO (_r__g_ = 0.05, _P_ = 0.90). We tested 30 epilepsy variants27 in our data and found that
only two also impact migraine (at _P_ < 3.3 × 10−4 = 0.05/30 variants × 5 phenotypes). The common (23.3%) intron variant rs59237858-T in _SCN1A_ that confers protection against epilepsy27
confers risk of migraine (OR = 1.031, _P_ = 8.6 × 10−6) in our data, and rs62151809-T (44.7%) near _TMEM182_ confers risk of epilepsy27 and of VD in our data (OR = 1.047, _P_ = 8.5 × 10−6).
None of the 30 epilepsy variants associate with MO or BRH (Supplementary Table 11). Conversely, of the 44 variants reported here, only p.Arg217ProfsTer8 associates with epilepsy. GWAS
META-ANALYSIS OF MA-PROXY PHENOTYPE YIELDS NEW MA-ASSOCIATED LOCI Besides the known MA-associated variant in _CACNA1A_, we found four other variants associating with the MA-proxy VD, all new
to migraine (Table 1). The first, rs11166276-C, is in a TF-binding site near _PALMD_ (OR = 0.926, _P_ = 5.1 × 10−14). It is in complete LD with rs7543130 that also associates protectively
with aortic valve stenosis28. Secondly, in _ABO_, the frameshift variant rs8176719-TC associates with VD (OR = 1.081, _P_ = 3.0 × 10−13). This variant contributes to determining the non-O
blood groups29, and variants in high LD associates with various coagulation factors and risk of venous thromboembolism (Supplementary Table 12). This variant associates with MA (OR = 1.030,
_P_ = 0.015) and overall migraine (OR = 1.020, _P_ = 1.5 × 10−3; Supplementary Table 7). Thirdly, a variant upstream of _LRRK2_, rs10748014-T, associates with VD (OR = 1.073, _P_ = 5.6 ×
10−12). _LRRK2_ encodes leucine-rich repeat kinase 2, a gene harboring common risk variants for inherited Parkinson’s disease (PD)30, none of which are in LD with rs10748014 (Supplementary
Table 12). This variant also associates with MA (OR = 1.065, _P_ = 8.4 × 10−8) and weakly with overall migraine (OR = 1.012, _P_ = 0.048), and we detected no association with MO or PD.
Finally, in a regulatory region near _HACD4/IFNB1_ is an association with rs77778288-C (frequency = 12.9%, OR = 1.097, _P_ = 4.9 × 10−10). _IFNB1_ encodes interferon β 1, which is used to
treat multiple sclerosis and can induce headaches31. We compared the effects of these VD variants on MA and all migraine in effect–effect plots (Fig. 2). Based on the slope derived from a
weighted regression through the origin, overall MA and migraine effect estimates are 73% and 29%, respectively, of VD effect estimates, and no associations were detected for MO, which is in
line with our estimates of genetic correlation between these traits. MIGRAINE SUBTYPE CLASSIFICATION OF LEAD VARIANTS We used a similar approach discussed in ref. 11 to study the effects of
43 lead variants on the migraine subtypes adjusting for sample overlap (_PRRT2_ excluded as it has larger effects than other variants and is shown to be an MA-associated variant; Methods).
We find that the new variants in _ABO_, _LRRK2_ and _PALMD_, and the previously reported11 MA-associated variant in _CACNA1A_ are classified as MA-associated variants, and 13 variants are
classified as MO-associated variants (bold in Tables 1 and 2; Fig. 3 and Supplementary Fig. 4). All MO-associated variants are in known migraine loci except the new MO-associated variant
rs71642605-C in _MANEAL_. We find that one of the MO-associated variants, rs12684144-C in _ASTN2_, confers protection against VD (OR = 0.956, _P_ = 0.00017) but risk of MO (OR = 1.073, _P_ =
1.5 × 10−5). In line with only 30% of migraineurs experiencing aura3, its association with overall migraine confers risk (OR = 1.055, _P_ = 1.3 × 10−14). PROTEIN-ALTERING VARIANTS IN _NGF_
AND _SCN11A_ Among new variants associated with overall migraine is the common missense variant rs6330-A (p.Ala35Val) in _NGF_ (OR = 1.035, _P_ = 2.1 × 10−8). _NGF_ encodes nerve growth
factor that is involved in regulating growth and differentiation of sympathetic and certain sensory neurons (https://www.ncbi.nlm.nih.gov/gene). _NGF_ is at 1p13.2 and nearby is _TSPAN2_,
harboring a previously reported11 migraine-associated variant (rs2078371) that is, however, uncorrelated (_r_2 = 0.02) with rs6330. Conditional analysis shows that the effects of rs6330-A on
migraine are significant when adjusting for rs2078371 (Table 2). In _SCN11A_, another common (25%) missense variant, rs33985936-T (p.Val909Ile), associates with overall migraine (OR =
1.041, _P_ = 3.4 × 10−9). _SCN11A_ encodes Nav1.9, which is highly expressed in nociceptive neurons of dorsal root and trigeminal ganglia32,33. Rare loss-of-function (LOF) variants in
_SCN11A_ can lead to both extremely painful and completely pain-insensitive disorders32,33. We looked for LOF variants in _SCN11A_ and found them at very low frequency in all datasets
studied, with the highest in the UK at a combined frequency of 0.13%, which is two orders of magnitude higher than in other cohorts. We used a genome-wide burden test combining the effects
of these rare variants on migraine in the UK, and at a threshold of _P_ = 2.5 × 10−6 (_P_ = 0.05/20,000 genes34 tested), they associate with strong protection against overall migraine (OR =
0.650, _P_ = 3.9 × 10−7) and other severe headaches and are not driven by a single variant (Table 4 and Supplementary Note 2). A RARE VARIANT TARGETING _KCNK5_ WITH PROTECTIVE EFFECTS In the
GWAS meta-analysis of BRH, there is an association with a large protective effect (OR = 0.697, _P_ = 7.6 × 10−14) with the rare (0.67%) intergenic variant rs72854118-G located in a
regulatory region between two potassium channel genes, _KCNK5_ and _KCNK17_. The variant also protects against clinically defined migraine (OR = 0.836, _P_ = 9.7 × 10−7), but does not
associate with migraine subtypes, MA, MO or VD (_P_ > 0.05). Two additional variants in high LD are at this locus, rs72854120 and rs72851880 (Supplementary Fig. 2). A common (28.1%)
intronic variant in _KCNK5_ was previously reported11 to be associated with migraine (rs10456100, OR = 1.051, _P_ = 9.2 × 10−19), but is uncorrelated with rs72854118 (_r_2 = 0.002).
rs72854118-G is reported in weak association with decreased diastolic blood pressure (_β_ = −0.07, _P_ = 2.7 × 10−7)35, and in a GWAS meta-analysis of self-reported migraine and headaches
combined, one of two correlated SNPs, rs72854120-C, shows borderline association, more so with headaches than migraine (_Z_migraine = −2.68, _Z_headache = −5.49, _P_ = 2.8 × 10−8)36.
Inspection of effect–effect plots of BRH versus clinically defined migraine for all 44 lead variants shows that rs72854118-G effects on BRH far exceed its migraine effects (Fig. 4 and
Supplementary Fig. 5). We performed a phenoscan in 1,000 GWAS meta-analyses at deCODE Genetics (_P_ threshold = 0.05/1,000 = 5.0 × 10−5) and observed that rs72854118-G also confers
substantial protection against brain aneurysms (OR = 0.470, _P_ = 1.8 × 10−8) and coronary artery disease (CAD) requiring bypass surgery (OR = 0.725, _P_ = 9.3 × 10−8), but associates more
weakly with CAD in general (OR = 0.900, _P_ = 1.9 × 10−5) and systolic blood pressure (effect = −0.054 s.d., _P_ = 2.0 × 10−5; Supplementary Table 15). Of 17 known brain aneurysm variants37,
3 are in migraine loci (_FHL5_, _SLC24A3_ and _PLCE1_). Plotting effects of the brain aneurysm variants (including rs72854118) on brain aneurysms versus effects on migraine and BRH, we find
this variant is an outlier in both and confers larger protective effects against brain aneurysms than other brain aneurysm variants (Supplementary Fig. 5). COLOCALIZATION HIGHLIGHTS NEW
MIGRAINE AND AURA GENES We performed systemic functional annotation of the 44 lead variants and variants in high LD (_r__2_ ≥ 0.8) and studied their association with mRNA sequence data
(expression quantitative trait loci (eQTL)) and with protein levels in plasma38 (protein quantitative trait loci (pQTL); Methods; Supplementary Tables 16–19). Results are summarized in
Supplementary Fig. 6. For the lead variants, we find 144 eQTLs, of which 16 implicate a specific gene (Supplementary Table 17). Variant rs4768221-G, in complete LD with rs10748014-T (VD
association OR = 1.073, _P_ = 1.2 × 10−12) upstream of _LRRK2_, consistently associates with VD and is the top ranking eQTL for this gene in blood. The allele associated with increased risk
of VD associates with reduced _LRRK2_ expression in blood (_β_ = −0.74 s.d., _P_ = 1.3 × 10−1,260). The lead BRH variant near _KCNK5_ rs72854118, but not the other correlated variants at
this locus, is found within a distal enhancer-like sequence (dELS) as defined by ENCODE’s catalog of candidate _cis_-regulatory elements39, and the gene target for this regulatory element is
_KCNK5_ (Supplementary Tables 20 and 21 and Supplementary Note 3). The variant is too rare to be studied in Genotype-Tissue Expression (GTEx, which includes only three carriers;
Supplementary Fig. 7), and its expression coverage in tissues available to us is too low for conclusive results. Three variants (or variants with _r_2 ≥ 0.8) represent top _cis_ pQTLs at
their respective loci in Icelandic SomaScan plasma protein association data and two variants in the UK Olink data (Supplementary Table 19). These proteomic methods differ in protein
profiles, but in both datasets are pQTL variants correlating with the migraine variant rs1359155039-TAAAAAAAAA upstream of _LATS1_ that associates with reduced migraine risk and increased
LRP11 plasma levels (_β_ = 0.58 s.d., _P_ = 10−1,140 and _β_ = 0.59 s.d., _P_ = 10−2,140 in Iceland and UK, respectively). LRP11 is predicted to be located in plasma membrane and involved in
several processes, including response to heat and cold (https://www.ncbi.nlm.nih.gov/gene). We do not have RNA expression or protein data for enough carriers of the rare _PRRT2_ variants to
detect transcription or protein associations. However, on the basis of previous functional studies40, the gene’s known function as a key component of the Ca2+-dependent neurotransmitter
release machinery41, and its reported links to rare paroxysmal brain disorders including infantile convulsions, the movement disorder PKD and FHM42, in addition to the findings in this
current study, we conclude that _PRRT2_ is also a risk gene for the common forms of MA and epilepsy. Finally, we scanned the GWAS catalog (https://www.ebi.ac.uk/gwas/) for associations with
lead variants identified in this study (or _r_2 ≥ 0.8). Results are presented in Supplementary Table 12. PATHWAY ANALYSIS HIGHLIGHTS NGF-RELATED PROCESSES For the 22 genes with evidence
supporting their role in migraine or subtypes, we performed a protein network analysis (https://reactome.org). Among the top 67 relevant pathways identified, 13 involve NGF processing,
including TrkA activation by NGF, previously studied in the context of pain and pain therapeutics43. Interestingly, pathways involved in phase-4 resting potential and cardiac conduction
involve the products of both _KCNK5_ and _SCN11A_, with the products of both _LRRK2_ and _LRPI_ interacting in the cardiac conduction pathway (Supplementary Data and Supplementary Table 22).
GENETIC DRUG TARGET ANALYSIS We performed a genetic drug target analysis for the 22 genes for which we have evidence of function pointing to the gene in addition to the established MA gene
_CACNA1A_. Drugs at various levels of development target four genes that associate with MA (_PRRT2_, _ABO_, _LRRK2_ and _CACNA1A_), none associated with MO, and four genes that associate
with overall migraine or severe headaches (_KCNK5_, _NGF_, _SCN11A_ and _TRPM8_; Supplementary Table 23 and Supplementary Note 5). Targeting _PRRT2_ is bryostatin, a powerful protein kinase
C agonist that was originally developed to prevent tumor growth, but in preclinical studies has also shown promising effects as a restorative synapse drug that is currently in trials to
treat Alzheimer’s disease44. Several voltage-gated Ca+2 channel blockers have been developed against _CACNA1A_, but have not been tested in migraine. Targeting _TRPM8_, cutaneous menthol
treatment has been found to alleviate migraine headaches45. Targeting _SCN11A_ (and other voltage-gated sodium transporter genes), intranasal lidocaine can be effective in treating acute
migraine46, and intravenous lidocaine infusion is suggested for treating refractory chronic migraine47. Drugs targeting other genes have not been tested for migraine, but β-nerve growth
factor inhibitors (antibodies) that target _NGF_ (fasinumab, tanezumab and fulranumab) are widely studied in the context of various other chronic pain conditions (for example, sciatica, low
back pain and abdominal pain; www.ClinicalTrials.gov). DISCUSSION Whether MA and MO are different diseases or part of a migraine continuum has long been debated48,49. Little is known about
the genetics underlying migraine subtypes as most prior studies have focused on migraine in general. Here we have identified several new associations supporting the distinct pathogenesis of
MA and MO. In terms of MA, variants in _PRRT2_, _PALMD_, _CACNA1A_, _ABO_ and _LRRK2_ associate with MA (VD) over MO. Of these, two genes have the highest expression in the cerebellum
(_PRRT2_ and _CACNA1A_), and in both are rare autosomal dominant variants reported to cause rare forms of movement disorders and hemiplegic migraine (https://www.omim.org/). This is of
interest in light of the characteristic cortical spreading depression observed in MA but not MO4,5. Both _ABO_ and _PALMD_ are widely expressed in tissues, and both harbor variants
associated with cardiovascular disorders. Indeed, the link between migraine and cardiovascular disease is well established50. Drugs targeting these genes are in various phases of
development, but for indications other than migraine. Five drugs target _CACNA1A_ for seven indications, including anxiety, insomnia and cardiovascular disease, and targeting _LRRK2_ is a
trial drug DNL201 (ClinicalTrials.gov identifier: NCT0371070, https://clinicaltrials.gov/study/NCT03710707) that shows promising therapeutic potential against PD51. LRRK2 is especially
abundant in dopamine-innervated areas and dopaminergic neurons of the substantia nigra30. Increased LRRK2 kinase activity is thought to impair lysosomal function and thus contribute to the
pathogenesis of PD52. However, consistent with our results showing that the variant in _LRRK2_ associates with increased risk of VD (MA) and with reduced LRRK2 mRNA expression, the main
adverse effects of this LRRK2 inhibitor in healthy individuals were headache (40% of participants) and nausea (13%), the main symptoms of migraine, and dizziness (in 13%)51. While _LRRK2_’s
expression is highest in brain areas associated with PD pathology, it is also expressed in other neurons and glial cells of the human brain53. Considerable pleiomorphism can occur among
_LRRK2_ carriers sharing the same pathogenic variant, even within the same family54. Indeed, _LRRK2_ has been dubbed the ‘Rosetta stone’ of Parkinsonism, perhaps providing a common link
between various neurological diseases55. Our GWAS meta-analysis identified six variants associated with MO, all in previously reported migraine loci. However, by the subtype stratification
of all lead variants, we detect 13 variants that impact MO over MA. These MO-associated variants are in or near genes with various functions, such as muscle cell development and
differentiation (_MEF2D_, _FGF6_ and _LRP1_) and intracellular calcium homeostasis (_MRVI1_ and _SLC24A3_). Several are in genes highly expressed in arteries (_MEF2D_, _LRP1_, _ADAMTSL4_,
_SUGCT_, _MRVI1_ and _MRPS6_) and in brain (_MEF2D_, _ARAP2_, _PHACTR1_ and _SLC24A3_). Of these, only _LRP1_ is currently a drug target (https://platform.opentargets.org). _LRP1_ encodes
low-density lipoprotein receptor-related protein 1, and an LRP1 binding agent is in trials to treat various brain tumors. Our results highlight three genes in or near which rare variants
show large and informative effects. Firstly, the rare insertion (p.Arg217ProfsTer8) in _PRRT2_ that associates with large effects on epilepsy and MA provides new insights into these
comorbid56 and genetically correlated diseases. _PRRT2_ is a four-exon gene that encodes a 340 amino acid protein with two predicted transmembrane domains25. Both the insertion and rarer
deletion lead to premature termination of around one-third of PRRT2, resulting in nonsense-mediated decay40. Due to the founder effect in Iceland, we have power to show the pleiotropic
effect of these LOF variants. Not only can they lead to rare neurological disorders, but they also confer substantial risk of common forms of MA and epilepsy, both of which are paroxysmal
brain diseases frequently experienced with aura57,58. _PRRT2_ is widely expressed in the brain, particularly in the cerebellum25,59. It is enriched in presynaptic terminals, is regulated by
Ca+2 release and interacts with SNAP-25 and synaptogamin41. The mutant PRRT2 of the truncating variants leads to increased glutamate release and subsequent neuronal hyperexcitability60. A
study of three Nav1 subunits (Nav1.1 encoded by _SCN1A_, Nav1.2 encoded by _SCN2A_ and Nav1.6 encoded by _SCN8A_) expressed in human embryonic kidney cell lines (HEK-293) demonstrated that
PRRT2 directly interacts with and negatively modulates Nav1.2 and Nav1.6, which generate action potentials in excitatory neurons, but does not affect Nav1.1 channels, which generate action
potentials in inhibitory neurons61. Lack of PRRT2 leads to hyperactivity of Nav1.2 and Nav1.6 in homozygous _PRRT2_ knockout (human and mouse) neurons61. The authors of that study suggest
that the lack of PRRT2 effects on Nav1.1 may enhance excitation/inhibition imbalance and trigger hyper-synchronized activity in neuronal networks61. Interestingly, we find that the only
epilepsy variant in our data that also associates with migraine is rs59237858 in _SCN1A_, the gene that encodes Nav1.1. Secondly, in the context of Nav1 channels, it is of interest that we
find both common and rare variants in _SCN11A_ that impact migraine risk. _SCN11A_ encodes Nav1.9 that is expressed in primary sensory neurons in peripheral and trigeminal ganglia62 and is
known to have a substantial role in pain perception62. Compared to other sodium channels, Nav1.9 generates a persistent current regulated by G-protein pathways63. Whether Nav1.9 is also
affected by _PRRT2_, like Nav1.2 and Nav1.6 (ref. 61), is not known. Currently in various stages of development are 63 drugs targeting _SCN11A_ (most unspecific blockers of all Nav
subtypes), with 341 indications, including headache, epilepsy and pain in general (https://genetics.opentargets.org/gene/ENSG00000168356). Increasing specificity of Nav subtype channel
blockers and studying their protein interactions seems key to harnessing their therapeutic potential64,65. Thirdly, the rare intergenic rs72854118-G near _KCNK5_ and _KCNK17_ is another
variant providing insight into the pathogenesis of migraine. Previous studies have assigned this variant to _KCNK17_ and reported weak associations with reduced blood pressure35 and
protection against self-reported headaches and migraine36. However, we find that rs72854118, but not its correlated variants at this locus, is in a _cis_-regulatory region targeting _KCNK5_.
_KCNK5_ encodes TWIK-related acid-sensitive potassium channel 2, primarily expressed in kidney (GTEx, https://gtexportal.org) but also in T cells, suggesting a role in the immune system66.
We find that the variant also confers protection against brain aneurysms and severe occlusive CAD, but associates weakly with blood pressure. Although hypertension is a risk factor for both
aneurysms and CAD, it is not a conclusive risk factor for migraine67. The observed association with brain aneurysms begs the question whether in some cases undetected brain aneurysms could
be misclassified as migraine68. According to the Open Targets Platform, no drugs are in development that target _KCNK5_. In all, our findings are consistent with the results of previous GWAS
analyses that have established migraine as a complex neurovascular brain disorder13,69. However, our results also highlight several distinct biological pathways involved in MA and MO that
warrant further study. In summary, we contribute new insights into both general and specific mechanisms underlying migraine and its subtypes, especially to the visual aura associated with
migraine attacks. Our results also emphasize the importance of assessing disease subtypes and proxies to improve understanding of complex genetic signals. METHODS ETHICS STATEMENT All human
research was approved by the relevant ethics review boards and conducted according to the Declaration of Helsinki. All participants provided written and informed consent as described per the
study population below. STUDY POPULATIONS Cases and controls were defined from six study populations. ICELAND About 155,000, or close to half of the Icelandic population of 340,000, have
participated in an ongoing nationwide research program at deCODE Genetics71,72. Participants donated blood or buccal samples after signing informed consents allowing the use of their samples
and data in various studies approved by the National Bioethics Committee (NBC). The data used here were analyzed under a study on the genetics of migraine (NBC; 19-158-V3,
VSNb2019090003/03.01) following review by the Icelandic Data Protection Authority. DENMARK Danish samples and data were obtained in collaboration with the Copenhagen Hospital Biobank Study15
and the DBDS16. CHB is a research biobank, which contains samples obtained during diagnostic procedures on hospitalized and outpatients in the Danish Capital Region hospitals. Data analysis
within this study was performed under the ‘Genetics of pain and degenerative diseases’ protocol, approved by the Danish Data Protection Agency (P-2019-51) and the National Committee on
Health Research Ethics (NVK-18038012). The DBDS Genomic Cohort is a nationwide study of ~110,000 blood donors16. The Danish Data Protection Agency (P-2019-99) and the National Committee on
Health Research Ethics (NVK-1700407) approved the studies under which data on DBDS participants were obtained for this study. UK Since 2006, the UK Biobank resource has collected extensive
phenotype and genotype data from ~500,000 participants recruited in the age range of 40–69 from across the UK after signing an informed consent for the use of their data in genetic
studies17. The North West Research Ethics Committee reviewed and approved the UK Biobank’s scientific protocol and operational procedures (REC Reference: 06/MRE08/65). This study was
conducted using the UK Biobank Resource (application 42256). FINLAND The FinnGen study20 consists of samples collected from the Finnish biobanks and phenotype data collected at Finland’s
national health registers. The Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District evaluated and approved the FinnGen research project. The project complies with
existing legislation (in particular the Biobank Law and the Personal Data Act). The official data controller of the study is the University of Helsinki. The summary statistics for FinnGen’s
migraine GWAS were imported from a source available to consortium partners (Release 6: https://r6.finngen.fi/). US Participants from the US were recruited via ongoing studies conducted at
Intermountain Healthcare (https://intermountainhealthcare.org). These studies include the Intermountain Inspire Registry and the HerediGene: Population study18. The latter is a large-scale
collaboration between Intermountain Healthcare, deCODE Genetics and Amgen. The Intermountain Healthcare Institutional Review Board approved this study, and all participants provided written
informed consent and samples for genotyping. NORWAY Data on Norwegian migraine cases and controls were obtained from the HUSK study, a population-based study carried out in Hordaland county
in Western Norway19. In 1992–1993, all Hordaland County residents born between 1950 and 1952, all Bergen residents born between 1925 and 1927 and three neighboring municipalities and a
random sample of individuals born between 1926 and 1949 were invited to participate. In total, 18,044 individuals participated, of which 17,561 provided blood samples for genotyping, of
which 10,000 were genotyped at deCODE Genetics. All participants signed informed consents, and the study was approved and carried out by the National Health Screening Service, Oslo (now the
Norwegian Institute of Public Health) in cooperation with the University of Bergen19. PHENOTYPE DEFINITIONS Cases with migraine and the migraine subtypes with and without aura were in all
cohorts but Norway (using self-reported migraine from questionnaires), mainly defined by International Classification of Diseases 10th Revision (ICD-10) codes (or comparable codes from
earlier versions of ICD) representing MA (code G43.1, MO (G43.0) and overall migraine (G43). Diagnostic codes were assigned by physicians and captured through both inpatient and outpatient
diagnostic registries. As triptan medications (Anatomical Therapeutic Chemical code N02CC) are used to prevent/treat migraine attacks, individuals who had received triptan subscriptions were
identified in data from drug registries (Iceland, Denmark, Finland and the UK) and added to migraine cases (without subtype). Both proxy phenotypes used in this study were based on
validated questionnaire items selected for the headache section of UK Biobank’s pain questionnaire (https://biobank.ctsu.ox.ac.uk/crystal/ukb/docs/pain_questionnaire.pdf), which was designed
in consultation with a group of leaders in pain research. The headache section is based on questions used in the American Migraine Prevalence and Prevention study73. For the MA-proxy
phenotype used in this study (VD preceding headaches), we defined cases and controls from questionnaire data obtained in the studies conducted in Iceland, Denmark and the UK Biobank.
Questions used in Icelandic and Danish cohorts were comparable to the question answered by participants in the UK Biobank (data field 120065: data description: visual changes before or near
the onset of headaches, Question: ‘I develop visual changes such as spots, lines and heat waves or graying out of my vision’). Responses ‘Yes’ were compared to responses ‘No.’ Such defined
cases with, and controls without, headache-related VD had all previously responded ‘Yes’ to a question on headaches as asked in the UK Biobank survey (data field 120053: data description:
bad and/or recurring headaches at any time in life, Question: ‘Have you ever had bad and/or recurring headaches at any time in your life?’). We used this UK Biobank data field 120053 as a
migraine proxy, defining comparable severity qualified headache questions in Icelandic and Danish questionnaire datasets for the GWAS meta-analysis. GENOTYPING AND WHOLE-GENOME SEQUENCING
ICELAND At deCODE Genetics, 63,118 Icelandic samples have been whole-genome sequenced (WGS) using GAIIx, HiSeq, HiSeqX and NovaSeq Illumina technology71,72 to a mean depth of 38×. Genotypes
of single-nucleotide polymorphisms (SNPs) and insertions/deletions (indels) were identified and called jointly by Graphtyper74. The effects of sequence variants on protein-coding genes were
annotated using the variant effect predictor (VEP) using protein-coding transcripts from RefSeq. Including all sequenced samples, 155,250 samples from Icelandic participants have been
genotyped using various Illumina SNP arrays71,72. The chip-typed individuals were long-range phased75, and the variants identified in the WGS Icelanders imputed into the chip-typed
individuals. Additionally, genotype probabilities for 285,644 ungenotyped close relatives of chip-typed individuals were calculated based on extensive encrypted genealogy data compiled by
deCODE Genetics (an unencrypted version is publicly available to all Icelandic citizens at https://www.islendingabok.is/english). All variants tested were required to have imputation
information over 0.8. DENMARK Danish samples from both CHB and DBDS were genotyped at deCODE Genetics using Illumina Infinium Global Screening Array. Individual genotype arrays were
discarded if the total yield was below 98%. Variants were derived from sequencing 25,215 Scandinavian samples (8,360 Danish) using NovaSeq Illumina technology. Only samples with a
genome-wide average coverage of over 20× were used. The genotypes of SNPs and indels were called jointly by Graphtyper74. Variants with a missing rate >2% were discarded. The genotyped
samples were phased using Eagle (version 2.4.1) and high-quality variants imputed into 270,627 genotyped Danes using haplotype sharing in a Hidden Markov Model based on a Li and Stephens
model76 similar to the one used in IMPUTE2 (ref. 77). UK In the UK Biobank dataset, the first 50,000 participants were genotyped using a custom-made Affymetrix chip, UK BiLEVE Axiom78, and
the remaining participants using the Affymetrix UK Biobank Axiom array17. We used existing long-range phasing of the SNP chip-genotyped samples17. We excluded SNP and indel sequence variants
in which at least 50% of samples had no coverage (genotype quality (GQ) score = 0), if the Hardy–Weinberg _P_ value was <10−30 or if heterozygous excess <0.05 or >1.5. At deCODE
Genetics, a collaborative effort was recently performed to whole-genome sequence 150,119 samples from the UK Biobank, allowing us to create a haplotype reference panel, which was then
imputed into the UK Biobank chip-genotyped dataset, as previously described elsewhere79. US Samples from the US (Intermountain dataset) were genotyped using Illumina Global Screening Array
chips (_n_ = 28,279) and WGS using NovaSeq Illumina technology (_n_ = 16,621). Samples were filtered on 98% variant yield and any duplicates were removed. Over 245 million high-quality
sequence variants and indels, sequenced to a mean depth of 20×, were identified using Graphtyper74. Quality-controlled chip genotype data were phased using SHAPEIT4 (ref. 80). A phased
haplotype reference panel was prepared from the sequence variants using the long-range phased chip-genotyped samples using in-house tools and methods described previously71,72. NORWAY
Norwegian samples were genotyped on Illumina SNP arrays (OmniExpress or Global Screening Array). The chip-genotyping QC and imputation of the Norwegian dataset were performed at deCODE
Genetics in Iceland using the same methods as described above for the Icelandic samples. The imputation for Norwegian samples is based on a haplotype reference panel of 25,215 samples of
European ancestry, of which 3,336 are Norwegian. FINLAND A custom-made FinnGen ThermoFisher Axiom array (>650,000 SNPs) was used to genotype FinnGen samples at the Thermo Fisher
Scientific genotyping service facility in San Diego. Genotype calls were made with the AxiomGT1 algorithm (https://finngen.gitbook.io/documentation/methods/genotype-imputation). The FinnGen
Release 6 used in this study contains 260,405 genotyped individuals after quality control (QC). Individuals with ambiguous sex, high genotype missingness (>5%), excess heterozygosity (±4
s.d.) or non-Finnish ancestry were excluded, as were variants with high missingness (>2%), low Hardy–Weinberg equilibrium (<1 × 10−6) or minor allele count (<3). Imputation was
performed using the Finnish population-specific and high coverage (25–30 times) WGS backbone and the population-specific SISu v3 imputation reference panel with Beagle 4.1. More than 16
million variants have been imputed in the Finnish dataset (https://www.finngen.fi/en/access_results). GENETIC ANCESTRY FILTERING AND PRINCIPAL COMPONENTS For the UK Biobank, we used a
British–Irish ancestry subset defined previously79. Procedures to account for ancestry in FinnGen20 and Iceland72 have also been previously described. Genetic ancestry analysis to identify
subsets of individuals with similar ancestry was performed for the Danish, Intermountain and Norwegian datasets separately. ADMIXTURE (v1.23)81 was run in supervised mode using the 1000
Genomes populations82 CEU (Utah residents with Northern and Western European ancestry), CHB (Han Chinese in Beijing, China), ITU (Indian Telugu in the UK), PEL (Peruvian in Lima, Peru) and
YRI (Yoruba in Ibadan, Nigeria) as training samples. These training samples had themselves been filtered for ancestry outliers using principal component analysis (PCA) and unsupervised
ADMIXTURE. For the Danish and Intermountain datasets, samples assigned <0.93 CEU were excluded. We performed a different filtering procedure for the Norwegian dataset to include
individuals with Finnish and Saami ancestry, who are common in Norway83. To identify such individuals, we first selected candidates those assigned between 0.5 and 0.93 CEU ancestry. We then
merged these individuals with the Human Origins dataset and calculated _F_ statistics84 of the form _f_3 (Mbuti; candidate individual, X), where X was each of the Human Origins populations
Nganasan, Pima, Han and Norwegian. In these _F_3 statistics, we identified a clear cluster of individuals with excess affinity to Nganasan and Norwegian over Pima and Han. In available
metadata, we observed that these individuals were highly enriched for locations of residence in Finnmark and officially designated Saami villages. These genetic and demographic features
match expectations for individuals of Saami or Finnish ancestry. Except for this cluster, we excluded all other Norwegian individuals assigned <0.93 CEU ancestry. Genetic principal
components for use as covariates in association analysis were obtained using bigsnpr85. ASSOCIATION TESTING AND META-ANALYSIS Using software developed at deCODE Genetics72, we applied
logistic regression assuming an additive model to test for genome-wide associations between sequence variants and migraine phenotypes. Association results from FinnGen were imported (Release
6: http://r6.finngen.fi). For the Icelandic data, the model included sex, county of birth, current age or age at death (first-order and second-order terms included), blood sample
availability for the individual and an indicator function for the overlap of the lifetime of the individual with the time span of phenotype collection. To include imputed but ungenotyped
individuals, we used county of birth as a proxy covariate for the first PCs in our analysis because county of birth has been shown to be in concordance with the first PC in Iceland86. For
the Danish, Norwegian, UK and US data, the covariates were sex, age, expected allele count and 20 PCs to adjust for population stratification. The association analysis of the imported
Finnish data was adjusted for sex, age, the genotyping batch and the first ten PCs. We used LD score regression intercepts22 to adjust the _χ_2 statistics and avoid inflation due to cryptic
relatedness and stratification, using a set of 1.1 million variants. _P_ values were calculated from the adjusted _χ_2 results. All statistical tests were two-sided unless otherwise
indicated. For the meta-analyses, we combined GWASs from the respective cohorts with summary statistics from Finland using a fixed-effects inverse-variance method based on effect estimates
and s.e. in which each dataset was assumed to have a common OR but allowed to have different population frequencies for alleles and genotypes. The total number of variants included in the
meta-analyses was between 68 and 80 million variants. Sequence variants were mapped to the NCBI Build 38 and matched on position and alleles to harmonize the datasets. The threshold for
genome-wide significance was corrected for multiple testing with a weighted Bonferroni adjustment that controls for the family-wise error rate, using as weights the enrichment of variant
classes with predicted functional impact among association signals21. The significance threshold then becomes 2.5 × 10−7 for high-impact variants (including stop-gained, frameshift, splice
acceptor or donor), 5.0 × 10−8 for moderate-impact variants (including missense, splice-region variants and in-frame indels), 4.5 × 10−9 for low-impact variants, 2.3 × 10−9 for other DNase I
hypersensitivity sites (DHS) variants and 7.5 × 10−10 for other non-DHS variants21. In a random-effects method, a likelihood ratio test was performed in all genome-wide associations to test
the heterogeneity of the effect estimate in the four datasets; the null hypothesis is that the effects are the same in all datasets, and the alternative hypothesis is that the effects
differ between datasets. The primary signal at each genomic locus was defined as the sequence variant with the lowest Bonferroni-adjusted _P_ value using the adjusted significance thresholds
described above. Conditional analysis was used to identify possible secondary signals within 500 kb from the primary signal. This was done using genotype data for the Icelandic, Norwegian,
Danish, UK and US datasets and an approximate conditional analysis implemented in GCTA software87 for the Finnish summary data. Adjusted _P_ values and ORs were combined using a
fixed-effects inverse-variance method. Class-specific genome-wide significance thresholds were also used for the secondary signals. Manhattan plots were generated using topr package in R.
For burden testing, we used the UK Biobank whole-exome sequenced dataset, consisting of 400,912 whole-exome sequenced White British (individuals identified by PCA analyses)88,89 who enrolled
in the study between 2006 and 2010 throughout the UK and were aged 38–65 years at recruitment. A wide range of phenotypic data has been provided by the UK Biobank primarily from hospital
records and increasingly from general practitioners from the UK. For the Icelandic, US and Danish cohorts, we used the phenotypes and WGS and imputation data previously described. We used
VEP90 to attribute predicted consequences to the variants sequenced in each dataset. We classified as high-impact variants those predicted as start-lost, stop-gain, stop-lost, splice donor,
splice acceptor or frameshift, collectively called LOF variants. For case–control analyses, we used logistic regression under an additive model to test for association between LOF gene
burdens and phenotypes, in which disease status was the dependent variable and genotype counts as the independent variable, using likelihood ratio test to compute two-sided _P_ values.
Individuals were coded 1 if they carried any of the LOF variants in the autosomal gene being tested and 0 otherwise. For the UK Biobank association testing, 20 PCs were used to adjust for
population substructure, and age and sex were included as covariates in the logistic regression model. We further included variables indicating sequencing batches to remove batch effects.
For these analyses, we used software developed at deCODE Genetics72. GENETIC CORRELATIONS Using cross-trait LD score regression22, we estimated the genetic correlation between each of the
migraine and proxy (BRH) and migraine subtype phenotypes (MO, MA and VD) defined in this study, in addition to epilepsy. In this analysis, we used results for about 1.2 million well-imputed
variants, and for LD information, we used precomputed LD scores for European populations (downloaded from https://data.broadinstitute.org/alkesgroup/LDSCORE/eur_w_ld_chr.tar.bz2). To avoid
bias due to sample overlap, we used the Icelandic and Danish cohorts combined to test for correlation with the respective phenotypes in the other remaining datasets combined. Finally, we
meta-analyzed the results of the two correlation analyses for each correlation for a combined correlation estimation. The significance level for the correlation estimates was determined
using a simple Bonferroni correction for the number of meta-analyzed correlations, and hence significance was set at _P_ < 0.0033 (0.05/15). IDENTIFICATION AND CONFIRMATION OF RARE
_PRRT2_ VARIANTS The variants in the _PRRT2_ gene are in a stretch of nine C’s, with one extra C in carriers of the insertion (p.Arg217ProfsTer8) and one missing C in carriers of the
deletion (p.Arg217GlufsTer12). This imposes a technical challenge for accurate whole-genome sequence calling. Therefore, all potential carriers of both variants were analyzed with Sanger
sequencing. Primers were designed using Primer 3 software. Following PCR, cycle sequencing reactions were performed in both directions on MJ Research PTC-225 thermal cyclers, using the
BigDye Terminator Cycle Sequencing Kit v3.1 (Life Technologies) and Ampure XP and CleanSeq kits (Agencourt) for cleanup of the PCR products and cycle sequencing reactions. Sequencing
products were loaded onto the 3730 XL DNA Analyzer (Applied Biosystems) and analyzed with Sequencher 5.0 software (Gene Codes Corporation). Based on the sequencing results, the variants were
then re-imputed into the respective cohorts. MIGRAINE SUBTYPE ANALYSIS OF LEAD VARIANTS To classify our lead variants by migraine subtype, we plotted their effects on MA versus MO and VD
versus MO using the method applied in ref. 11. This method requires a correlation parameter between MO and MA (MO and VD) to account for sample overlap, and previously this parameter was
estimated from GWAS summary statistics11, using empirical Pearson correlation of effect size estimates of common variants (MAF > 0.05), which do not show a strong association with either
of the migraine subtypes studied (_P_ > 1 × 10−4)91. In our data, this estimate of the correlation parameter was _r__ij_ = 0.59 between MO and MA and _r__ij_ = 0.198 between MO and VD
(estimated using 7,858,264 markers), which is considerably larger than if we estimated the sample overlap directly using counts of cases, controls and the counts of overlaps in these groups
between phenotypes70 (from all cohorts except the summary statistics from FinnGen), where we get _r__ij_ = 0.023 for MO and MA and _r__ij_ = 0.012 for MO and VD. As the latter estimates are
more conservative, we used those in the subtype analysis. Finally, we tested whether the effect sizes between MA and MO (and VD and MO) were equal at a Bonferroni corrected significance
threshold of _P_ = 0.05/43 (as we excluded from the 44 lead variants the MA variant in _PRRT2_) performed by using normal approximation and accounting for the correlation in effect size
difference estimators. As pointed out in ref. 11, this subtype classification method takes into account the different statistical power of the migraine subtype GWASs, which is an advantage
compared to simply comparing subtype effects. For the subtype analysis, we followed the R code available at https://github.com/mjpirinen/migraine-meta. FUNCTIONAL DATA AND COLOCALIZATION
ANALYSIS To highlight genes whose products potentially mediate the observed associations with migraine and migraine subtypes, we annotated the associations detected in this study (Tables 1
and 2) as well as variants in high LD (_r_2 ≥ 0.8 and within ±1 Mb) that are predicted to affect coding or splicing of a protein (VEP using RefSeq gene set), mRNA expression (top local eQTL,
_cis_-eQTL) in multiple tissues from deCODE, GTEx (https://www.gtexportal.org) and other public datasets (see Supplementary Table 18 for eQTL data sources) and/or plasma protein levels (top
pQTL) identified in large proteomic datasets from Iceland and the UK. The Icelandic proteomics data were analyzed using the SomaLogic SOMAscan proteomics assay that scans 4,907 aptamers,
measuring 4,719 proteins in samples from 35,559 Icelanders with the genetic information available at deCODE Genetics38. Plasma protein levels were standardized and adjusted for year of
birth, sex and year of sample collection (2000–2019)38. The UK proteomics dataset was analyzed using the Olink proteomics assay characterizing 1,463 proteins in 54,306 participants in the UK
Biobank92. RNA sequencing was performed on whole blood from 17,848 Icelanders and on subcutaneous adipose tissue from 769 Icelanders, respectively38. Gene expression was computed based on
personalized transcript abundances using kallisto93. Association between sequence variants and gene expression (_cis_-eQTL) was tested using a generalized linear regression, assuming
additive genetic effect and normal quantile gene expression estimates, adjusting for measurements of sequencing artifacts, demographic variables, blood composition and PCs94. The gene
expression PCs were computed per chromosome using a leave-one-chromosome-out method. All variants within 1 Mb of each gene were tested. We performed gene-based enrichment analysis using the
GENE2FUNC tool in FUMA95. The genes were tested for over-representation in different gene sets, including Gene Ontology cellular components (MsigDB c5) and GWAS Catalog-reported genes.
GENETIC DRUG TARGET ANALYSIS Using sources from the Drug-Gene Interaction Database96, Open Targets97 and the National Institutes of Health’s Illuminating the Druggable Genome98, we performed
a genetic drug target analysis for the 22 genes for which we have evidence of function pointing to the gene (Supplementary Fig. 6), in addition to the established MA gene _CACNA1A_.
REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Our previously described Icelandic
population whole-genome sequence data have been deposited at the European Variant Archive under accession PRJEB15197. The GWAS summary statistics for the migraine GWAS meta-analyses are
available at https://www.decode.com/summarydata/. FinnGen data are publicly available and were downloaded from https://www.finngen.fi/en/access_results. The UKB data were downloaded under
application 42256. Proteomics data and protein mapping to UniProt identifiers and gene names were provided by SomaLogic and Olink. Other data generated or analyzed in this study are included
in the article and its Supplementary Information. URLs for other external data used are as follows: precomputed LD scores for European populations,
https://data.broadinstitute.org/alkesgroup/LDSCORE/eur_w_ld_chr.tar.bz2; GWAS Catalog, https://www.ebi.ac.uk/gwas/; GTEx project, https://gtexportal.org/home/. URL sources for expression
data can be found in Supplementary Table 18. CODE AVAILABILITY We used publicly available software that is available on request under the following URLs: GraphTyper (v2.0-beta, GNU GPLv3
license), https://github.com/DecodeGenetics/graphtyper; Eagle (version 2.4.1), http://www.hsph.harvard.edu/alkes-price/software/; SHAPEIT4, https://odelaneau.github.io/shapeit4/; ADMIXTURE
(v1.23), https://dalexander.github.io/admixture/; BOLT-LMM (v.2.1), http://www.hsph.harvard.edu/alkes-price/software/; R (version 3.6.3), https://www.r-project.org/; R package ggplot for
visualization (version 3.3.3), https://ggplot2.tidyverse.org/; Ensembl v.87, https://www.ensembl.org/index.html; IMPUTE2 v.2.3.1, https://mathgen.stats.ox.ac.uk/impute/impute_v2.html; dbSNP
v.140, http://www.ncbi.nlm.nih.gov/SNP/; kallisto v.0.46, https://github.com/pachterlab/kallisto; for subtype stratification analysis, we used R code available at
https://github.com/mjpirinen/migraine-meta; MAGMA (v1.08), http://ctglab.nl/software/magma; VEP (release 100), https://github.com/Ensembl/ensembl-vep; FUMA, https://fuma.ctglab.nl/;
Sequencher 5.0, https://sequencher.software.informer.com/5.0/; NCBI Build 38, https://www.ncbi.nlm.nih.gov/. No custom code was written for this study. REFERENCES * Lipton, R. B. &
Bigal, M. E. The epidemiology of migraine. _Am. J. Med._ 118, 3S–10S (2005). PubMed Google Scholar * Headache Classification Committee of the International Headache Society (IHS) The
International Classification of Headache Disorders, 3rd edition. _Cephalalgia_ 38, 1–211 (2018). * Rasmussen, B. K. & Olesen, J. Migraine with aura and migraine without aura: an
epidemiological study. _Cephalalgia_ 12, 221–228 (1992). Article CAS PubMed Google Scholar * Lauritzen, M. Pathophysiology of the migraine aura. The spreading depression theory. _Brain_
117, 199–210 (1994). Article PubMed Google Scholar * Lai, J. & Dilli, E. Migraine aura: updates in pathophysiology and management. _Curr. Neurol. Neurosci. Rep._ 20, 17 (2020).
Article PubMed Google Scholar * Olesen, J., Tfelt-Hansen, P., Henriksen, L. & Larsen, B. The common migraine attack may not be initiated by cerebral ischaemia. _Lancet_ 2, 438–440
(1981). Article CAS PubMed Google Scholar * Sanchez del Rio, M. et al. Perfusion weighted imaging during migraine: spontaneous visual aura and headache. _Cephalalgia_ 19, 701–707 (1999).
Article CAS PubMed Google Scholar * Riant, F. et al. Hemiplegic migraine associated with _PRRT2_ variations: a clinical and genetic study. _Neurology_ 98, e51–e61 (2022). Article CAS
PubMed Google Scholar * De Vries, T., Villalon, C. M. & MaassenVanDenBrink, A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. _Pharmacol. Ther._ 211, 107528
(2020). Article PubMed Google Scholar * Dodick, D. W. et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. _Cephalalgia_ 38, 1026–1037 (2018). Article PubMed
Google Scholar * Hautakangas, H. et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. _Nat. Genet._ 54, 152–160 (2022). Article
CAS PubMed PubMed Central Google Scholar * Sacco, S. et al. Burden and attitude to resistant and refractory migraine: a survey from the European Headache Federation with the
endorsement of the European Migraine & Headache Alliance. _J. Headache Pain_ 22, 39 (2021). Article PubMed PubMed Central Google Scholar * Hautakangas, H. et al. A genome-wide
meta-analysis of migraine with over 102,000 cases identifies 124 risk loci and provides first genetic insights to new migraine therapeutics targeting CGRP pathway. In _Proceedings of 2019
Annual Meeting of American Society of Human Genetics_ (ASHG, 2019). * Katsarava, Z., Mania, M., Lampl, C., Herberhold, J. & Steiner, T. J. Poor medical care for people with migraine in
Europe—evidence from the Eurolight study. _J. Headache Pain_ 19, 10 (2018). Article PubMed PubMed Central Google Scholar * Sorensen, E. et al. Data resource profile: the Copenhagen
Hospital Biobank (CHB). _Int J. Epidemiol._ 50, 719–720 (2021). Article PubMed Google Scholar * Hansen, T. F. et al. DBDS Genomic Cohort, a prospective and comprehensive resource for
integrative and temporal analysis of genetic, environmental and lifestyle factors affecting health of blood donors. _BMJ Open_ 9, e028401 (2019). Article PubMed PubMed Central Google
Scholar * Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. _Nature_ 562, 203–209 (2018). * Azriel, E. et al. Utilizing public health frameworks and
partnerships to ensure equity in DNA-based population screening. _Front. Genet._ 13, 886755 (2022). Article CAS PubMed PubMed Central Google Scholar * Refsum, H. et al. The Hordaland
Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. _J. Nutr._ 136, 1731S–1740S (2006). Article CAS PubMed Google Scholar *
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. _Nature_ 613, 508–518 (2023). * Sveinbjornsson, G. et al. Weighting sequence variants based
on their annotation increases power of whole-genome association studies. _Nat. Genet._ 48, 314–317 (2016). Article CAS PubMed Google Scholar * Bulik-Sullivan, B. K. et al. LD score
regression distinguishes confounding from polygenicity in genome-wide association studies. _Nat. Genet._ 47, 291–295 (2015). Article CAS PubMed PubMed Central Google Scholar *
Ebrahimi-Fakhari, D., Saffari, A., Westenberger, A. & Klein, C. The evolving spectrum of PRRT2-associated paroxysmal diseases. _Brain_ 138, 3476–3495 (2015). Article PubMed Google
Scholar * Park, B. M., Kim, Y. O., Kim, M. K. & Woo, Y. J. A novel frameshift mutation of PRRT2 in a family with infantile convulsions and choreathetosis syndrome: c.640delinsCC
(p.Ala214ProfsTer11). _J. Genet. Med._ 16, 19–22 (2019). Article Google Scholar * Chen, W. J. et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal
kinesigenic dyskinesia. _Nat. Genet._ 43, 1252–1255 (2011). Article CAS PubMed Google Scholar * Kaushik, J. S., Bala, K. & Dubey, R. Paroxysmal kinesigenic dyskinesia. _Indian
Pediatr._ 55, 74 (2018). Article PubMed Google Scholar * International League Against Epilepsy Consortium on Complex Epilepsies. GWAS meta-analysis of over 29,000 people with epilepsy
reveals 26 risk loci and subtype-specific genetic architecture. _Nat. Genet._ 55, 1471–1482 (2023). * Helgadottir, A. et al. Genome-wide analysis yields new loci associating with aortic
valve stenosis. _Nat. Commun._ 9, 987 (2018). Article PubMed PubMed Central Google Scholar * Wang, M., Gao, J., Liu, J., Zhao, X. & Lei, Y. Genomic association vs. serological
determination of ABO blood types in a Chinese cohort, with application in Mendelian randomization. _Genes (Basel)_ 12, 959 (2021). Article CAS PubMed Google Scholar * Gandhi, P. N.,
Wang, X., Zhu, X., Chen, S. G. & Wilson-Delfosse, A. L. The Roc domain of leucine-rich repeat kinase 2 is sufficient for interaction with microtubules. _J. Neurosci. Res._ 86, 1711–1720
(2008). Article CAS PubMed PubMed Central Google Scholar * Elmazny, A. et al. Interferon-β-induced headache in patients with multiple sclerosis: frequency and characterization. _J.
Pain. Res._ 13, 537–545 (2020). Article CAS PubMed PubMed Central Google Scholar * Ginanneschi, F. et al. SCN11A variant as possible pain generator in sensory axonal neuropathy.
_Neurol. Sci._ 40, 1295–1297 (2019). Article PubMed Google Scholar * Leipold, E. et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. _Nat. Genet._ 45,
1399–1404 (2013). Article CAS PubMed Google Scholar * Lonsdale, J. et al. The Genotype-Tissue Expression (GTEx) project. _Nat. Genet._ 45, 580–585 (2013). Article CAS Google Scholar *
Hoffmann, T. J. et al. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. _Nat. Genet._ 49, 54–64 (2017). Article CAS
PubMed Google Scholar * Meng, W. et al. A meta-analysis of the genome-wide association studies on two genetically correlated phenotypes suggests four new risk loci for headaches.
_Phenomics_ 3, 64–76 (2022). Article PubMed PubMed Central Google Scholar * Bakker, M. K. & Ruigrok, Y. M. Genetics of intracranial aneurysms. _Stroke_ 52, 3004–3012 (2021). Article
PubMed Google Scholar * Ferkingstad, E. et al. Large-scale integration of the plasma proteome with genetics and disease. _Nat. Genet._ 53, 1712–1721 (2021). Article CAS PubMed Google
Scholar * ENCODE Project Consortium et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. _Nature_ 583, 699–710 (2020). * Wu, L. et al. PRRT2 truncated mutations
lead to nonsense-mediated mRNA decay in paroxysmal kinesigenic dyskinesia. _Parkinsonism Relat. Disord._ 20, 1399–1404 (2014). Article PubMed Google Scholar * Valente, P. et al. PRRT2 is
a key component of the Ca2+-dependent neurotransmitter release machinery. _Cell Rep._ 15, 117–131 (2016). Article CAS PubMed PubMed Central Google Scholar * Zhao, S. Y. et al.
Functional study and pathogenicity classification of PRRT2 missense variants in PRRT2-related disorders. _CNS Neurosci. Ther._ 26, 39–46 (2020). Article CAS PubMed Google Scholar *
Watson, J. J., Allen, S. J. & Dawbarn, D. Targeting nerve growth factor in pain: what is the therapeutic potential? _BioDrugs_ 22, 349–359 (2008). Article CAS PubMed Google Scholar *
Sun, M.-K. & Alkon, D. L. Bryostatin-1: pharmacology and therapeutic potential as a CNS drug. _CNS Drug Rev._ 12, 1–8 (2006). Article CAS PubMed PubMed Central Google Scholar *
Borhani Haghighi, A. et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over
study. _Int J. Clin. Pract._ 64, 451–456 (2010). Article CAS PubMed Google Scholar * Chi, P. W. et al. Intranasal lidocaine for acute migraine: a meta-analysis of randomized controlled
trials. _PLoS ONE_ 14, e0224285 (2019). Article CAS PubMed PubMed Central Google Scholar * Schwenk, E. S. et al. Lidocaine infusions for refractory chronic migraine: a retrospective
analysis. _Reg. Anesth. Pain Med._ 47, 408–413 (2022). Article PubMed Google Scholar * Olesen, J. The international classification of headache disorders. _Headache_ 48, 691–693 (2008).
Article PubMed Google Scholar * Olesen, J. ICHD-3 β is published. Use it immediately. _Cephalalgia_ 33, 627–628 (2013). Article PubMed Google Scholar * Winsvold, B. S. et al. Shared
genetic risk between migraine and coronary artery disease: a genome-wide analysis of common variants. _PLoS ONE_ 12, e0185663 (2017). Article PubMed PubMed Central Google Scholar *
Jennings, D. et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. _Sci. Transl. Med._ 14, eabj2658 (2022). Article CAS PubMed Google Scholar
* Schapansky, J. et al. Familial knockin mutation of LRRK2 causes lysosomal dysfunction and accumulation of endogenous insoluble α-synuclein in neurons. _Neurobiol. Dis._ 111, 26–35 (2018).
Article CAS PubMed Google Scholar * Miklossy, J. et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. _J. Neuropathol. Exp. Neurol._ 65, 953–963 (2006).
Article CAS PubMed Google Scholar * Zimprich, A. et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. _Neuron_ 44, 601–607 (2004). Article CAS
PubMed Google Scholar * Dachsel, J. C. & Farrer, M. J. LRRK2 and Parkinson disease. _Arch. Neurol._ 67, 542–547 (2010). Article PubMed Google Scholar * Kanner, A. M. Management of
psychiatric and neurological comorbidities in epilepsy. _Nat. Rev. Neurol._ 12, 106–116 (2016). Article CAS PubMed Google Scholar * Noebels, J. L., Avoli, M., Rogawski, M. A., Olsen, R.
W. & Delgado-Escueta A. V. (eds.) _Jasper’s Basic Mechanisms of the Epilepsies_ 4th edn (National Center for Biotechnology Information, 2012). * Baldin, E., Ludvigsson, P., Mixa, O.
& Hesdorffer, D. C. Prevalence of recurrent symptoms and their association with epilepsy and febrile seizure in school-aged children: a community-based survey in Iceland. _Epilepsy
Behav._ 23, 315–319 (2012). Article PubMed Google Scholar * Lee, H. Y. et al. Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. _Cell Rep._
1, 2–12 (2012). Article CAS PubMed Google Scholar * Li, M. et al. PRRT2 mutant leads to dysfunction of glutamate signaling. _Int. J. Mol. Sci._ 16, 9134–9151 (2015). Article CAS PubMed
PubMed Central Google Scholar * Fruscione, F. et al. PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. _Brain_ 141, 1000–1016 (2018). Article
PubMed PubMed Central Google Scholar * Baker, M. D. & Nassar, M. A. Painful and painless mutations of SCN9A and SCN11A voltage-gated sodium channels. _Pflugers Arch._ 472, 865–880
(2020). Article CAS PubMed PubMed Central Google Scholar * Cummins, T. R. et al. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory
neurons. _J. Neurosci._ 19, RC43 (1999). Article CAS PubMed PubMed Central Google Scholar * Braden, K., Stratton, H. J., Salvemini, D. & Khanna, R. Small molecule targeting NaV1.7
via inhibition of the CRMP2-Ubc9 interaction reduces and prevents pain chronification in a mouse model of oxaliplatin-induced neuropathic pain. _Neurobiol. Pain_ 11, 100082 (2022). Article
CAS PubMed Google Scholar * Cai, S. et al. Selective targeting of NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces pain in rodents. _Sci. Transl. Med._ 13, eabh1314 (2021).
Article CAS PubMed Google Scholar * Bittner, S. et al. Upregulation of K2P5.1 potassium channels in multiple sclerosis. _Ann. Neurol._ 68, 58–69 (2010). Article CAS PubMed Google
Scholar * Hagen, K. et al. Blood pressure and risk of headache: a prospective study of 22 685 adults in Norway. _J. Neurol. Neurosurg. Psychiatry_ 72, 463–466 (2002). CAS PubMed PubMed
Central Google Scholar * Lebedeva, E. R., Gurary, N. M., Sakovich, V. P. & Olesen, J. Migraine before rupture of intracranial aneurysms. _J. Headache Pain_ 14, 15 (2013). Article
PubMed PubMed Central Google Scholar * Gormley, P. et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. _Nat. Genet._ 48, 856–866 (2016). Article
CAS PubMed PubMed Central Google Scholar * Bhattacharjee, S. et al. A subset-based approach improves power and interpretation for the combined analysis of genetic association studies of
heterogeneous traits. _Am. J. Hum. Genet._ 90, 821–835 (2012). Article CAS PubMed PubMed Central Google Scholar * Jonsson, H. et al. Whole genome characterization of sequence diversity
of 15,220 Icelanders. _Sci. Data_ 4, 170115 (2017). Article CAS PubMed PubMed Central Google Scholar * Gudbjartsson, D. F. et al. Large-scale whole-genome sequencing of the Icelandic
population. _Nat. Genet._ 47, 435–444 (2015). Article CAS PubMed Google Scholar * Lipton, R. B. et al. Migraine prevalence, disease burden, and the need for preventive therapy.
_Neurology_ 68, 343–349 (2007). Article CAS PubMed Google Scholar * Eggertsson, H. P. et al. Graphtyper enables population-scale genotyping using pangenome graphs. _Nat. Genet._ 49,
1654–1660 (2017). Article CAS PubMed Google Scholar * Kong, A. et al. Detection of sharing by descent, long-range phasing and haplotype imputation. _Nat. Genet._ 40, 1068–1075 (2008).
Article CAS PubMed PubMed Central Google Scholar * Li, N. & Stephens, M. Modeling linkage disequilibrium and identifying recombination hotspots using single-nucleotide polymorphism
data. _Genetics_ 165, 2213–2233 (2003). Article CAS PubMed PubMed Central Google Scholar * Howie, B. N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation
method for the next generation of genome-wide association studies. _PLoS Genet._ 5, e1000529 (2009). Article PubMed Central Google Scholar * Wain, L. V. et al. Novel insights into the
genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. _Lancet Respir. Med._ 3, 769–781 (2015).
Article PubMed PubMed Central Google Scholar * Halldorsson, B. V. et al. The sequences of 150,119 genomes in the UK Biobank. _Nature_ 607, 732–740 (2022). Article CAS PubMed PubMed
Central Google Scholar * Delaneau, O., Zagury, J. F., Robinson, M. R., Marchini, J. L. & Dermitzakis, E. T. Accurate, scalable and integrative haplotype estimation. _Nat. Commun._ 10,
5436 (2019). Article PubMed PubMed Central Google Scholar * Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. _Genome Res._
19, 1655–1664 (2009). Article CAS PubMed PubMed Central Google Scholar * Auton, A. et al. A global reference for human genetic variation. _Nature_ 526, 68–74 (2015). Article PubMed
Google Scholar * Mattingsdal, M. et al. The genetic structure of Norway. _Eur. J. Hum. Genet._ 29, 1710–1718 (2021). Article CAS PubMed PubMed Central Google Scholar * Patterson, N. et
al. Ancient admixture in human history. _Genetics_ 192, 1065–1093 (2012). Article PubMed PubMed Central Google Scholar * Privé, F., Aschard, H., Ziyatdinov, A. & Blum, M. G. B.
Efficient analysis of large-scale genome-wide data with two R packages: bigstatsr and bigsnpr. _Bioinformatics_ 34, 2781–2787 (2018). Article PubMed PubMed Central Google Scholar *
Price, A. L. et al. The impact of divergence time on the nature of population structure: an example from Iceland. _PLoS Genet._ 5, e1000505 (2009). Article PubMed PubMed Central Google
Scholar * Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. _Nat. Genet._ 44, S1–S3 (2012).
Article Google Scholar * Backman, J. D. et al. Exome sequencing and analysis of 454,787 UK Biobank participants. _Nature_ 599, 628–634 (2021). Article CAS PubMed PubMed Central Google
Scholar * Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. _Nature_ 562, 203–209 (2018). Article CAS PubMed PubMed Central Google Scholar * McLaren,
W. et al. The Ensembl variant effect predictor. _Genome Biol._ 17, 122 (2016). Article PubMed PubMed Central Google Scholar * Cichonska, A. et al. metaCCA: summary statistics-based
multivariate meta-analysis of genome-wide association studies using canonical correlation analysis. _Bioinformatics_ 32, 1981–1989 (2016). Article CAS PubMed PubMed Central Google
Scholar * Sun, B. B. et al. Plasma proteomic associations with genetics and health in the UK Biobank. _Nature_ 622, 329–338 (2023). * Bray, N. L., Pimentel, H., Melsted, P. & Pachter,
L. Near-optimal probabilistic RNA-seq quantification. _Nat. Biotechnol._ 34, 525–527 (2016). Article CAS PubMed Google Scholar * Stegle, O., Parts, L., Piipari, M., Winn, J. &
Durbin, R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. _Nat. Protoc._ 7, 500–507 (2012). Article
CAS PubMed PubMed Central Google Scholar * Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. _Nat.
Commun._ 8, 1826 (2017). Article PubMed PubMed Central Google Scholar * Freshour, S. L. et al. Integration of the drug–gene interaction database (DGIdb 4.0) with open crowdsource
efforts. _Nucleic Acids Res._ 49, D1144–D1151 (2021). Article CAS PubMed Google Scholar * Ochoa, D. et al. The next-generation Open Targets Platform: reimagined, redesigned, rebuilt.
_Nucleic Acids Res._ 51, D1353–D1359 (2022). Article PubMed Central Google Scholar * Nguyen, D.-T. et al. Pharos: collating protein information to shed light on the druggable genome.
_Nucleic Acids Res._ 45, D995–D1002 (2016). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank all participants who contributed data and samples
used in this study. Their contributions are essential for research such as reported here. We thank all investigators and colleagues who collaborated on the many aspects of this study,
including data collection, sample handling, phenotypic characterization of clinical samples, genotyping and analysis of the whole-genome association data. We acknowledge participants and
investigators of the FinnGen study20 and the UK Biobank study. This research has been conducted using the UK Biobank Resource, a major biomedical database (application 42256,
https://www.ukbiobank.ac.uk/). The financial support from the European Commission to the painFACT project to T.E.T. (H2020-2020-848099) is acknowledged, as is support from the Novo Nordisk
Foundation, DBDS Consortium (grants NNF17OC0027594 and NNF14CC0001). The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the
National Institutes of Health (commonfund.nih.gov/GTEx). Additional funds were provided by the NCI, NHGRI, NHLBI, NIDA, NIMH and NINDS. Donors were enrolled at Biospecimen Source Sites
funded by NCI\Leidos Biomedical Research, Inc. subcontracts to the National Disease Research Interchange (10XS170), GTEx Project March 5, 2014 version Page 5 of 8 Roswell Park Cancer
Institute (10XS171), and Science Care, Inc. (X10S172). The Laboratory, Data Analysis, and Coordinating Center (LDACC) was funded through a contract (HHSN268201000029C) to the The Broad
Institute, Inc. Biorepository operations were funded through a Leidos Biomedical Research, Inc. subcontract to Van Andel Research Institute (10ST1035). Additional data repository and project
management were provided by Leidos Biomedical Research, Inc. (HHSN261200800001E). The Brain Bank was supported supplements to University of Miami grant DA006227. Statistical Methods
development grants were made to the University of Geneva (MH090941 and MH101814), the University of Chicago (MH090951, MH090937, MH101825 and MH101820), the University of North Carolina -
Chapel Hill (MH090936), North Carolina State University (MH101819), Harvard University (MH090948), Stanford University (MH101782), Washington University (MH101810) and to the University of
Pennsylvania (MH101822). The datasets used for the analyses described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number
phs000424.v9.p2. AUTHOR INFORMATION Author notes * These authors contributed equally: Gyda Bjornsdottir, Mona A. Chalmer. * These authors jointly supervised this work: Thomas F. Hansen, Kari
Stefansson. AUTHORS AND AFFILIATIONS * deCODE Genetics/Amgen, Inc., Reykjavik, Iceland Gyda Bjornsdottir, Lilja Stefansdottir, Astros Th. Skuladottir, Gudmundur Einarsson, Margret
Andresdottir, Doruk Beyter, Egil Ferkingstad, Solveig Gretarsdottir, Bjarni V. Halldorsson, Gisli H. Halldorsson, Anna Helgadottir, Hannes Helgason, Grimur Hjorleifsson Eldjarn, Adalbjorg
Jonasdottir, Aslaug Jonasdottir, Ingileif Jonsdottir, Sigrun H. Lund, Olafur Th. Magnusson, Pall Melsted, Kristjan H. S. Moore, Asmundur Oddsson, Pall I. Olason, Asgeir Sigurdsson, Olafur A.
Stefansson, Jona Saemundsdottir, Gardar Sveinbjornsson, Vinicius Tragante, Unnur Unnsteinsdottir, G. Bragi Walters, Florian Zink, Gisli Masson, Unnur Thorsteinsdottir, Hilma Holm, Daniel F.
Gudbjartsson, Gudmar Thorleifsson, Patrick Sulem, Hreinn Stefansson, Thorgeir E. Thorgeirsson & Kari Stefansson * Danish Headache Center, Department of Neurology, Copenhagen University
Hospital, Rigshospitalet-Glostrup, Copenhagen, Denmark Mona A. Chalmer, Lisette J. A. Kogelman, Jes Olesen & Thomas F. Hansen * Reykjavik University, School of Technology, Reykjavik,
Iceland Bjarni V. Halldorsson * School of Engineering and Natural Sciences, University of Iceland, Reykjavik, Iceland Gisli H. Halldorsson, Hannes Helgason, Pall Melsted & Daniel F.
Gudbjartsson * Faculty of Medicine, School of Health Sciences, University of Iceland, Reykjavik, Iceland Ingileif Jonsdottir, Unnur Thorsteinsdottir & Kari Stefansson * Intermountain
Heart Institute, Salt Lake City, UT, USA Kirk U. Knowlton * Intermountain Healthcare, Saint George, UT, USA Lincoln D. Nadauld * Faculty of Physical Sciences, School of Engineering and
Natural Sciences, University of Iceland, Reykjavik, Iceland Sigrun H. Lund * NORMENT, Centre for Mental Disorders Research, Division of Mental Health and Addiction, Oslo University Hospital,
and Institute of Clinical Medicine, University of Oslo, Oslo, Norway Linn Rødevand & Ole A. Andreassen * Department of Global Public Health and Primary Care, University of Bergen,
Bergen, Norway Jannicke Igland & Rolv T. Lie * Department of Health and Social Science, Centre for Evidence-Based Practice, Western Norway University of Applied Science, Bergen, Norway
Jannicke Igland * Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway Rolv T. Lie * Department of Biomedicine, University of Bergen, Bergen, Norway Jan Haavik
* Division of Psychiatry, Haukeland University Hospital, Bergen, Norway Jan Haavik * Novo Nordisk Foundation Center for Protein Research, Faculty of Health and Medical Sciences, University
of Copenhagen, Copenhagen, Denmark Karina Banasik, Søren Brunak, Kristoffer Burgdorf, Ioannis Louloudis, Agnete Lundgaard, Alexander P. Henriksen, David Westergaard & Thomas F. Hansen *
Department of Clinical Immunology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark Maria Didriksen, Erik Sørensen, Kristoffer Burgdorf, Joseph Dowsett, Bjarke Feenstra,
Frank Geller, Rikke L. Jacobsen, Margit A. H. Larsen, Christina Mikkelsen, Ioanna Nissen, Michael Swinn, Lise W. Thørner & Sisse R. Ostrowski * Department of Clinical Immunology, Odense
University Hospital, Odense, Denmark Mie T. Bruun & Mie T. Bruun * Department of Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark Christian Erikstrup, Jens K. Boldsen,
Khoa M. Dinh, Lotte Hindhede, Katrine Kaspersen, Bertram D. Kjerulf & Susan Mikkelsen * Department of Clinical Medicine Health, Aarhus University, Aarhus, Denmark Christian Erikstrup *
Department of Clinical Immunology, Aalborg University Hospital, Aalborg, Denmark Kaspar R. Nielsen * Department of Clinical Medicine, Aalborg University, Aalborg, Denmark Kaspar R. Nielsen *
Department of Clinical Immunology, Zealand University Hospital, Køge, Denmark Ole B. Pedersen, Jakob Bay & Thorsten Brodersen * Department of Clinical Medicine, Faculty of Health and
Medical Sciences, University of Copenhagen, Copenhagen, Denmark Ole B. Pedersen, Thomas Werge & Sisse R. Ostrowski * Statens Serum Institut, Copenhagen, Denmark Henrik Ullum, Bjarke
Feenstra & Frank Geller * Department of Pediatrics, Landspitali University Hostpital, Reykjavik, Iceland Petur Ludvigsson & Olafur Thorarensen * Heilsuklasinn Clinic, Reykjavik,
Iceland Anna Bjornsdottir * Laeknasetrid Clinic, Reykjavik, Iceland Gudrun R. Sigurdardottir & Olafur A. Sveinsson * Department of Neurology, Landspitali University Hospital, Reykjavik,
Iceland Olafur A. Sveinsson * Danish Cancer Society Research Center, Copenhagen, Denmark Henrik Hjalgrim & Klaus Rostgaard * Department of Dermatology, Zealand University Hospital,
Roskilde, Denmark Gregor Jemec * Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark Mette Nyegaard & Palle D. Rohde * Institute of
Biological Psychiatry, Mental Health Centre, Sct. Hans, Copenhagen University Hospital, Roskilde, Denmark Thomas Werge Authors * Gyda Bjornsdottir View author publications You can also
search for this author inPubMed Google Scholar * Mona A. Chalmer View author publications You can also search for this author inPubMed Google Scholar * Lilja Stefansdottir View author
publications You can also search for this author inPubMed Google Scholar * Astros Th. Skuladottir View author publications You can also search for this author inPubMed Google Scholar *
Gudmundur Einarsson View author publications You can also search for this author inPubMed Google Scholar * Margret Andresdottir View author publications You can also search for this author
inPubMed Google Scholar * Doruk Beyter View author publications You can also search for this author inPubMed Google Scholar * Egil Ferkingstad View author publications You can also search
for this author inPubMed Google Scholar * Solveig Gretarsdottir View author publications You can also search for this author inPubMed Google Scholar * Bjarni V. Halldorsson View author
publications You can also search for this author inPubMed Google Scholar * Gisli H. Halldorsson View author publications You can also search for this author inPubMed Google Scholar * Anna
Helgadottir View author publications You can also search for this author inPubMed Google Scholar * Hannes Helgason View author publications You can also search for this author inPubMed
Google Scholar * Grimur Hjorleifsson Eldjarn View author publications You can also search for this author inPubMed Google Scholar * Adalbjorg Jonasdottir View author publications You can
also search for this author inPubMed Google Scholar * Aslaug Jonasdottir View author publications You can also search for this author inPubMed Google Scholar * Ingileif Jonsdottir View
author publications You can also search for this author inPubMed Google Scholar * Kirk U. Knowlton View author publications You can also search for this author inPubMed Google Scholar *
Lincoln D. Nadauld View author publications You can also search for this author inPubMed Google Scholar * Sigrun H. Lund View author publications You can also search for this author inPubMed
Google Scholar * Olafur Th. Magnusson View author publications You can also search for this author inPubMed Google Scholar * Pall Melsted View author publications You can also search for
this author inPubMed Google Scholar * Kristjan H. S. Moore View author publications You can also search for this author inPubMed Google Scholar * Asmundur Oddsson View author publications
You can also search for this author inPubMed Google Scholar * Pall I. Olason View author publications You can also search for this author inPubMed Google Scholar * Asgeir Sigurdsson View
author publications You can also search for this author inPubMed Google Scholar * Olafur A. Stefansson View author publications You can also search for this author inPubMed Google Scholar *
Jona Saemundsdottir View author publications You can also search for this author inPubMed Google Scholar * Gardar Sveinbjornsson View author publications You can also search for this author
inPubMed Google Scholar * Vinicius Tragante View author publications You can also search for this author inPubMed Google Scholar * Unnur Unnsteinsdottir View author publications You can also
search for this author inPubMed Google Scholar * G. Bragi Walters View author publications You can also search for this author inPubMed Google Scholar * Florian Zink View author
publications You can also search for this author inPubMed Google Scholar * Linn Rødevand View author publications You can also search for this author inPubMed Google Scholar * Ole A.
Andreassen View author publications You can also search for this author inPubMed Google Scholar * Jannicke Igland View author publications You can also search for this author inPubMed Google
Scholar * Rolv T. Lie View author publications You can also search for this author inPubMed Google Scholar * Jan Haavik View author publications You can also search for this author inPubMed
Google Scholar * Karina Banasik View author publications You can also search for this author inPubMed Google Scholar * Søren Brunak View author publications You can also search for this
author inPubMed Google Scholar * Maria Didriksen View author publications You can also search for this author inPubMed Google Scholar * Mie T. Bruun View author publications You can also
search for this author inPubMed Google Scholar * Christian Erikstrup View author publications You can also search for this author inPubMed Google Scholar * Lisette J. A. Kogelman View author
publications You can also search for this author inPubMed Google Scholar * Kaspar R. Nielsen View author publications You can also search for this author inPubMed Google Scholar * Erik
Sørensen View author publications You can also search for this author inPubMed Google Scholar * Ole B. Pedersen View author publications You can also search for this author inPubMed Google
Scholar * Henrik Ullum View author publications You can also search for this author inPubMed Google Scholar * Gisli Masson View author publications You can also search for this author
inPubMed Google Scholar * Unnur Thorsteinsdottir View author publications You can also search for this author inPubMed Google Scholar * Jes Olesen View author publications You can also
search for this author inPubMed Google Scholar * Petur Ludvigsson View author publications You can also search for this author inPubMed Google Scholar * Olafur Thorarensen View author
publications You can also search for this author inPubMed Google Scholar * Anna Bjornsdottir View author publications You can also search for this author inPubMed Google Scholar * Gudrun R.
Sigurdardottir View author publications You can also search for this author inPubMed Google Scholar * Olafur A. Sveinsson View author publications You can also search for this author
inPubMed Google Scholar * Sisse R. Ostrowski View author publications You can also search for this author inPubMed Google Scholar * Hilma Holm View author publications You can also search
for this author inPubMed Google Scholar * Daniel F. Gudbjartsson View author publications You can also search for this author inPubMed Google Scholar * Gudmar Thorleifsson View author
publications You can also search for this author inPubMed Google Scholar * Patrick Sulem View author publications You can also search for this author inPubMed Google Scholar * Hreinn
Stefansson View author publications You can also search for this author inPubMed Google Scholar * Thorgeir E. Thorgeirsson View author publications You can also search for this author
inPubMed Google Scholar * Thomas F. Hansen View author publications You can also search for this author inPubMed Google Scholar * Kari Stefansson View author publications You can also search
for this author inPubMed Google Scholar CONSORTIA DBDS GENETIC CONSORTIUM * Karina Banasik * , Jakob Bay * , Jens K. Boldsen * , Thorsten Brodersen * , Søren Brunak * , Kristoffer Burgdorf
* , Mona A. Chalmer * , Maria Didriksen * , Khoa M. Dinh * , Joseph Dowsett * , Christian Erikstrup * , Bjarke Feenstra * , Frank Geller * , Daniel F. Gudbjartsson * , Thomas F. Hansen * ,
Lotte Hindhede * , Henrik Hjalgrim * , Rikke L. Jacobsen * , Gregor Jemec * , Katrine Kaspersen * , Bertram D. Kjerulf * , Lisette J. A. Kogelman * , Margit A. H. Larsen * , Ioannis
Louloudis * , Agnete Lundgaard * , Susan Mikkelsen * , Christina Mikkelsen * , Kaspar R. Nielsen * , Ioanna Nissen * , Mette Nyegaard * , Sisse R. Ostrowski * , Ole B. Pedersen * , Alexander
P. Henriksen * , Palle D. Rohde * , Klaus Rostgaard * , Michael Swinn * , Kari Stefansson * , Hreinn Stefansson * , Erik Sørensen * , Unnur Thorsteinsdottir * , Lise W. Thørner * , Mie T.
Bruun * , Thomas Werge * & David Westergaard CONTRIBUTIONS O.B.P. ([email protected]) is the representative for the DBDS Genetic Consortium. G.B., M.A.C., L.S., A.Th.S., G.E.,
E.F., S.G., B.V.H., A.H., Adalbjorg Jonasdottir, Aslaug Jonasdottir, I.J., G.M., K.H.S.M., O.Th.M., P.I.O., A.S., O.A. Stefansson, G.S., V.T., U.U., G.B.W., F.Z., U.T., S.R.O., H. Holm,
D.F.G., G.T., P.S., H.S., T.E.T., T.F.H. and K.S. designed the study, analyzed data and interpreted results. G.B., M.A., A.H., I.J., A.O., J.S., U.U., G.B.W., U.T., H. Holm, D.F.G., P.S.,
H.S., T.E.T. and K.S. collected and analyzed Icelandic phenotypes and samples for the study. G.B., A.Th.S., D.B., E.F., G.H.H., H. Helgason, S.H.L., P.M., A.S., O.A. Stefansson, H. Holm,
G.H.E., D.F.G., G.T., P.S., H.S., T.E.T., T.F.H. and K.S. performed and/or interpreted results from functional studies, transcriptomics, proteomics and gene set enrichment. O.A.A., J.H.,
J.I., R.T.L. and L.R. designed, collected, contributed and interpreted Norwegian study data. The DBDS Genetic Consortium, M.A.C., K.B., S.B., M.D., M.T.B., C.E., L.J.A.K., K.R.N., E.S.,
O.B.P., H.U., J.O., S.R.O. and T.F.H. designed, collected, contributed and interpreted Danish study data. L.D.N. and K.U.K designed, collected, contributed and interpreted the US study data.
G.B., M.A.C., L.S., A.Th.S., E.F., S.G., A.H., Adalbjorg Jonasdottir, Aslaug Jonasdottir, A.S., A.B., A.O., G.R.S., P.L., O.T., O.A. Sveinsson, H. Holm, G.T., P.S., H.S., T.E.T., T.F.H. and
K.S. drafted the manuscript with input and comments from other authors who all reviewed and contributed to the final version of the manuscript. CORRESPONDING AUTHORS Correspondence to Gyda
Bjornsdottir or Kari Stefansson. ETHICS DECLARATIONS COMPETING INTERESTS Authors affiliated with deCODE Genetics/Amgen declare competing financial interests as employees. The remaining
authors declare no competing financial interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Genetics_ thanks Guy Rouleau and Ynte Ruigrok for their contribution to the peer review of this
work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION Supplementary Figs. 1–7 and Supplementary Notes 1–5. REPORTING SUMMARY SUPPLEMENTARY TABLES Supplementary Tables 1–23. SUPPLEMENTARY DATA Reactome gene pathway
report. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Bjornsdottir, G., Chalmer, M.A., Stefansdottir, L. _et al._ Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without
aura. _Nat Genet_ 55, 1843–1853 (2023). https://doi.org/10.1038/s41588-023-01538-0 Download citation * Received: 02 December 2022 * Accepted: 18 September 2023 * Published: 26 October 2023 *
Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41588-023-01538-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
