Biased modulators of nmda receptors control channel opening and ion selectivity
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

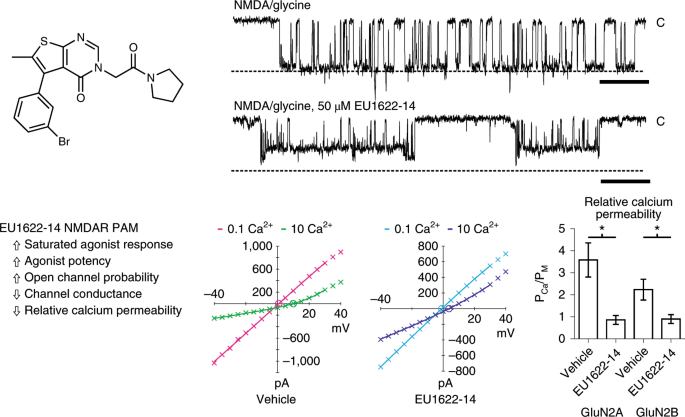
ABSTRACT Allosteric modulators of ion channels typically alter the transitions rates between conformational states without changing the properties of the open pore. Here we describe a new
class of positive allosteric modulators of _N_-methyl d-aspartate receptors (NMDARs) that mediate a calcium-permeable component of glutamatergic synaptic transmission and play essential
roles in learning, memory and cognition, as well as neurological disease. EU1622-14 increases agonist potency and channel-open probability, slows receptor deactivation and decreases both
single-channel conductance and calcium permeability. The unique functional selectivity of this chemical probe reveals a mechanism for enhancing NMDAR function while limiting excess calcium
influx, and shows that allosteric modulators can act as biased modulators of ion-channel permeation. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ALLOSTERIC COMPETITION AND INHIBITION IN AMPA RECEPTORS Article
Open access 04 June 2024 GLUN2A AND GLUN2B NMDA RECEPTORS USE DISTINCT ALLOSTERIC ROUTES Article Open access 05 August 2021 BI-DIRECTIONAL ALLOSTERIC PATHWAY IN NMDA RECEPTOR ACTIVATION AND
MODULATION Article Open access 13 October 2024 DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. CODE
AVAILABILITY The code that support the analysis of the findings contained in this study are available from the corresponding author upon reasonable request. REFERENCES * Traynelis, S. F. et
al. Glutamate receptor ion channels: structure, regulation, and function. _Pharm. Rev._ 62, 405–496 (2010). Article CAS PubMed PubMed Central Google Scholar * Coyle, J. T., Tsai, G.
& Goff, D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. _Ann. N. Y. Acad. Sci._ 1003, 318–327 (2003). Article CAS PubMed Google Scholar
* Heresco-Levy, U., Javitt, D. C., Ermilov, M., Silipo, G. & Shimoni, J. Double-blind, placebo-controlled, crossover trial of d-cycloserine adjuvant therapy for treatment-resistant
schizophrenia. _Int. J. Neuropsychopharmacol._ 1, 131–135 (1998). Article PubMed Google Scholar * Hu, C., Chen, W., Myers, S. J., Yuan, H. & Traynelis, S. F. Human GRIN2B variants in
neurodevelopmental disorders. _J. Pharm. Sci._ 132, 115–121 (2016). Article CAS Google Scholar * Ingram, D. K. et al. New pharmacological strategies for cognitive enhancement using a rat
model of age-related memory impairment. _Ann. N. Y. Acad. Sci._ 717, 16–32 (1994). Article CAS PubMed Google Scholar * Javitt, D. C. Management of negative symptoms of schizophrenia.
_Curr. Psychiatry Rep._ 3, 413–417 (2001). Article CAS PubMed Google Scholar * Yuan, H., Low, C. M., Moody, O. A., Jenkins, A. & Traynelis, S. F. Ionotropic GABA and glutamate
receptor mutations and human neurologic diseases. _Mol. Pharmacol._ 88, 203–217 (2015). Article CAS PubMed PubMed Central Google Scholar * Choi, D. W. Excitotoxic cell death. _J.
Neurobiol._ 23, 1261–1276 (1992). Article CAS PubMed Google Scholar * Parsons, M. P. & Raymond, L. A. Extrasynaptic NMDA receptor involvement in central nervous system disorders.
_Neuron_ 82, 279–293 (2014). Article CAS PubMed Google Scholar * Gonzalez, J. et al. NMDARs in neurological diseases: a potential therapeutic target. _Int J. Neurosci._ 125, 315–327
(2015). Article CAS PubMed Google Scholar * Collingridge, G. L. et al. The NMDA receptor as a target for cognitive enhancement. _Neuropharmacology_ 64, 13–26 (2013). Article CAS PubMed
Google Scholar * Schade, S. & Paulus, W. d-Cycloserine in neuropsychiatric diseases: a systematic review. _Int. J. Neuropsychopharmacol._ 19, pyv102 (2016). Article CAS PubMed
Google Scholar * Chopra, D. A. et al. A single-channel mechanism for pharmacological potentiation of GluN1/GluN2A NMDA receptors. _Sci. Rep._ 7, 6933 (2017). Article CAS PubMed PubMed
Central Google Scholar * Hackos, D. H. et al. Positive allosteric modulators of GluN2A-containing NMDARs with distinct modes of action and impacts on circuit function. _Neuron_ 89, 983–999
(2016). Article CAS PubMed Google Scholar * Khatri, A. et al. Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator. _Mol.
Pharmacol._ 86, 548–560 (2014). Article CAS PubMed PubMed Central Google Scholar * Perszyk, R. E. et al. GluN2D-Containing _N_-methyl-d-aspartate receptors mediate synaptic transmission
in hippocampal interneurons and regulate interneuron activity. _Mol. Pharmacol._ 90, 689–702 (2016). Article CAS PubMed PubMed Central Google Scholar * Sapkota, K. et al. Mechanism and
properties of positive allosteric modulation of _N_-methyl-d-aspartate receptors by 6-alkyl 2-naphthoic acid derivatives. _Neuropharmacology_ 125, 64–79 (2017). Article CAS PubMed PubMed
Central Google Scholar * Strong, K. L. et al. The structure–activity relationship of a tetrahydroisoquinoline class of _N_-methyl-d-aspartate receptor modulators that potentiates
GluN2B-containing _N_-methyl-d-aspartate receptors. _J. Med. Chem._ 60, 5556–5585 (2017). Article CAS PubMed PubMed Central Google Scholar * Wang, T. M. et al. A novel NMDA receptor
positive allosteric modulator that acts via the transmembrane domain. _Neuropharmacology_ 121, 204–218 (2017). Article CAS PubMed Google Scholar * Mullasseril, P. et al. A
subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. _Nat. Commun._ 1, 90 (2010). Article CAS PubMed Google Scholar * Hansen, K. B. et al. Implementation of a
fluorescence-based screening assay identifies histamine H3 receptor antagonists clobenpropit and iodophenpropit as subunit-selective _N_-methyl-d-aspartate receptor antagonists. _J.
Pharmacol. Exp. Ther._ 333, 650–662 (2010). Article CAS PubMed PubMed Central Google Scholar * Ogden, K. K. et al. Molecular mechanism of disease-associated mutations in the pre-M1
helix of NMDA receptors and potential rescue pharmacology. _PLoS Genet._ 13, e1006536 (2017). Article CAS PubMed PubMed Central Google Scholar * Karakas, E. & Furukawa, H. Crystal
structure of a heterotetrameric NMDA receptor ion channel. _Science_ 344, 992–997 (2014). Article CAS PubMed PubMed Central Google Scholar * Lee, C. H. et al. NMDA receptor structures
reveal subunit arrangement and pore architecture. _Nature_ 511, 191–197 (2014). Article CAS PubMed PubMed Central Google Scholar * Gibb, A. J. et al. A structurally derived model of
subunit-dependent NMDA receptor function. _J. Physiol._ 596, 4057–4089 (2018). Article CAS PubMed PubMed Central Google Scholar * Ogden, K. K. & Traynelis, S. F. Contribution of the
M1 transmembrane helix and pre-M1 region to positive allosteric modulation and gating of _N_-methyl-d-aspartate receptors. _Mol. Pharmacol._ 83, 1045–1056 (2013). Article CAS PubMed
PubMed Central Google Scholar * Sobolevsky, A. I., Prodromou, M. L., Yelshansky, M. V. & Wollmuth, L. P. Subunit-specific contribution of pore-forming domains to NMDA receptor channel
structure and gating. _J. Gen. Physiol._ 129, 509–525 (2007). Article CAS PubMed PubMed Central Google Scholar * Kazi, R. et al. Asynchronous movements prior to pore opening in NMDA
receptors. _J. Neurosci._ 33, 12052–12066 (2013). Article CAS PubMed PubMed Central Google Scholar * Talukder, I., Borker, P. & Wollmuth, L. P. Specific sites within the
ligand-binding domain and ion channel linkers modulate NMDA receptor gating. _J. Neurosci._ 30, 11792–11804 (2010). Article CAS PubMed PubMed Central Google Scholar * Perszyk, R et al.
An NMDAR positive and negative allosteric modulator series share a binding site and are interconverted by methyl groups. _Elife_ 7, e34711 (2018). Article PubMed PubMed Central Google
Scholar * Swanger, S. A. et al. A novel negative allosteric modulator selective for GluN2C/2D-containing NMDA receptors inhibits synaptic transmission in hippocampal interneurons. _ACS
Chem. Neurosci._ 9, 306–319 (2018). Article CAS PubMed Google Scholar * Zhu, S. et al. Mechanism of NMDA receptor inhibition and activation. _Cell_ 165, 704–714 (2016). Article CAS
PubMed PubMed Central Google Scholar * Twomey, E. C. & Sobolevsky, A. I. Structural mechanisms of gating in ionotropic glutamate receptors. _Biochemistry_ 57, 267–276 (2018). Article
CAS PubMed Google Scholar * Watanabe, J., Beck, C., Kuner, T., Premkumar, L. S. & Wollmuth, L. P. DRPEER: a motif in the extracellular vestibule conferring high Ca2+ flux rates in
NMDA receptor channels. _J. Neurosci._ 22, 10209–10216 (2002). Article CAS PubMed PubMed Central Google Scholar * Schewe, M. et al. A pharmacological master key mechanism that unlocks
the selectivity filter gate in K+ channels. _J. Sci._ 363, 875–880 (2019). CAS Google Scholar * Traynelis, S. F. & Jaramillo, F. Getting the most out of noise in the central nervous
system. _Trends Neurosci._ 21, 137–145 (1998). Article CAS PubMed Google Scholar * Lester, R. A., Clements, J. D., Westbrook, G. L. & Jahr, C. E. Channel kinetics determine the time
course of NMDA receptor-mediated synaptic currents. _Nature_ 346, 565–567 (1990). Article CAS PubMed Google Scholar * Mizuta, I., Katayama, M., Watanabe, M., Mishina, M. & Ishii, K.
Developmental expression of NMDA receptor subunits and the emergence of glutamate neurotoxicity in primary cultures of murine cerebral cortical neurons. _Cell. Mol. Life Sci._ 54, 721–725
(1998). Article CAS PubMed Google Scholar * Erreger, K. et al. Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing _N_-methyl-d-aspartate glutamate receptors.
_Mol. Pharmacol._ 72, 907–920 (2007). Article CAS PubMed Google Scholar * Dravid, S. M., Prakash, A. & Traynelis, S. F. Activation of recombinant NR1/NR2C NMDA receptors. _J.
Physiol._ 586, 4425–4439 (2008). Article CAS PubMed PubMed Central Google Scholar * Wyllie, D. J., Behe, P., Nassar, M., Schoepfer, R. & Colquhoun, D. Single-channel currents from
recombinant NMDA NR1a/NR2D receptors expressed in _Xenopus_ oocytes. _Proc. Biol. Sci._ 263, 1079–1086 (1996). Article CAS PubMed Google Scholar * Premkumar, L. S. & Auerbach, A.
Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. _Neuron_ 16, 869–880 (1996). Article CAS PubMed Google Scholar * Lewis, C.
A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. _J. Physiol._ 286, 417–445 (1979). Article
CAS PubMed PubMed Central Google Scholar * Jatzke, C., Watanabe, J. & Wollmuth, L. P. Voltage and concentration dependence of Ca2+ permeability in recombinant glutamate receptor
subtypes. _J. Physiol._ 538, 25–39 (2002). Article CAS PubMed PubMed Central Google Scholar * Siegler Retchless, B., Gao, W. & Johnson, J. W. A single GluN2 subunit residue controls
NMDA receptor channel properties via intersubunit interaction. _Nat. Neurosci._ 15, 406–413 (2012). Article CAS PubMed Google Scholar * Wollmuth, L. P. & Sakmann, B. Different
mechanisms of Ca2+ transport in NMDA and Ca2+-permeable AMPA glutamate receptor channels. _J. Gen. Physiol._ 112, 623–636 (1998). Article CAS PubMed PubMed Central Google Scholar *
Woodhull, A. M. Ionic blockage of sodium channels in nerve. _J. Gen. Physiol._ 61, 687–708 (1973). Article CAS PubMed PubMed Central Google Scholar * Rosenmund, C., Stern-Bach, Y. &
Stevens, C. F. The tetrameric structure of a glutamate receptor channel. _Science_ 280, 1596–1599 (1998). Article CAS PubMed Google Scholar * Zheng, J. & Sigworth, F. J. Selectivity
changes during activation of mutant shaker potassium channels. _J. Gen. Physiol._ 110, 101–117 (1997). Article CAS PubMed PubMed Central Google Scholar * Ikonomidou, C. & Turski,
L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? _Lancet Neurol._ 1, 383–386 (2002). Article CAS PubMed Google Scholar * Colquhoun, D.
& Sigworth, F. J. In _Single-Channel Recording_ (Eds Sakmann, B. & Neher, E.) 483–587 (Springer, 1995). * Neher, E. Correction for liquid junction potentials in patch clamp
experiments. _Methods Enzymol._ 207, 123–131 (1992). Article CAS PubMed Google Scholar * Burnashev, N., Zhou, Z., Neher, E. & Sakmann, B. Fractional calcium currents through
recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. _J. Physiol._ 485, 403–418 (1995). Article CAS PubMed PubMed Central Google Scholar * Paoletti, P., Neyton, J.
& Ascher, P. Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+. _Neuron_ 15, 1109–1120 (1995). Article CAS PubMed Google Scholar *
Traynelis, S. F. Software-based correction of single compartment series resistance errors. _J. Neurosci. Methods_ 86, 25–34 (1998). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS The authors thank K. Ogden for help with analytical software development and Y. Du and the Emory Chemical Biology Discovery Center for their invaluable assistance. This work
was supported by the NINDS (NS065371 and NS111619, to S.F.T.), NICHD (HD082373, to H.Y.), NIMH (MH109026, to G.B.), Citizens United for Research in Epilepsy (to S.A.S.) and the Emory
University Research Committee (to S.A.S.). AUTHOR INFORMATION Author notes * Gabriela Fernandez-Cuervo Present address: Department of Pathology, Stanford University School of Medicine,
Stanford, CA, USA AUTHORS AND AFFILIATIONS * Department of Pharmacology and Chemical Biology, Emory University School of Medicine, Atlanta, GA, USA Riley E. Perszyk, Sharon A. Swanger, Chris
Shelley, Alpa Khatri, Jing Zhang, Phuong Le, Hongjie Yuan & Stephen F. Traynelis * Virginia Tech Carilion Research Institute, Roanoke, VA, USA Sharon A. Swanger * Department of Internal
Medicine, Virginia Tech Carilion School of Medicine, Roanoke, VA, USA Sharon A. Swanger * Department of Biomedical Sciences and Pathobiology, Virginia-Maryland School of Veterinary
Medicine, Virginia Tech, Blacksburg, VA, USA Sharon A. Swanger * Department of Biology, University of the South, Sewanee, TN, USA Chris Shelley * Department of Chemistry, Emory University,
Atlanta, GA, USA Gabriela Fernandez-Cuervo, Matthew P. Epplin, Ethel Garnier-Amblard, Pavan Kumar Reddy Gangireddy, David S. Menaldino, Dennis C. Liotta & Lanny S. Liebeskind *
Department of Physiology, Emory University, Atlanta, GA, USA Pernille Bülow * Department of Cell Biology, Emory University, Atlanta, GA, USA Pernille Bülow & Gary J. Bassell Authors *
Riley E. Perszyk View author publications You can also search for this author inPubMed Google Scholar * Sharon A. Swanger View author publications You can also search for this author
inPubMed Google Scholar * Chris Shelley View author publications You can also search for this author inPubMed Google Scholar * Alpa Khatri View author publications You can also search for
this author inPubMed Google Scholar * Gabriela Fernandez-Cuervo View author publications You can also search for this author inPubMed Google Scholar * Matthew P. Epplin View author
publications You can also search for this author inPubMed Google Scholar * Jing Zhang View author publications You can also search for this author inPubMed Google Scholar * Phuong Le View
author publications You can also search for this author inPubMed Google Scholar * Pernille Bülow View author publications You can also search for this author inPubMed Google Scholar * Ethel
Garnier-Amblard View author publications You can also search for this author inPubMed Google Scholar * Pavan Kumar Reddy Gangireddy View author publications You can also search for this
author inPubMed Google Scholar * Gary J. Bassell View author publications You can also search for this author inPubMed Google Scholar * Hongjie Yuan View author publications You can also
search for this author inPubMed Google Scholar * David S. Menaldino View author publications You can also search for this author inPubMed Google Scholar * Dennis C. Liotta View author
publications You can also search for this author inPubMed Google Scholar * Lanny S. Liebeskind View author publications You can also search for this author inPubMed Google Scholar * Stephen
F. Traynelis View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The authors contributed in the following manner: conceptualization (S.F.T.,
R.E.P. and S.A.S.); data curation and formal analysis (R.E.P., S.A.S., C.S., A.K., G.F.-C., H.Y. and S.F.T.); funding acquisition (S.A.S., S.F.T., H.Y., D.C.L. and G.J.B.); investigation
(R.E.P., S.A.S., C.S., A.K., J.Z., P.L., E.G.-A., G.F.-C., M.P.E., D.S.M. and P.B.); development or design of methodology (R.E.P., S.A.S., S.F.T., E.G.-A., G.F.-C., M.P.E., G.J.B., D.S.M.,
D.C.L. and L.S.L.); project administration (R.E.P., S.F.T. and S.A.S.); provision of reagents, materials and analysis tools (P.K.R.G., E.G.-A., G.F.-C., P.B., G.J.B., M.P.E., D.S.M., D.C.L.
and L.S.L.); software (R.E.P. and S.F.T.); supervision (S.F.T., L.S.L., D.C.L. and G.J.B.); verification (R.E.P., S.A.S., C.S., A.K., G.F.-C., M.P.E. and D.S.M.); and visualization and
writing (all authors). CORRESPONDING AUTHOR Correspondence to Stephen F. Traynelis. ETHICS DECLARATIONS COMPETING INTERESTS Several authors have competing interests. S.F.T. is a consultant
for Janssen Pharmaceuticals, principal investigator on research grants from Janssen and Allergan to Emory University School of Medicine, a member of the scientific advisory board for Sage
Therapeutics, the GRIN2B Foundation, and the CureGRIN Foundation, co-founder of NeurOp and receives royalties for software. D.C.L. is a member of the Board of Directors for NeurOp. D.C.L.,
D.S.M., E.G.A., G.F.C., P.K.R.G., L.S.L., M.P.E., S.F.T. are co-inventors on Emory-owned intellectual property that includes allosteric modulators of NMDA receptor function. H.Y. is
principal investigator on a research grant from Sage Therapeutics to Emory University School of Medicine. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard
to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–11, Supplementary Tables 1–12 and
Synthetic Procedures. REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Perszyk, R.E., Swanger, S.A., Shelley, C. _et al._ Biased
modulators of NMDA receptors control channel opening and ion selectivity. _Nat Chem Biol_ 16, 188–196 (2020). https://doi.org/10.1038/s41589-019-0449-5 Download citation * Received: 28
December 2018 * Revised: 08 November 2019 * Accepted: 04 December 2019 * Published: 20 January 2020 * Issue Date: February 2020 * DOI: https://doi.org/10.1038/s41589-019-0449-5 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
