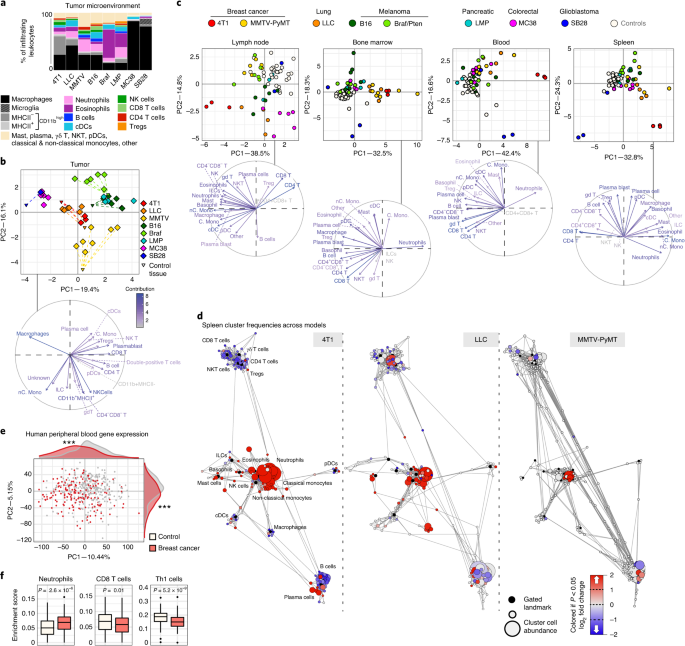
Systemic dysfunction and plasticity of the immune macroenvironment in cancer models
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Understanding of the factors governing immune responses in cancer remains incomplete, limiting patient benefit. In this study, we used mass cytometry to define the systemic immune
landscape in response to tumor development across five tissues in eight mouse tumor models. Systemic immunity was dramatically altered across models and time, with consistent findings in the
peripheral blood of patients with breast cancer. Changes in peripheral tissues differed from those in the tumor microenvironment. Mice with tumor-experienced immune systems mounted dampened
responses to orthogonal challenges, including reduced T cell activation during viral or bacterial infection. Antigen-presenting cells (APCs) mounted weaker responses in this context,
whereas promoting APC activation rescued T cell activity. Systemic immune changes were reversed with surgical tumor resection, and many were prevented by interleukin-1 or granulocyte
colony-stimulating factor blockade, revealing remarkable plasticity in the systemic immune state. These results demonstrate that tumor development dynamically reshapes the composition and
function of the immune macroenvironment. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this
journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now
Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS SYSTEMIC IMMUNITY IN CANCER Article 09 April 2021 AN EX VIVO TUMOR FRAGMENT PLATFORM TO DISSECT RESPONSE TO PD-1 BLOCKADE IN CANCER Article 08
July 2021 CONVERGING AND EVOLVING IMMUNO-GENOMIC ROUTES TOWARD IMMUNE ESCAPE IN BREAST CANCER Article Open access 21 February 2024 DATA AVAILABILITY All mass cytometry data are publicly
available at https://premium.cytobank.org/cytobank/projects/2433/ or by request to the senior author without restrictions. CODE AVAILABILITY The updated Statistical Scaffold package is
available at https://github.com/SpitzerLab/statisticalScaffold. CHANGE HISTORY * _ 02 APRIL 2024 A Correction to this paper has been published: https://doi.org/10.1038/s41591-024-02947-2 _
REFERENCES * Philip, M. et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. _Nature_ 545, 452–456 (2017). Article CAS PubMed PubMed Central Google
Scholar * Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. _Cell_ 168, 487–502 (2017). Article CAS PubMed PubMed Central Google Scholar *
Fransen, M. F. et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. _JCI Insight_ 3, 1–7 (2018). Article Google Scholar * Tang, H. et al. PD-L1 on host cells is
essential for PD-L1 blockade-mediated tumor regression. _J. Clin. Invest._ 128, 580–588 (2018). Article PubMed PubMed Central Google Scholar * Chamoto, K. et al. Mitochondrial activation
chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. _PNAS_ 114, E761–E770 (2017). Article CAS PubMed PubMed Central Google Scholar *
Mathios, D. et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. _Sci. Transl. Med._ 8, 370ra180 (2016). Article PubMed PubMed Central
Google Scholar * Lin, H. et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. _J. Clin. Invest._ 128, 805–815 (2018). Article PubMed
PubMed Central Google Scholar * Curiel, T. J. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. _Nat. Med._ 9, 562–567 (2003). Article CAS PubMed
Google Scholar * Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. _Nat. Med._ 25, 1251–1259 (2019). Article CAS PubMed PubMed Central Google
Scholar * McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. _Nat. Cell Biol._ 16, 717–727 (2014).
Article CAS PubMed PubMed Central Google Scholar * Zhang, S. et al. The role of myeloid-derived suppressor cells in patients with solid tumors: a meta-analysis. _PLoS ONE_ 11, e0164514
(2016). Article PubMed PubMed Central Google Scholar * Casbon, A.-J. et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate
immunosuppressive neutrophils. _Proc. Natl Acad. Sci. USA_ 112, E566–E575 (2015). Article CAS PubMed PubMed Central Google Scholar * Meyer, M. A. et al. Breast and pancreatic cancer
interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. _Nat. Commun._ 9, 1–19 (2018). Article Google Scholar * Barnstorf, I. et al. Chronic virus infection
compromises memory bystander T cell function in an IL-6/ STAT1-dependent manner. _J. Exp. Med._ 216, 571–586 (2019). Article CAS PubMed PubMed Central Google Scholar * Snell, L. M. et
al. CD8+ T cell priming in established chronic viral infection preferentially directs differentiation of memory-like cells for sustained immunity. _Immunity_ 49, 678–694 (2018). * Osborne,
L. C. et al. Virus-helminth coinfection reveals a microbiota-independent mechanism of immunomodulation. _Science_ 345, 578–582 (2014). Article CAS PubMed PubMed Central Google Scholar *
Danna, E. A. et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. _Cancer Res._ 64, 2205–2211 (2004). Article CAS
PubMed Google Scholar * Ghochikyan, A. et al. Primary 4T1 tumor resection provides critical ‘window of opportunity’ for immunotherapy. _Clin. Exp. Metastasis_ 31, 185–198 (2014). Article
CAS PubMed Google Scholar * Mosely, S. I. S. et al. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. _Cancer Immunol. Res._ 5, 29–41 (2017).
Article CAS PubMed Google Scholar * Westcott, P. M. K. et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. _Nature_ 517, 489–492 (2015). Article
CAS PubMed Google Scholar * Zeitouni, B. et al. Abstract 1840: Whole-exome somatic mutation analysis of mouse cancer models and implications for preclinical immunomodulatory drug
development. In _Proceedings of the 107th Annual Meeting of the American Association for Cancer Research_ https://doi.org/10.1158/1538-7445.AM2017-1840 (AACR, 2017). * Heinzel, F. P.,
Sadick, M. D., Holaday, B. J., Coffman, R. L. & Locksley, R. M. Reciprocal expression of interferon γ or interleukin 4 during the resolution or progression of murine leishmaniasis.
Evidence for expansion of distinct helper T cell subsets. _J. Exp. Med._ 169, 59–72 (1989). Article CAS PubMed Google Scholar * Kather, J. N. et al. Topography of cancer-associated
immune cells in human solid tumors. _eLife_ 7, e36967 (2018). * Spitzer, M. H. et al. An interactive reference framework for modeling a dynamic immune system. _Science_ 349, 1259425 (2015).
Article PubMed PubMed Central Google Scholar * Anz, D. et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. _Int. J. Cancer_ 129, 2417–2426 (2011). Article CAS PubMed
Google Scholar * Ross, E. A. et al. CD31 is required on CD4+ T cells to promote T cell survival during _Salmonella_ infection. _J. Immunol._ 187, 1553–1565 (2011). Article CAS PubMed
Google Scholar * Hänninen, A., Maksimow, M., Alam, C., Morgan, D. J. & Jalkanen, S. Ly6C supports preferential homing of central memory CD8+ T cells into lymph nodes. _Eur. J. Immunol._
41, 634–644 (2011). Article PubMed Google Scholar * Fourcade, J. et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in
melanoma patients. _J. Exp. Med._ 207, 2175–2186 (2010). Article CAS PubMed PubMed Central Google Scholar * Mita, Y. et al. Crucial role of CD69 in anti-tumor immunity through
regulating the exhaustion of tumor-infiltrating T cells. _Int. Immunol._ 30, 559–567 (2018). Article CAS PubMed Google Scholar * Sun, C., Mezzadra, R. & Schumacher, T. N. Regulation
and function of the PD-L1 checkpoint. _Immunity_ 48, 434–452 (2018). Article CAS PubMed PubMed Central Google Scholar * Bianchini, M. et al. PD-L1 expression on nonclassical monocytes
reveals their origin and immunoregulatory function. _Sci. Immunol._ 4, eaar3054 (2019). Article CAS PubMed Google Scholar * Busch, D. H., Pilip, I. M., Vijh, S. & Pamer, E. G.
Coordinate regulation of complex T cell populations responding to bacterial infection. _Immunity_ 8, 353–362 (1998). Article CAS PubMed Google Scholar * Kaech, S. M. & Ahmed, R.
Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. _Nat. Immunol._ 2, 415–422 (2001). Article CAS PubMed PubMed Central Google
Scholar * Herndler-Brandstetter, D. et al. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. _Immunity_ 48,
716–729 (2018). Article CAS PubMed PubMed Central Google Scholar * Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous
cell-associated antigens. _Immunity_ 17, 211–220 (2002). Article CAS PubMed PubMed Central Google Scholar * Gabrilovich, D. I., Corak, J., Ciernik, I. F., Kavanaugh, D. & Carbone,
D. P. Decreased antigen presentation by dendritic cells in patients with breast cancer. _Clin. Cancer Res._ 3, 483–490 (1997). CAS PubMed Google Scholar * Coffelt, S. B. et al.
IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. _Nature_ 522, 345–348 (2015). Article CAS PubMed PubMed Central Google Scholar * Wu, W.-C. et
al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. _Proc. Natl Acad. Sci. USA_ 111, 4221–4226 (2014). Article CAS PubMed PubMed Central Google
Scholar * Apte, R. N. et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. _Eur. J. Cancer_
42, 751–759 (2006). Article CAS PubMed Google Scholar * Wu, T. C. et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast
cancer. _Cancer Res._ 78, 5243–5258 (2018). Article CAS PubMed PubMed Central Google Scholar * Singer, C. F. et al. Interleukin-1ɑ protein secretion in breast cancer is associated with
poor differentiation and estrogen receptor ɑ negativity. _Int. J. Gynecol. Cancer_ 16, 556–559 (2006). Article PubMed Google Scholar * Pickup, M., Novitskiy, S. & Moses, H. L. The
roles of TGFβ in the tumour microenvironment. _Nat. Rev. Cancer_ 13, 788–799 (2013). Article CAS PubMed PubMed Central Google Scholar * Mariathasan, S. et al. TGFβ attenuates tumour
response to PD-L1 blockade by contributing to exclusion of T cells. _Nature_ 554, 544–548 (2018). Article CAS PubMed PubMed Central Google Scholar * Suzuki, A. et al. IL-1 production as
a regulator of G-CSF and IL-6 production in CSF-producing cell lines. _Br. J. Cancer_ 65, 515–518 (1992). Article CAS PubMed PubMed Central Google Scholar * Mittal, R., Wagener, M.,
Breed, E. R., Liang, Z. & Yoseph, B. P. Phenotypic T cell exhaustion in a murine model of bacterial infection in the setting of pre-existing malignancy. _PLoS ONE_ 9, 93523 (2014).
Article Google Scholar * Xie, J. et al. Pre-existing malignancy results in increased prevalence of distinct populations of CD4+ T cells during sepsis. _PLoS ONE_ 13, e0191065 (2018).
Article PubMed PubMed Central Google Scholar * Russ, A. J. et al. Melanoma-induced suppression of tumor antigen-specific T cell expansion is comparable to suppression of global T cell
expansion. _Cell. Immunol._ 271, 104–109 (2011). Article CAS PubMed PubMed Central Google Scholar * Klastersky, J. & Aoun, M. Opportunistic infections in patients with cancer. _Ann.
Oncol._ 15, iv329–iv335 (2004). Article PubMed Google Scholar * Baluch, A. & Pasikhova, Y. Influenza vaccination in oncology patients. _Curr. Infect. Dis. Rep._ 15, 486–490 (2013).
Article PubMed Google Scholar * O’Hara, M. H. et al. Abstract CT004: A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP)
with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. _Clin. Trials_ https://doi.org/10.1158/1538-7445.am2019-ct004 (2019). * Zuckerman,
N. S. et al. Altered local and systemic immune profiles underlie lymph node metastasis in breast cancer patients. _Int. J. Cancer_ 132, 2537–2547 (2012). Article PubMed PubMed Central
Google Scholar * Wang, L. et al. Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. _Nat. Immunol._ 20, 1220–1230 (2019). Article CAS
PubMed PubMed Central Google Scholar * Kosaka, A., Ohkuri, T., Program, B. T. & Okada, H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib
induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. _Cancer Immunol. Immunother._ 63, 847–857 (2014). Article CAS PubMed PubMed Central Google
Scholar * Tseng, W. W. et al. Development of an orthotopic model of invasive pancreatic cancer in an immunocompetent murine host. _Clin. Cancer Res._ 16, 3684–3695 (2010). Article CAS
PubMed PubMed Central Google Scholar * Kathryn, E. et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. _J. Immunol._ 168, 1528–1532
(2002). Article Google Scholar * Zunder, E. R. et al. Palladium-based mass-tag cell barcoding with a doublet-filtering scheme and single cell deconvolution algorithm. _Nat. Protoc._ 10,
316–333 (2015). Article CAS PubMed PubMed Central Google Scholar * Finck, R. et al. Normalization of mass cytometry data with bead standards. _Cytometry A_ 83, 483–494 (2013). Article
PubMed PubMed Central Google Scholar * Bair, E. & Tibshirani, R. Semi-supervised methods to predict patient survival from gene expression data. _PLoS Biol._ 2, 0511–0522 (2004).
Article CAS Google Scholar * Dumeaux, V. et al. Interactions between the tumor and the blood systemic response of breast cancer patients. _PLoS Comput. Biol_. 13, e1005680 (2017). * Aran,
D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. _Genome Biol._ 18, 1–14 (2017). Article Google Scholar Download references
ACKNOWLEDGEMENTS We thank the UCSF Flow Cytometry Core and S. Tamaki for CyTOF maintenance and M.H. Barcellos-Hoff, R. Levine, H. Okada, E. Engleman and J. Bluestone for cell lines,
transgenic mice and reagents. We thank L. Lanier, Z. Werb, M.H. Barcellos-Hoff and L. Fong for insightful feedback. This work was supported by National Institutes of Health (NIH) grants
DP5OD023056 and P50CA097257 (UCSF Brain Tumor SPORE Developmental Research Program), funds from the UCSF Program for Breakthrough Biomedical Research and investigator funding from the Parker
Institute for Cancer Immunotherapy to M.H.S. and by NIH grant S10OD018040, which enabled procurement of the mass cytometer used in this study. This study makes use of data generated by the
Norwegian Women and Cancer Study. A full list of investigators who contributed to the generation of the data is available at http://site.uit.no/nowac/. Funding for the project was provided
by European Research Council grant ERC-2008-AdG 232997. The Norwegian Women and Cancer Study group is not responsible for the analysis or interpretation of the data presented. AUTHOR
INFORMATION Author notes * These authors contributed equally: Breanna M. Allen, Kamir J. Hiam. AUTHORS AND AFFILIATIONS * Graduate Program in Biomedical Sciences, University of California,
San Francisco, San Francisco, CA, USA Breanna M. Allen, Kamir J. Hiam, Cassandra E. Burnett, Anthony Venida, Rachel DeBarge & Matthew H. Spitzer * Departments of Otolaryngology and
Microbiology & Immunology, Helen Diller Family Comprehensive Cancer Center, Parker Institute for Cancer Immunotherapy, Chan Zuckerberg Biohub, University of California, San Francisco,
San Francisco, CA, USA Breanna M. Allen, Kamir J. Hiam, Cassandra E. Burnett, Rachel DeBarge, Iliana Tenvooren, Diana M. Marquez, Nam Woo Cho & Matthew H. Spitzer * Department of
Anatomy, University of California San Francisco, San Francisco, CA, USA Anthony Venida * Department of Radiation Oncology, University of California San Francisco, San Francisco, CA, USA Nam
Woo Cho * Department of Pathology, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel Yaron Carmi Authors * Breanna M. Allen View author publications You can also search for
this author inPubMed Google Scholar * Kamir J. Hiam View author publications You can also search for this author inPubMed Google Scholar * Cassandra E. Burnett View author publications You
can also search for this author inPubMed Google Scholar * Anthony Venida View author publications You can also search for this author inPubMed Google Scholar * Rachel DeBarge View author
publications You can also search for this author inPubMed Google Scholar * Iliana Tenvooren View author publications You can also search for this author inPubMed Google Scholar * Diana M.
Marquez View author publications You can also search for this author inPubMed Google Scholar * Nam Woo Cho View author publications You can also search for this author inPubMed Google
Scholar * Yaron Carmi View author publications You can also search for this author inPubMed Google Scholar * Matthew H. Spitzer View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS Conceptualization: B.M.A, K.J.H., Y.C. and M.H.S.; experimental methodology: B.M.A., K.J.H., C.E.B., A.V., R.D., I.T., D.M.M., N.W.C., Y.C. and M.H.S.;
computational methodology: B.M.A. and M.H.S.; investigation: all authors; writing, original draft: B.M.A.; writing, review and editing: all authors; funding acquisition: M.H.S.; supervision:
M.H.S. CORRESPONDING AUTHOR Correspondence to Matthew H. Spitzer. ETHICS DECLARATIONS COMPETING INTERESTS M.H.S. receives research funding from Roche/Genentech, Bristol-Myers Squibb and
Valitor and has been a paid consultant for Five Prime Therapeutics, Ono Pharmaceutical and January Inc. ADDITIONAL INFORMATION PEER REVIEW INFORMATION Saheli Sadanand was the primary editor
on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 MAIN MASS CYTOMETRY GATING SCHEME. A, Main gating strategy for identifying major
immune cell populations from mass cytometry datasets. EXTENDED DATA FIG. 2 SYSTEMIC IMMUNITY IS DISTINCTLY REMODELED ACROSS TUMOR MODELS. A, Relative abundance of total leukocytes
infiltrating the TME across eight tumor models. B-F, Scaffold maps of spleen cell frequencies across five distinct tumor models, SB28 glioblastoma (B), MC38 colorectal (C), LMP pancreatic
(D), B16 melanoma (E), and Braf/PTEN melanoma (F), comparing late stage tumor burden to their respective health littermate controls. G, Heatmaps of the log2 adjusted fold change in bulk
immune cell frequencies across all five tissues, where relevant, across all models. H, Heatmaps of the log2 adjusted fold change in bulk immune cell frequencies comparing the parental LLC
and engineered LLC expressing reporters GFP and Luciferase, with cell labels in g. Lower inset shows Pearson’s correlation between these systemic immune features. EXTENDED DATA FIG. 3
SYSTEMIC IMMUNITY IS DISTINCTLY REMODELED OVER TUMOR DEVELOPMENT. A, Pearson’s correlation between MMTV-PyMT primary tumor size and change in systemic immune composition, measured as
Aitchison distance. B, Degree of systemic immune change by Aitchison distance over tumor growth (left) and after removing the contribution of primary tumor size by linear regression (right).
C, Percent of PyMT expressing metastatic cancer cells in the lung (green) and primary draining lymph node (blue). D, Pearson’s correlation between lung or lymph node metastasis and the
residual changes in systemic immune composition after regressing out primary tumor burden. E, Heatmap of the log2 adjusted fold change in bulk spleen immune cell frequencies for each 400mm2
tumor-bearing mouse, ranging from 0 to high metastatic disease. F, Pearson’s correlation between tumor mass and absolute number of infiltrating leukocytes in 4T1 breast tumors. G, Spleen
immune absolute cell counts, adjusted absolute cell counts per mg of tissue, and unadjusted immune frequencies at each time point for neutrophils, B cells and T cells of the 4T1 breast tumor
model. H, PCA of relative immune cell frequencies from each major immune tissue over time in the MMTV-PyMT breast tumor model. Vectors designate progression from control (first point) to 25
mm2, 50mm2, 125mm2, and 400mm2 (last point, arrowhead). I, Scaffold maps of immune cell frequencies in the spleen at each time point of 4T1 tumor burden, colored by log2 fold change in
frequency compared to the previous time point. EXTENDED DATA FIG. 4 IMMUNITY IS DISTINCTLY REMODELED BY COMPARTMENT OVER TUMOR DEVELOPMENT. A-D, Scaffold maps of immune cell frequencies over
4T1 tumor progression in the tumor draining lymph node (A) blood (B), bone marrow (C), and tumor (D), colored by fold change from the previous time point. EXTENDED DATA FIG. 5 TUMOR GROWTH
SHIFTS THE SYSTEMIC T CELL COMPOSITION ACROSS MODELS. A-B, PCA of T cell cluster frequencies across lymphoid tissues over tumor development for the 4T1 (A) and MMTV-PyMT (B) breast tumor
models. Vectors designate directional progression from control (first point) to late stage disease (last point, arrowhead). In A, tumor time points include day 7, 14, 21, and 35 after 4T1
cancer cell transplant. In B, tumor time points include tumor sizes of 25 mm2, 50 mm2, 125 mm2, and 400 mm2. C-E, CD3 + CD11b- leukocytes from all tissues clustered together from healthy and
MMTV-PyMT tumor-burdened animals at progressive tumor sizes. C, Heatmap of each T cell cluster frequency, by row, in each site and across the individual 2-3 animals per time point. D,
Stacked bar plot of the log2 fold change in cluster frequency between early (25 mm2) and late (400 mm2) disease time points, colored by tissue. E, Heatmap of the protein expression defining
each T cell cluster, column normalized to each protein’s maximum positive expression. F-H, Representative scatter plots of key proteins that define T cell clusters changing in frequency in
the designated site between early and late disease stage for CD8 T cells (F), Tregs (G), and CD4 T Cells (H). EXTENDED DATA FIG. 6 TUMOR GROWTH SHIFTS THE SYSTEMIC MONONUCLEAR PHAGOCYTE
COMPOSITION. A, CD3- CD19- leukocytes from all tissues clustered together from healthy and 4T1 tumor-burdened animals at progressive time points. _Left_, stacked bar plot of the log2 fold
change in cluster frequency between early (day 7) and late (day 35) times points, colored by tissue. _Right_, heatmap of the protein expression defining each cluster, column normalized to
each protein’s maximum positive expression. B, Curves of the mean cell frequencies over time in the 4T1 breast tumor model from designated mononuclear phagocyte cell types, colored by
tissue. C, PCA of the mononuclear phagocyte cell frequencies from each tissue over time in the 4T1 breast tumor model. Vectors designate progression from control (first point) to day 7, 14,
21, and 35 (last point, arrowhead). Coloring of tissues for a-c corresponds to labels in c. EXTENDED DATA FIG. 7 PD-1 AND PD-L1 EXPRESSION IS DYNAMIC OVER TUMOR GROWTH. A, Distribution of
PD-1 and PD-L1 signal intensities on tumor infiltrating leukocytes over time in the 4T1 or LLC tumor models. Coloring of time points for a-d corresponds to legend in a. B, Percent of total
infiltrating leukocytes (_left of dashed line_) or CD45−, non-endothelial cells (_right of dashed line_) with high PD-1 or PD-L1 expression in the 4T1 or LLC tumor models. C, Percent of
leukocytes with high PD-1 or PD-L1 expression over time and across tissues, 4T1 model. D, Pearson’s correlation between median PD-L1 signal intensity on blood versus tumor infiltrating
leukocytes, 4T1 model. E, Percent of each major immune cell subset expressing high PD-1 or PD-L1 in the tumor, blood, and spleen, identified manually. Cell subsets below 0.2% of total
leukocytes were not included, X. Bars ordered by time point, beginning at healthy control. Double positive PD-1/PD-L1 expression was rare and not illustrated. p*< 0.05, One-Way ANOVA,
with Tukey correction versus control tissue or healthy mammary fat pad (blue in b-c, fill corresponding to bar color in e), or versus day 7 (green in b-c). EXTENDED DATA FIG. 8 TUMOR BURDEN
INDUCES TISSUE-SPECIFIC CHANGES IN IMMUNE CELL CYCLING. A-B, Log2 fold change in bulk Ki67 expressing leukocytes in each tissue tissues for 4T1, LLC and MMTV tumors (A), and over 4T1 tumor
progression (B). p*< 0.05, One-Way ANOVA, with Tukey correction versus control. C-D, Statistical Scaffold maps of Ki67 expression in immune cells of the tumor draining lymph node
comparing control to day 21 (C) and the Spleen over time (D) in 4T1 tumor burdened animals. E, Percent of increasing clusters (red, total of 56), decreasing clusters (blue, total of 90), or
unchanged cluster that have corresponding changes in cell cycle markers Ki67 and cleaved Caspase-3. EXTENDED DATA FIG. 9 TUMOR DRIVEN DEFICITS IN T CELL RESPONSES ARE CELL-EXTRINSIC. A,
Quantification of bulk CD8+ T cell populations in the spleen of healthy or LLC tumor-burdened mice after 7 days of _Lm_ infection, Two-Way ANOVA with Bonferroni correction. B, Expression of
inflammatory cytokines, INFy, IL-2, and TNFa in splenic CD8 T Cells isolated from control or LLC tumor-burdened mice after _in vitro_ differentiation with CD3, CD28 and IL-2, and
re-stimulation with BrefeldinA and PMA Ionomycin. C, Scatter plots of CD11b and Ly6G showing expected neutrophilia in OT-I TCR transgenic mice with LLC tumor burden. D, Histograms of CD80,
CD86, and CD83 signal intensity on cDCs from healthy or LLC tumor-burdened mice at day 2 of _Lm_-OVA infection. E, Median signal intensity of CD80, PD-L1 and CD54 activation markers on
splenic cDCs from healthy or LLC tumor-burdened mice compared to IL-12p70 or CD40 treatment at day 7 of _Lm_-OVA infection. F, Median signal intensity of PD-L1 on splenic cDCs from untreated
or CD40 treated LLC tumor-burdened (day 21) mice. G, Quantification of splenic CD8 + T cell proliferation in healthy, untreated or CTLA-4 treated LLC tumor-burdened animals in response to 7
days of _Lm_-OVA infection. p*<0.05, two-tailed t-test. EXTENDED DATA FIG. 10 TUMOR RESECTION RESETS SYSTEMIC IMMUNE ORGANIZATION AND FUNCTION. A-C, Statistical scaffold maps of spleen
immune cell frequencies (A) and proliferation by Ki67 expression (B) in 4T1 resected mice, and of spleen immune cell frequencies in MC38 resected mice (C) compared to healthy control. Insets
show resected mice compared to tumor-burdened mice. D-E, Heatmap of the log2 fold changes in splenic immune cell frequencies for local or lung recurrences from control mice (D), and for
IL-1, G-CSF, or TGFβ blockade from untreated LLC tumor-burdened mice (E). F-G, Heatmaps of T cell cluster expression profiles and log2 fold change from control for LLC (F) and 4T1 (G) for
the spleen and draining lymph node. H, Median signal intensity of CD86 and PD-L1 on splenic cDCs from healthy, LLC tumor-burdened, resected, or resected mice with local recurrence at day 7
of _Lm_-OVA infection. I, Concentration of circulating cytokines, IL-1α and G-CSF from healthy, LLC tumor-burdened, resected, or resected mice with local recurrence. J, Concentration of
cytokines, IL-1α, IL-1β and G-CSF from _in vitro_ cell culture media conditioned with LLC cancer cells. K, Concentration of circulating G-CSF from control or LLC tumor-bearing mice, or LLC
tumor-bearing mice treated with either IL-1 or G-CSF blocking antibodies. p*<0.05, two-tailed t-test. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Tables 1–3
REPORTING SUMMARY RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the
author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Allen, B.M., Hiam, K.J., Burnett, C.E. _et al._ Systemic dysfunction and plasticity of the immune macroenvironment in cancer
models. _Nat Med_ 26, 1125–1134 (2020). https://doi.org/10.1038/s41591-020-0892-6 Download citation * Received: 30 September 2019 * Accepted: 17 April 2020 * Published: 25 May 2020 * Issue
Date: 01 July 2020 * DOI: https://doi.org/10.1038/s41591-020-0892-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
