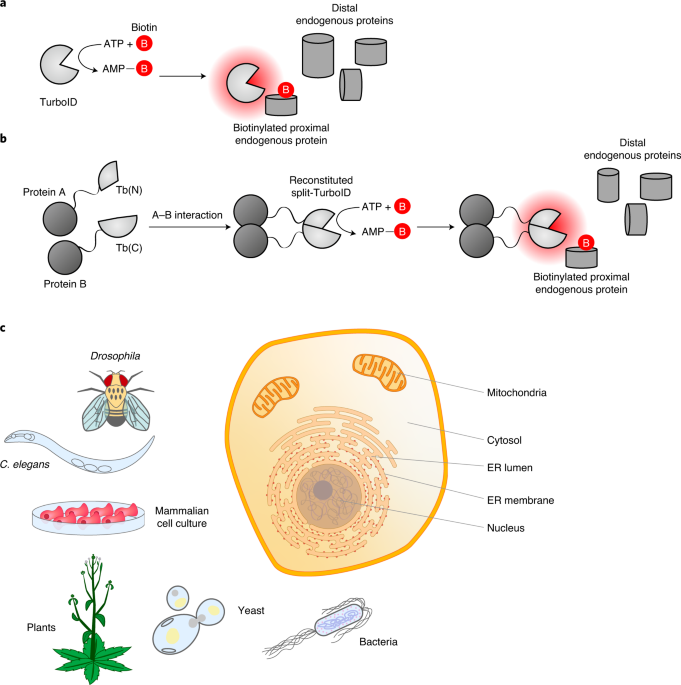
Proximity labeling in mammalian cells with turboid and split-turboid
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT This protocol describes the use of TurboID and split-TurboID in proximity labeling applications for mapping protein–protein interactions and subcellular proteomes in live mammalian
cells. TurboID is an engineered biotin ligase that uses ATP to convert biotin into biotin–AMP, a reactive intermediate that covalently labels proximal proteins. Optimized using directed
evolution, TurboID has substantially higher activity than previously described biotin ligase–related proximity labeling methods, such as BioID, enabling higher temporal resolution and
broader application in vivo. Split-TurboID consists of two inactive fragments of TurboID that can be reconstituted through protein–protein interactions or organelle–organelle interactions,
which can facilitate greater targeting specificity than full-length enzymes alone. Proteins biotinylated by TurboID or split-TurboID are then enriched with streptavidin beads and identified
by mass spectrometry. Here, we describe fusion construct design and characterization (variable timing), proteomic sample preparation (5–7 d), mass spectrometric data acquisition (2 d), and
proteomic data analysis (1 week). Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal
Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS OFF-THE-SHELF PROXIMITY BIOTINYLATION USING PROTA-TURBOID Article 12 October 2022 OFF-THE-SHELF PROXIMITY BIOTINYLATION FOR INTERACTION PROTEOMICS
Article Open access 18 August 2021 ENGINEERING OF ULTRAID, A COMPACT AND HYPERACTIVE ENZYME FOR PROXIMITY-DEPENDENT BIOTINYLATION IN LIVING CELLS Article Open access 04 July 2022 DATA
AVAILABILITY The data presented in this paper have been previously published, and associated raw data are provided in the original articles6,7. REFERENCES * Huber, L. A., Pfaller, K. &
Vietor, I. Organelle proteomics: implications for subcellular fractionation in proteomics. _Circ. Res._ 92, 962–968 (2003). Article CAS PubMed Google Scholar * Puig, O. et al. The tandem
affinity purification (TAP) method: a general procedure of protein complex purification. _Methods_ 24, 218–229 (2001). Article CAS PubMed Google Scholar * Stasyk, T. & Huber, L. A.
Zooming in: fractionation strategies in proteomics. _Proteomics_ 4, 3704–3716 (2004). Article CAS PubMed Google Scholar * Lee, W. C. & Lee, K. H. Applications of affinity
chromatography in proteomics. _Anal. Biochem._ 324, 1–10 (2004). Article CAS PubMed Google Scholar * Gingras, A. C., Abe, K. T. & Raught, B. Getting to know the neighborhood: using
proximity-dependent biotinylation to characterize protein complexes and map organelles. _Curr. Opin. Chem. Biol._ 48, 44–54 (2019). Article CAS PubMed Google Scholar * Branon, T. C. et
al. Efficient proximity labeling in living cells and organisms with TurboID. _Nat. Biotechnol._ 36, 880–887 (2018). Article CAS PubMed PubMed Central Google Scholar * Cho, K. F. et al.
Split-TurboID enables contact-dependent proximity labeling in cells. _Proc. Natl Acad. Sci. USA_ 117, 12143–12154 (2020). Article CAS PubMed PubMed Central Google Scholar * Udeshi, N.
D. et al. Antibodies to biotin enable large-scale detection of biotinylation sites on proteins. _Nat. Methods_ 14, 1167–1170 (2017). Article CAS PubMed PubMed Central Google Scholar *
Fazal, F. M. et al. Atlas of subcellular RNA localization revealed by APEX-Seq. _Cell_ 178, 473–490.e26 (2019). Article CAS PubMed PubMed Central Google Scholar * Myers, S. A. et al.
Discovery of proteins associated with a predefined genomic locus via dCas9-APEX-mediated proximity labeling. _Nat. Methods_ 15, 437–439 (2018). Article CAS PubMed PubMed Central Google
Scholar * Michalski, A. et al. Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. _Mol. Cell. Proteom._ 10, M111.011015
(2011). Article Google Scholar * Eliuk, S. & Makarov, A. Evolution of Orbitrap mass spectrometry instrumentation. _Annu. Rev. Anal. Chem._ 8, 61–80 (2015). Article Google Scholar *
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. _Nat. Protoc._ 11, 2301–2319 (2016). Article CAS PubMed Google
Scholar * Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. _Nat. Methods_ 12, 51–54 (2014). Article CAS PubMed PubMed Central Google Scholar
* Rhee, H. W. et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. _Science_ 339, 1328–1331 (2013). Article CAS PubMed PubMed Central
Google Scholar * Mortensen, A. & Skibsted, L. H. Importance of carotenoid structure in radical-scavenging reactions. _J. Agric. Food Chem._ 45, 2970–2977 (1997). Article CAS Google
Scholar * Wishart, J. F. & Rao, B. S. M. _Recent Trends in Radiation Chemistry_ (World Scientific, 2010). https://doi.org/10.1142/7413 * Martell, J. D. et al. Engineered ascorbate
peroxidase as a genetically encoded reporter for electron microscopy. _Nat. Biotechnol._ 30, 1143–1148 (2012). Article CAS PubMed PubMed Central Google Scholar * Rodríguez-López, J. N.
et al. Mechanism of reaction of hydrogen peroxide with horseradish peroxidase: identification of intermediates in the catalytic cycle. _J. Am. Chem. Soc._ 123, 11838–11847 (2001). Article
PubMed CAS Google Scholar * Loh, K. H. et al. Proteomic analysis of unbounded cellular compartments: synaptic clefts. _Cell_ 166, 1295–1307.e21 (2016). Article CAS PubMed PubMed
Central Google Scholar * Bar, D. Z. et al. Biotinylation by antibody recognition—a method for proximity labeling. _Nat. Methods_ 15, 127–133 (2018). Article CAS PubMed Google Scholar *
Honke, K. & Kotani, N. The enzyme-mediated activation of radical source reaction: a new approach to identify partners of a given molecule in membrane microdomains. _J. Neurochem_ 116,
690–695 (2011). Article CAS PubMed Google Scholar * Li, X.-W. et al. New insights into the DT40 B cell receptor cluster using a proteomic proximity labeling assay. _J. Biol. Chem._ 289,
14434–14447 (2014). Article CAS PubMed PubMed Central Google Scholar * Paek, J. et al. Multidimensional tracking of GPCR signaling via peroxidase-catalyzed proximity labeling. _Cell_
169, 338–349.e11 (2017). Article CAS PubMed PubMed Central Google Scholar * Lobingier, B. T. et al. An approach to spatiotemporally resolve protein interaction networks in living cells.
_Cell_ 169, 350–360.e12 (2017). Article CAS PubMed PubMed Central Google Scholar * Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein
identifies proximal and interacting proteins in mammalian cells. _J. Cell Biol._ 196, 801–810 (2012). Article CAS PubMed PubMed Central Google Scholar * Kim, D. I. et al. An improved
smaller biotin ligase for BioID proximity labeling. _Mol. Biol. Cell_ 27, 1188–1196 (2016). Article CAS PubMed PubMed Central Google Scholar * Ramanathan, M. et al. RNA-protein
interaction detection in living cells. _Nat. Methods_ 15, 207–212 (2018). Article CAS PubMed PubMed Central Google Scholar * Choi-Rhee, E., Schulman, H. & Cronan, J. E. Promiscuous
protein biotinylation by _Escherichia coli_ biotin protein ligase. _Protein Sci._ 13, 3043–3050 (2008). Article CAS Google Scholar * Kim, D. I. et al. Probing nuclear pore complex
architecture with proximity-dependent biotinylation. _Proc. Natl Acad. Sci. USA_ 111, E2453–E2461 (2014). Article CAS PubMed PubMed Central Google Scholar * Kido, K. et al. Airid, a
novel proximity biotinylation enzyme, for analysis of protein–protein interactions. _eLife_ 9, e54983 (2020). Article CAS PubMed PubMed Central Google Scholar * Birendra, K. C. et al.
VRK2A is an A-type lamin-dependent nuclear envelope kinase that phosphorylates BAF. _Mol. Biol. Cell_ 28, 2241–2250 (2017). Article CAS PubMed Central Google Scholar * Redwine, W. B. et
al. The human cytoplasmic dynein interactome reveals novel activators of motility. _eLife_ 6, e28257 (2017). Article PubMed PubMed Central Google Scholar * Jung, E. M. et al. Arid1b
haploinsufficiency disrupts cortical interneuron development and mouse behavior. _Nat. Neurosci._ 20, 1694–1707 (2017). Article CAS PubMed PubMed Central Google Scholar * Mair, A., Xu,
S. L., Branon, T. C., Ting, A. Y. & Bergmann, D. C. Proximity labeling of protein complexes and cell type specific organellar proteomes in _Arabidopsis_ enabled by TurboID. _eLife_ 8,
e47864 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y. et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated
immunity. _Nat. Commun._ 10, 3252 (2019). Article CAS PubMed PubMed Central Google Scholar * Larochelle, M., Bergeron, D., Arcand, B. & Bachand, F. Proximity-dependent biotinylation
mediated by TurboID to identify protein-protein interaction networks in yeast. _J. Cell Sci._ 132, jcs232249 (2019). Article CAS PubMed Google Scholar * Struk, S. et al. Exploring the
protein–protein interaction landscape in plants. _Plant Cell Environ._ 42, 387–409 (2019). Article CAS PubMed Google Scholar * Opitz, N. et al. Capturing the Asc1p/receptor for activated
C kinase 1 (RACK1) microenvironment at the head region of the 40s ribosome with quantitative BioID in yeast. _Mol. Cell. Proteom._ 16, 2199–2218 (2017). Article CAS Google Scholar *
Uezu, A. et al. Identification of an elaborate complex mediating postsynaptic inhibition. _Science_ 353, 1123–1129 (2016). Article CAS PubMed PubMed Central Google Scholar * Lin, Q. et
al. Screening of proximal and interacting proteins in rice protoplasts by proximity-dependent biotinylation. _Front. Plant Sci._ 8, 749 (2017). Article PubMed PubMed Central Google
Scholar * Khan, M., Youn, J. Y., Gingras, A. C., Subramaniam, R. & Desveaux, D. In planta proximity dependent biotin identification (BioID). _Sci. Rep._ 8, 1123 (2018). Article CAS
Google Scholar * Conlan, B., Stoll, T., Gorman, J. J., Saur, I. & Rathjen, J. P. Development of a rapid in planta bioid system as a probe for plasma membrane-associated immunity
proteins. _Front. Plant Sci._ 9, 1882 (2018). Article PubMed PubMed Central Google Scholar * Roux, K. J., Kim, D. I., Burke, B. & May, D. G. BioID: a screen for protein-protein
interactions. _Curr. Protoc. Protein Sci._ 91, 19.23.1–19.23.15 (2018). CAS Google Scholar * May, D. G., Scott, K. L., Campos, A. R. & Roux, K. J. Comparative application of BioID and
TurboID for protein-proximity biotinylation. _Cells_ 9, 1070 (2020). Article PubMed Central CAS Google Scholar * Chapman-Smith, A. & Cronan, J. E. Jr Molecular biology of biotin
attachment to proteins. _J. Nutr._ 129, 477S–484S (1999). Article CAS PubMed Google Scholar * Han, Y. et al. Directed evolution of split APEX2 peroxidase. _ACS Chem. Biol._ 14, 619–635
(2019). Article CAS PubMed PubMed Central Google Scholar * Martell, J. D. et al. A split horseradish peroxidase for the detection of intercellular protein-protein interactions and
sensitive visualization of synapses. _Nat. Biotechnol._ 34, 774–780 (2016). Article CAS PubMed PubMed Central Google Scholar * De Munter, S. et al. Split-BioID: a proximity
biotinylation assay for dimerization-dependent protein interactions. _FEBS Lett._ 591, 415–424 (2017). Article PubMed CAS Google Scholar * Schopp, I. M. et al. Split-BioID a conditional
proteomics approach to monitor the composition of spatiotemporally defined protein complexes. _Nat. Commun._ 8, 15690 (2017). Article CAS PubMed PubMed Central Google Scholar * Kwak, C.
et al. Contact-ID, a new tool for profiling organelle contact site, reveals proteins of mitochondrial-associated membrane formation. _Proc. Natl Acad. Sci. USA_ 117, 12109–12120 (2020).
Article CAS PubMed PubMed Central Google Scholar * McClellan, D. et al. Growth factor independence 1B-mediated transcriptional repression and lineage allocation require lysine-specific
demethylase 1-dependent recruitment of the BHC complex. _Mol. Cell. Biol._ 39, e00020-19 (2019). Article PubMed PubMed Central Google Scholar * Lambert, J. P. et al. Interactome rewiring
following pharmacological targeting of BET bromodomains. _Mol. Cell_ 73, 621–638.e17 (2019). Article CAS PubMed PubMed Central Google Scholar * Dingar, D. et al. BioID identifies novel
c-MYC interacting partners in cultured cells and xenograft tumors. _J. Proteom._ 118, 95–111 (2015). Article CAS Google Scholar * Couzens, A. L. et al. Protein interaction network of the
mammalian hippo pathway reveals mechanisms of kinase-phosphatase interactions. _Sci. Signal._ 6, rs15–rs15 (2013). Article PubMed CAS Google Scholar * Gupta, G. D. et al. A dynamic
protein interaction landscape of the human centrosome-cilium interface. _Cell_ 163, 1484–1499 (2015). Article CAS PubMed PubMed Central Google Scholar * Youn, J. Y. et al. High-density
proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. _Mol. Cell_ 69, 517–532.e11 (2018). Article CAS PubMed Google Scholar * Firat-Karalar, E.
N., Rauniyar, N., Yates, J. R. & Stearns, T. Proximity interactions among centrosome components identify regulators of centriole duplication. _Curr. Biol._ 24, 664–670 (2014). Article
CAS PubMed PubMed Central Google Scholar * Chou, C. C. et al. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. _Nat. Neurosci._ 21, 228–239
(2018). Article CAS PubMed PubMed Central Google Scholar * Kabeiseman, E. J., Cichos, K. H. & Moore, E. R. The eukaryotic signal sequence, YGRL, targets the chlamydial inclusion.
_Front. Cell. Infect. Microbiol._ 4, 129 (2014). Article PubMed PubMed Central CAS Google Scholar * Mojica, S. A. et al. SINC, a type III secreted protein of _Chlamydia psittaci_,
targets the inner nuclear membrane of infected cells and uninfected neighbors. _Mol. Biol. Cell_ 26, 1918–1934 (2015). Article CAS PubMed PubMed Central Google Scholar * Le Sage, V.,
Cinti, A., Valiente-Echeverría, F. & Mouland, A. J. Proteomic analysis of HIV-1 Gag interacting partners using proximity-dependent biotinylation. _Virol. J._ 12, 138 (2015). Article
PubMed PubMed Central CAS Google Scholar * Ritchie, C., Cylinder, I., Platt, E. J. & Barklis, E. Analysis of HIV-1 Gag protein interactions via biotin ligase tagging. _J. Virol._ 89,
3988–4001 (2015). Article CAS PubMed PubMed Central Google Scholar * Kueck, T. et al. Serine phosphorylation of HIV-1 Vpu and its binding to tetherin regulates interaction with
clathrin adaptors. _PLoS Pathog._ 11, e1005141 (2015). Article PubMed PubMed Central CAS Google Scholar * Holthusen, K., Talaty, P. & Everly, D. N. Regulation of latent membrane
protein 1 signaling through interaction with cytoskeletal proteins. _J. Virol._ 89, 7277–7290 (2015). Article CAS PubMed PubMed Central Google Scholar * Coyaud, E. et al. Global
interactomics uncovers extensive organellar targeting by Zika virus. _Mol. Cell. Proteom._ 17, 2242–2255 (2018). Article CAS Google Scholar * Rider, M. A. et al. The interactome of EBV
LMP1 evaluated by proximity-based BioID approach. _Virology_ 516, 55–70 (2018). Article CAS PubMed Google Scholar * Cheerathodi, M. R. & Meckes, D. G. BioID combined with mass
spectrometry to study herpesvirus protein–protein interaction networks. _Methods Mol. Biol._ 2060, 327–341 (2020). Article CAS PubMed Google Scholar * Bradley, P. J., Rayatpisheh, S.,
Wohlschlegel, J. A. & Nadipuram, S. M. Using BioID for the identification of interacting and proximal proteins in subcellular compartments in _Toxoplasma gondii_. _Methods Mol. Biol._
2071, 323–346 (2020). Article CAS PubMed Google Scholar * Gillingham, A. K., Bertram, J., Begum, F. & Munro, S. In vivo identification of GTPase interactors by mitochondrial
relocalization and proximity biotinylation. _eLife_ 8, e45916 (2019). Article PubMed PubMed Central Google Scholar * Hoyer, M. J. et al. A novel class of ER membrane proteins regulates
ER-associated endosome fission. _Cell_ 175, 254–265.e14 (2018). Article CAS PubMed PubMed Central Google Scholar * van Vliet, A. R. et al. The ER stress sensor PERK coordinates
ER-plasma membrane contact site formation through interaction with filamin-A and F-actin remodeling. _Mol. Cell_ 65, 885–899.e6 (2017). Article PubMed CAS Google Scholar * Spence, E. F.
et al. In vivo proximity proteomics of nascent synapses reveals a novel regulator of cytoskeleton-mediated synaptic maturation. _Nat. Commun._ 10, 386 (2019). Article PubMed PubMed Central
CAS Google Scholar * Feng, W. et al. Identifying the cardiac dyad proteome in vivo by a BioID2 knock-in strategy. _Circulation_ 141, 940–942 (2020). Article PubMed PubMed Central
Google Scholar * Hung, V. et al. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. _Nat. Protoc._ 11, 456–475 (2016). Article CAS PubMed PubMed
Central Google Scholar * Hung, V. et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. _Mol. Cell_ 55, 332–341 (2014).
Article CAS PubMed PubMed Central Google Scholar * Han, S. et al. Proximity biotinylation as a method for mapping proteins associated with mtDNA in living cells. _Cell Chem. Biol._ 24,
404–414 (2017). Article CAS PubMed PubMed Central Google Scholar * Hung, V. et al. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by
proximity biotinylation. _eLife_ 6, e24463 (2017). Article PubMed PubMed Central Google Scholar * Mertins, P. et al. Reproducible workflow for multiplexed deep-scale proteome and
phosphoproteome analysis of tumor tissues by liquid chromatography-mass spectrometry. _Nat. Protoc._ 13, 1632–1661 (2018). Article CAS PubMed PubMed Central Google Scholar * Li, J. et
al. Cell-surface proteomic profiling in the fly brain uncovers wiring regulators. _Cell_ 180, 373–386.e15 (2020). Article CAS PubMed PubMed Central Google Scholar * Vandemoortele, G. et
al. A well-controlled BioID design for endogenous bait proteins. _J. Proteome Res._ 18, 95–106 (2019). CAS PubMed Google Scholar * Bian, Y. et al. Robust, reproducible and quantitative
analysis of thousands of proteomes by micro-flow LC–MS/MS. _Nat. Commun._ 11, 157 (2020). Article CAS PubMed PubMed Central Google Scholar * Käll, L., Krogh, A. & Sonnhammer, E. L.
L. A combined transmembrane topology and signal peptide prediction method. _J. Mol. Biol._ 338, 1027–1036 (2004). Article PubMed CAS Google Scholar * Ashburner, M. et al. Gene ontology:
tool for the unification of biology. _Nat. Genet._ 25, 25–29 (2000). Article CAS PubMed PubMed Central Google Scholar * Gene Ontology Consortium. Gene Ontology Consortium: going
forward. _Nucleic Acids Res._ 43, D1049–D1056 (2015). * Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for
proteomics using StageTips. _Nat. Protoc._ 2, 1896–1906 (2007). Article CAS PubMed Google Scholar * Bateman, A. et al. UniProt: the universal protein knowledgebase. _Nucleic Acids Res_
45, D158–D169 (2017). Article CAS Google Scholar * Lee, S. Y. et al. APEX fingerprinting reveals the subcellular localization of proteins of interest. _Cell Rep._ 15, 1837–1847 (2016).
Article CAS PubMed Google Scholar * Cho, I. T. et al. Ascorbate peroxidase proximity labeling coupled with biochemical fractionation identifies promoters of endoplasmic
reticulum–mitochondrial contacts. _J. Biol. Chem._ 292, 16382–16392 (2017). Article CAS PubMed PubMed Central Google Scholar * Cao, Q. et al. PAQR3 regulates endoplasmic
reticulum-to-Golgi trafficking of COPII vesicle via interaction with Sec13/Sec31 coat proteins. _iScience_ 9, 382–398 (2018). Article CAS PubMed PubMed Central Google Scholar * Le
Guerroué, F. et al. Autophagosomal content profiling reveals an LC3C-dependent piecemeal mitophagy pathway. _Mol. Cell_ 68, 786–796.e6 (2017). Article PubMed CAS Google Scholar * Mick,
D. U. et al. Proteomics of primary cilia by proximity labeling. _Dev. Cell_ 35, 497–512 (2015). Article CAS PubMed PubMed Central Google Scholar * Santin, Y. G. et al. In vivo TssA
proximity labelling during type VI secretion biogenesis reveals TagA as a protein that stops and holds the sheath. _Nat. Microbiol._ 3, 1304–1313 (2018). Article CAS PubMed Google Scholar
* Mannix, K. M., Starble, R. M., Kaufman, R. S. & Cooley, L. Proximity labeling reveals novel interactomes in live _Drosophila_ tissue. _Development_ 146, dev176644 (2019). Article
CAS PubMed PubMed Central Google Scholar * Liu, G. et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. _Nature_ 577, 695–700 (2020). Article CAS PubMed
PubMed Central Google Scholar * Chojnowski, A. et al. Progerin reduces LAP2α-telomere association in Hutchinson-Gilford progeria. _eLife_ 4, 1–21 (2015). Article Google Scholar *
Cross, S. H. et al. The nanophthalmos protein TMEM98 inhibits MYRF self-cleavage and is required for eye size specification. _PLoS Genet_ 16, e1008583 (2020). Article CAS PubMed PubMed
Central Google Scholar * Pagac, M. et al. SEIPIN regulates lipid droplet expansion and adipocyte development by modulating the activity of glycerol-3-phosphate acyltransferase. _Cell Rep._
17, 1546–1559 (2016). Article CAS PubMed PubMed Central Google Scholar * Cole, A. et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid
leukemia. _Cancer Cell_ 27, 864–876 (2015). Article CAS PubMed PubMed Central Google Scholar * Janer, A. et al. SLC 25A46 is required for mitochondrial lipid homeostasis and cristae
maintenance and is responsible for Leigh syndrome. _EMBO Mol. Med._ 8, 1019–1038 (2016). Article CAS PubMed PubMed Central Google Scholar * Antonicka, H. et al. A pseudouridine synthase
module is essential for mitochondrial protein synthesis and cell viability. _EMBO Rep._ 18, 28–38 (2017). Article CAS PubMed Google Scholar * Van Itallie, C. M. et al. Biotin ligase
tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell-cell and cell-substrate adhesion. _J. Cell Sci._ 127, 885–895 (2014).
PubMed PubMed Central Google Scholar * Guo, Z. et al. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. _Sci. Signal._ 7, rs7 (2014). Article PubMed
PubMed Central CAS Google Scholar * Hua, R. et al. VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. _J. Cell Biol._ 216, 367–377 (2017).
Article CAS PubMed PubMed Central Google Scholar * Chan, C. J. et al. BioID performed on Golgi enriched fractions identify C10orf76 as a GBF1 binding protein essential for Golgi
maintenance and secretion. _Mol. Cell. Proteom._ 18, 2285–2297 (2019). Article CAS Google Scholar * Opitz, N. et al. Capturing the Asc1p/ _R_ eceptor for _A_ ctivated _C K_ inase _1_
(RACK1) microenvironment at the head region of the 40S ribosome with quantitative BioID in yeast. _Mol. Cell. Proteom._ 16, 2199–2218 (2017). Article CAS Google Scholar * Domsch, K. et
al. The hox transcription factor ubx stabilizes lineage commitment by suppressing cellular plasticity in _Drosophila_. _eLife_ 8, e42675 (2019). Article PubMed PubMed Central Google
Scholar * Bagchi, P., Torres, M., Qi, L. & Tsai, B. Selective EMC subunits act as molecular tethers of intracellular organelles exploited during viral entry. _Nat. Commun._ 11, 1127
(2020). Article CAS PubMed PubMed Central Google Scholar * Yoshinaka, T. et al. Structural basis of mitochondrial scaffolds by prohibitin complexes: insight into a role of the
coiled-coil region. _iScience_ 19, 1065–1078 (2019). Article CAS PubMed PubMed Central Google Scholar * Callegari, S. et al. A MICOS–TIM22 association promotes carrier import into human
mitochondria. _J. Mol. Biol._ 431, 2835–2851 (2019). Article CAS PubMed Google Scholar * Chen, Z. et al. Global phosphoproteomic analysis reveals ARMC10 as an AMPK substrate that
regulates mitochondrial dynamics. _Nat. Commun._ 10, 104 (2019). Article PubMed PubMed Central CAS Google Scholar * Liu, L., Doray, B. & Kornfeld, S. Recycling of Golgi
glycosyltransferases requires direct binding to coatomer. _Proc. Natl Acad. Sci. USA._ 115, 8984–8989 (2018). Article CAS PubMed PubMed Central Google Scholar * Mirza, A. N. et al. LAP2
proteins chaperone GLI1 movement between the lamina and chromatin to regulate transcription. _Cell_ 176, 198–212.e15 (2019). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by NIH R01-DK121409 (to A.Y.T. and S.A.C.) and the Stanford Wu Tsai Neurosciences Institute Big Ideas Initiative (to A.Y.T.). K.F.C. was supported by
NIH Training Grant 2T32CA009302-41 and the Blavatnik Graduate Fellowship. T.C.B. is a Robert Black Fellow of the Damon Runyon Cancer Research Foundation (DRG-2391-20). A.Y.T. is an
investigator of the Chan Zuckerberg Biohub. AUTHOR INFORMATION Author notes * These authors contributed equally: Kelvin F. Cho, Tess C. Branon. AUTHORS AND AFFILIATIONS * Cancer Biology
Program, Stanford University, Stanford, CA, USA Kelvin F. Cho * Department of Genetics, Stanford University, Stanford, CA, USA Kelvin F. Cho & Alice Y. Ting * Department of Molecular and
Cell Biology, University of California, Berkeley, Berkeley, CA, USA Tess C. Branon * Broad Institute of MIT and Harvard, Cambridge, MA, USA Namrata D. Udeshi, Samuel A. Myers & Steven
A. Carr * Department of Biology, Stanford University, Stanford, CA, USA Alice Y. Ting * Department of Chemistry, Stanford University, Stanford, CA, USA Alice Y. Ting * Chan Zuckerberg
Biohub, San Francisco, CA, USA Alice Y. Ting Authors * Kelvin F. Cho View author publications You can also search for this author inPubMed Google Scholar * Tess C. Branon View author
publications You can also search for this author inPubMed Google Scholar * Namrata D. Udeshi View author publications You can also search for this author inPubMed Google Scholar * Samuel A.
Myers View author publications You can also search for this author inPubMed Google Scholar * Steven A. Carr View author publications You can also search for this author inPubMed Google
Scholar * Alice Y. Ting View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS K.F.C., T.C.B, N.D.U., S.A.M., S.A.C., and A.Y.T. contributed to
the writing and editing of the manuscript. CORRESPONDING AUTHOR Correspondence to Alice Y. Ting. ETHICS DECLARATIONS COMPETING INTERESTS A.Y.T. and T.C.B. have filed a patent application
covering some aspects of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RELATED LINKS KEY REFERENCES USING THIS PROTOCOL: Branon, T. C. et al. _Nat. Biotechnol_. 36, 880–887 (2018): https://www.nature.com/articles/nbt.4201 Cho, K. F. et al. _Proc.
Natl Acad. Sci. USA_ 117, 12143–12154 (2020): https://www.pnas.org/content/117/22/12143 SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLE 1 Human proteome of proteins,
annotated by whether each protein was previously detected in a PL proteomic experiment from our lab (regions include: mitochondrial matrix6,15, mitochondrial intermembrane space76,
mitochondrial nucleoid77, ER membrane6,7,78, outer mitochondrial membrane7,78, ER-mitochondria contact sites7,78, nucleus6, synaptic cleft20, and cytosol6,7,78). For each protein, the
compartment(s) in which they were detected are listed. SUPPLEMENTARY TABLE 2 Compilation of data from previous PL proteomic mapping experiments performed by our lab, categorized by
organelle/region of interest (each tab is a different subcellular compartment). In each tab, the relevant studies and corresponding enrichment ratios (SILAC, TMT, or iTRAQ) for proteins
detected above the respective cutoffs are provided. Data are included for the mitochondrial matrix6,15 (Tab 1), mitochondrial intermembrane space76 (Tab 2), mitochondrial nucleoid77 (Tab 3),
ER membrane6,7,78 (Tab 4), outer mitochondrial membrane7,78 (Tab 5), ER-mitochondria contact sites7,78 (Tab 6), nucleus6 (Tab 7), synaptic cleft20 (Tab 8), and cytosol6,7,78 (Tab 9). RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cho, K.F., Branon, T.C., Udeshi, N.D. _et al._ Proximity labeling in mammalian cells with TurboID and
split-TurboID. _Nat Protoc_ 15, 3971–3999 (2020). https://doi.org/10.1038/s41596-020-0399-0 Download citation * Received: 17 May 2020 * Accepted: 18 August 2020 * Published: 02 November 2020
* Issue Date: December 2020 * DOI: https://doi.org/10.1038/s41596-020-0399-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
