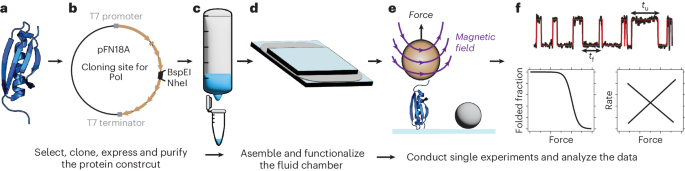
Single-molecule magnetic tweezers to probe the equilibrium dynamics of individual proteins at physiologically relevant forces and timescales
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The reversible unfolding and refolding of proteins is a regulatory mechanism of tissue elasticity and signalling used by cells to sense and adapt to extracellular and intracellular
mechanical forces. However, most of these proteins exhibit low mechanical stability, posing technical challenges to the characterization of their conformational dynamics under force. Here,
we detail step-by-step instructions for conducting single-protein nanomechanical experiments using ultra-stable magnetic tweezers, which enable the measurement of the equilibrium
conformational dynamics of single proteins under physiologically relevant low forces applied over biologically relevant timescales. We report the basic principles determining the functioning
of the magnetic tweezer instrument, review the protein design strategy and the fluid chamber preparation and detail the procedure to acquire and analyze the unfolding and refolding
trajectories of individual proteins under force. This technique adds to the toolbox of single-molecule nanomechanical techniques and will be of particular interest to those interested in
proteins involved in mechanosensing and mechanotransduction. The procedure takes 4 d to complete, plus an additional 6 d for protein cloning and production, requiring basic expertise in
molecular biology, surface chemistry and data analysis. KEY POINTS * Ultra-stable magnetic tweezers are used for measuring the conformational dynamics of individual proteins at
physiologically relevant low forces and over long timescales. * Magnetic fields are created by using either permanent magnets or a tape head, which generates precisely calibrated forces for
pulling single proteins tethered between a superparamagnetic bead and a functionalized glass substrate. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE ROLE OF SINGLE-PROTEIN ELASTICITY IN MECHANOBIOLOGY Article 24
October 2022 OPTICAL TWEEZERS IN SINGLE-MOLECULE BIOPHYSICS Article 25 March 2021 MAGNETIC TWEEZERS TO CAPTURE THE FAST-FOLDING Λ6-85 IN SLOW MOTION Article Open access 07 January 2025 DATA
AVAILABILITY Example data from Figs. 7 and 10 can be found as Supplementary Data. Modified pFN18a plasmids from Fig. 5 are available in Addgene (pFN18A-HaloTag-Biotin: Addgene plasmid
#206039; pFN18A-HaloTag-SpyCatcher Addgene plasmid #206041). Other data that support the plots within this paper are available from the corresponding author upon reasonable request. CODE
AVAILABILITY Scripts for the fluctuation analysis are included in the Supplementary Data. The data acquisition code can be accessed at https://doi.org/10.5281/zenodo.8092186. REFERENCES *
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. _Science_ 341, 1240104 (2013). Article PubMed PubMed Central Google Scholar *
De Belly, H., Paluch, E. K. & Chalut, K. J. Interplay between mechanics and signalling in regulating cell fate. _Nat. Rev. Mol. Cell Biol._ 23, 465–480 (2022). Article PubMed Google
Scholar * Iskratsch, T., Wolfenson, H. & Sheetz, M. P. Appreciating force and shape—the rise of mechanotransduction in cell biology. _Nat. Rev. Mol. Cell Biol._ 15, 825–833 (2014).
Article CAS PubMed PubMed Central Google Scholar * Paul, C. D., Mistriotis, P. & Konstantopoulos, K. Cancer cell motility: lessons from migration in confined spaces. _Nat. Rev.
Cancer_ 17, 131–140 (2017). Article CAS PubMed Google Scholar * Dumortier, J. G. et al. Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. _Science_
365, 465–468 (2019). Article CAS PubMed Google Scholar * Mongera, A. et al. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. _Nature_ 561, 401–405 (2018).
Article CAS PubMed PubMed Central Google Scholar * Pesce, M. et al. Cardiac fibroblasts and mechanosensation in heart development, health and disease. _Nat. Rev. Cardiol._ 20, 309–324
(2022). Article PubMed Google Scholar * Kefauver, J. M., Ward, A. B. & Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. _Nature_ 587,
567–576 (2020). Article CAS PubMed PubMed Central Google Scholar * Huse, M. Mechanical forces in the immune system. _Nat. Rev. Immunol._ 17, 679–690 (2017). Article CAS PubMed PubMed
Central Google Scholar * Romani, P., Valcarcel-Jimenez, L., Frezza, C. & Dupont, S. Crosstalk between mechanotransduction and metabolism. _Nat. Rev. Mol. Cell Biol._ 22, 22–38 (2021).
Article CAS PubMed Google Scholar * Romani, P. et al. Mitochondrial fission links ECM mechanotransduction to metabolic redox homeostasis and metastatic chemotherapy resistance. _Nat.
Cell Biol._ 24, 168–180 (2022). Article CAS PubMed PubMed Central Google Scholar * Jaalouk, D. E. & Lammerding, J. Mechanotransduction gone awry. _Nat. Rev. Mol. Cell Biol._ 10,
63–73 (2009). Article CAS PubMed PubMed Central Google Scholar * Chemla, Y. R. et al. Mechanism of force generation of a viral DNA packaging motor. _Cell_ 122, 683–692 (2005). Article
CAS PubMed Google Scholar * Ranade, S. S., Syeda, R. & Patapoutian, A. Mechanically activated ion channels. _Neuron_ 87, 1162–1179 (2015). Article CAS PubMed PubMed Central Google
Scholar * Neuman, K. C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. _Nat. Methods_ 5, 491–505 (2008). Article CAS
PubMed PubMed Central Google Scholar * Krieg, M. et al. Atomic force microscopy-based mechanobiology. _Nat. Rev. Phys._ 1, 41–57 (2018). Article Google Scholar * Bustamante, C. J.,
Chemla, Y. R., Liu, S. & Wang, M. D. Optical tweezers in single-molecule biophysics. _Nat. Rev. Methods Prim._ 1, 25 (2021). Article CAS Google Scholar * Popa, I., Kosuri, P.,
Alegre-Cebollada, J., Garcia-Manyes, S. & Fernandez, J. M. Force dependency of biochemical reactions measured by single-molecule force-clamp spectroscopy. _Nat. Protoc._ 8, 1261–1276
(2013). Article CAS PubMed PubMed Central Google Scholar * Li, H. et al. Reverse engineering of the giant muscle protein titin. _Nature_ 418, 998–1002 (2002). Article CAS PubMed
Google Scholar * Echelman, D. J., Lee, A. Q. & Fernandez, J. M. Mechanical forces regulate the reactivity of a thioester bond in a bacterial adhesin. _J. Biol. Chem._ 292, 8988–8997
(2017). Article CAS PubMed PubMed Central Google Scholar * Milles, L. F., Schulten, K., Gaub, H. E. & Bernardi, R. C. Molecular mechanism of extreme mechanostability in a pathogen
adhesin. _Science_ 359, 1527–1533 (2018). Article CAS PubMed PubMed Central Google Scholar * Mora, M., Stannard, A. & Garcia-Manyes, S. The nanomechanics of individual proteins.
_Chem. Soc. Rev._ 49, 6816–6832 (2020). Article CAS PubMed Google Scholar * Dudko, O. K., Hummer, G. & Szabo, A. Theory, analysis, and interpretation of single-molecule force
spectroscopy experiments. _Proc. Natl Acad. Sci. USA_ 105, 15755–15760 (2008). Article CAS PubMed PubMed Central Google Scholar * Cecconi, C., Shank, E. A., Bustamante, C. &
Marqusee, S. Direct observation of the three-state folding of a single protein molecule. _Science_ 309, 2057–2060 (2005). Article CAS PubMed Google Scholar * Stigler, J., Ziegler, F.,
Gieseke, A., Gebhardt, J. C. & Rief, M. The complex folding network of single calmodulin molecules. _Science_ 334, 512–516 (2011). Article CAS PubMed Google Scholar * Neupane, K.,
Manuel, A. P. & Woodside, M. T. Protein folding trajectories can be described quantitatively by one-dimensional diffusion over measured energy landscapes. _Nat. Phys._ 12, 700–703
(2016). Article CAS Google Scholar * Woodside, M. T. & Block, S. M. Reconstructing folding energy landscapes by single-molecule force spectroscopy. _Annu. Rev. Biophys._ 43, 19–39
(2014). Article CAS PubMed PubMed Central Google Scholar * Kaiser, C. M., Goldman, D. H., Chodera, J. D., Tinoco, I. Jr. & Bustamante, C. The ribosome modulates nascent protein
folding. _Science_ 334, 1723–1727 (2011). Article CAS PubMed PubMed Central Google Scholar * Lipfert, J., Kerssemakers, J. W., Jager, T. & Dekker, N. H. Magnetic torque tweezers:
measuring torsional stiffness in DNA and RecA-DNA filaments. _Nat. Methods_ 7, 977–980 (2010). Article CAS PubMed Google Scholar * Ding, F. et al. Single-molecule mechanical
identification and sequencing. _Nat. Methods_ 9, 367–372 (2012). Article CAS PubMed PubMed Central Google Scholar * Hodeib, S. et al. Single molecule studies of helicases with magnetic
tweezers. _Methods_ 105, 3–15 (2016). Article CAS PubMed Google Scholar * Lionnet, T. et al. Magnetic trap construction. _Cold Spring Harb. Protoc._ 2012, 133–138 (2012). Article PubMed
Google Scholar * Rivas-Pardo, J. A. et al. Work done by titin protein folding assists muscle contraction. _Cell Rep._ 14, 1339–1347 (2016). Article CAS PubMed PubMed Central Google
Scholar * Chen, H. et al. Dynamics of equilibrium folding and unfolding transitions of titin immunoglobulin domain under constant forces. _J. Am. Chem. Soc._ 137, 3540–3546 (2015). Article
CAS PubMed PubMed Central Google Scholar * Bauer, M. S. et al. A tethered ligand assay to probe SARS-CoV-2:ACE2 interactions. _Proc. Natl Acad. Sci. USA_ 119, e2114397119 (2022).
Article CAS PubMed PubMed Central Google Scholar * Yao, M. et al. The mechanical response of talin. _Nat. Commun._ 7, 11966 (2016). Article PubMed PubMed Central Google Scholar *
Popa, I. et al. A HaloTag anchored ruler for week-long studies of protein dynamics. _J. Am. Chem. Soc._ 138, 10546–10553 (2016). Article CAS PubMed PubMed Central Google Scholar * Lof,
A. et al. Multiplexed protein force spectroscopy reveals equilibrium protein folding dynamics and the low-force response of von Willebrand factor. _Proc. Natl Acad. Sci. USA_ 116,
18798–18807 (2019). Article PubMed PubMed Central Google Scholar * Zhao, X., Zeng, X., Lu, C. & Yan, J. Studying the mechanical responses of proteins using magnetic tweezers.
_Nanotechnology_ 28, 414002 (2017). Article PubMed Google Scholar * Choi, H. K. et al. Watching helical membrane proteins fold reveals a common N-to-C-terminal folding pathway. _Science_
366, 1150–1156 (2019). Article CAS PubMed PubMed Central Google Scholar * Choi, H. K. et al. Evolutionary balance between foldability and functionality of a glucose transporter. _Nat.
Chem. Biol._ 18, 713–723 (2022). Article CAS PubMed PubMed Central Google Scholar * Choi, H. K., Kim, H. G., Shon, M. J. & Yoon, T. Y. High-resolution single-molecule magnetic
tweezers. _Annu. Rev. Biochem._ 91, 33–59 (2022). Article CAS PubMed Google Scholar * Smith, S. B., Finzi, L. & Bustamante, C. Direct mechanical measurements of the elasticity of
single DNA molecules by using magnetic beads. _Science_ 258, 1122–1126 (1992). Article CAS PubMed Google Scholar * Strick, T. R., Allemand, J. F., Bensimon, D., Bensimon, A. &
Croquette, V. The elasticity of a single supercoiled DNA molecule. _Science_ 271, 1835–1837 (1996). Article CAS PubMed Google Scholar * Gosse, C. & Croquette, V. Magnetic tweezers:
micromanipulation and force measurement at the molecular level. _Biophys. J._ 82, 3314–3329 (2002). Article CAS PubMed PubMed Central Google Scholar * Maier, B., Bensimon, D. &
Croquette, V. Replication by a single DNA polymerase of a stretched single-stranded DNA. _Proc. Natl Acad. Sci. USA_ 97, 12002–12007 (2000). Article CAS PubMed PubMed Central Google
Scholar * Dekker, N. H. et al. The mechanism of type IA topoisomerases. _Proc. Natl Acad. Sci. USA_ 99, 12126–12131 (2002). Article CAS PubMed PubMed Central Google Scholar * Crut, A.,
Koster, D. A., Seidel, R., Wiggins, C. H. & Dekker, N. H. Fast dynamics of supercoiled DNA revealed by single-molecule experiments. _Proc. Natl Acad. Sci. USA_ 104, 11957–11962 (2007).
Article CAS PubMed PubMed Central Google Scholar * England, C. G., Luo, H. & Cai, W. HaloTag technology: a versatile platform for biomedical applications. _Bioconjug. Chem._ 26,
975–986 (2015). Article CAS PubMed PubMed Central Google Scholar * Zakeri, B. et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin.
_Proc. Natl Acad. Sci. USA_ 109, E690–E697 (2012). Article CAS PubMed PubMed Central Google Scholar * Tapia-Rojo, R., Eckels, E. C. & Fernandez, J. M. Ephemeral states in protein
folding under force captured with a magnetic tweezers design. _Proc. Natl Acad. Sci. USA_ 116, 7873–7878 (2019). Article CAS PubMed PubMed Central Google Scholar * Stannard, A. et al.
Molecular fluctuations as a ruler of force-induced protein conformations. _Nano Lett._ 21, 2953–2961 (2021). Article CAS PubMed PubMed Central Google Scholar * Tapia-Rojo, R.,
Alonso-Caballero, A. & Fernandez, J. M. Direct observation of a coil-to-helix contraction triggered by vinculin binding to talin. _Sci. Adv._ 6, eaaz4707 (2020). Article CAS PubMed
PubMed Central Google Scholar * Franz, F. et al. Allosteric activation of vinculin by talin. _Nat. Commun._ 14, 4311 (2023). Article CAS PubMed PubMed Central Google Scholar *
Tapia-Rojo, R. et al. Enhanced statistical sampling reveals microscopic complexity in the talin mechanosensor folding energy landscape. _Nat. Phys._ 19, 52–60 (2023). Article CAS PubMed
Google Scholar * Austen, K. et al. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. _Nat. Cell Biol._ 17, 1597–1606 (2015). Article CAS PubMed PubMed Central
Google Scholar * Riveline, D. et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and
ROCK-independent mechanism. _J. Cell Biol._ 153, 1175–1186 (2001). Article CAS PubMed PubMed Central Google Scholar * Grashoff, C. et al. Measuring mechanical tension across vinculin
reveals regulation of focal adhesion dynamics. _Nature_ 466, 263–266 (2010). Article CAS PubMed PubMed Central Google Scholar * Garcia-Manyes, S. et al. Single-molecule force
spectroscopy predicts a misfolded, domain-swapped conformation in human γD-crystallin protein. _J. Biol. Chem._ 291, 4226–4235 (2016). Article CAS PubMed Google Scholar * Mora, M. et al.
A single-molecule strategy to capture non-native intramolecular and intermolecular protein disulfide bridges. _Nano Lett._ 22, 3922–3930 (2022). Article CAS PubMed PubMed Central Google
Scholar * Petrosyan, R., Patra, S., Rezajooei, N., Garen, C. R. & Woodside, M. T. Unfolded and intermediate states of PrP play a key role in the mechanism of action of an antiprion
chaperone. _Proc. Natl Acad. Sci. USA_ 118, e2010213118 (2021). Article CAS PubMed PubMed Central Google Scholar * Gupta, A. N. et al. Pharmacological chaperone reshapes the energy
landscape for folding and aggregation of the prion protein. _Nat. Commun._ 7, 12058 (2016). Article CAS PubMed PubMed Central Google Scholar * Sen Mojumdar, S. et al. Partially native
intermediates mediate misfolding of SOD1 in single-molecule folding trajectories. _Nat. Commun._ 8, 1881 (2017). Article PubMed PubMed Central Google Scholar * Yao, M. et al.
Force-dependent conformational switch of alpha-catenin controls vinculin binding. _Nat. Commun._ 5, 4525 (2014). Article CAS PubMed Google Scholar * Dahal, N., Sharma, S., Phan, B., Eis,
A. & Popa, I. Mechanical regulation of talin through binding and history-dependent unfolding. _Sci. Adv._ 8, eabl7719 (2022). Article CAS PubMed Google Scholar * Kemmerich, F. E. et
al. Simultaneous single-molecule force and fluorescence sampling of DNA nanostructure conformations using magnetic tweezers. _Nano Lett._ 16, 381–386 (2016). Article CAS PubMed Google
Scholar * Ivanov, I. E. et al. Multimodal measurements of single-molecule dynamics using FluoRBT. _Biophys. J._ 114, 278–282 (2018). Article CAS PubMed Google Scholar * Tapia-Rojo, R.,
Alonso-Caballero, A. & Fernandez, J. M. Talin folding as the tuning fork of cellular mechanotransduction. _Proc. Natl Acad. Sci. USA_ 117, 21346–21353 (2020). Article CAS PubMed
PubMed Central Google Scholar * Alonso-Caballero, A. et al. Protein folding modulates the chemical reactivity of a Gram-positive adhesin. _Nat. Chem._ 13, 172–181 (2021). Article CAS
PubMed Google Scholar * Guo, H. A simple algorithm for fitting a gaussian function. In _Streamlining Digital Signal Processing_ 297–305 (John Wiley & Sons, 2012). * Fonnum, G.,
Johansson, C., Molteberg, A., Mørup, S. & Aksnes, E. Characterisation of Dynabeads® by magnetization measurements and Mössbauer spectroscopy. _J. Magn. Magn. Mater._ 293, 41–47 (2005).
Article CAS Google Scholar * Ostrofet, E., Papini, F. S. & Dulin, D. Correction-free force calibration for magnetic tweezers experiments. _Sci. Rep._ 8, 15920 (2018). Article PubMed
PubMed Central Google Scholar * Buschow, K. H. J., Long, G. J. & Grandjean, F. _High Density Digital Recording_ (Springer, 1993). * Liu, R., Garcia-Manyes, S., Sarkar, A., Badilla,
C. L. & Fernandez, J. M. Mechanical characterization of protein L in the low-force regime by electromagnetic tweezers/evanescent nanometry. _Biophys. J._ 96, 3810–3821 (2009). Article
CAS PubMed PubMed Central Google Scholar * Valle-Orero, J. et al. Proteins breaking bad: a free energy perspective. _J. Phys. Chem. Lett._ 8, 3642–3647 (2017). Article CAS PubMed
PubMed Central Google Scholar * Alegre-Cebollada, J., Badilla, C. L. & Fernandez, J. M. Isopeptide bonds block the mechanical extension of pili in pathogenic _Streptococcus pyogenes_.
_J. Biol. Chem._ 285, 11235–11242 (2010). Article CAS PubMed PubMed Central Google Scholar * Schlierf, M., Li, H. & Fernandez, J. M. The unfolding kinetics of ubiquitin captured
with single-molecule force-clamp techniques. _Proc. Natl Acad. Sci. USA_ 101, 7299–7304 (2004). Article CAS PubMed PubMed Central Google Scholar * Evans, E. & Ritchie, K. Dynamic
strength of molecular adhesion bonds. _Biophys. J._ 72, 1541–1555 (1997). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y., Jiao, J. & Rebane, A. A. Hidden Markov
modeling with detailed balance and its application to single protein folding. _Biophys. J._ 111, 2110–2124 (2016). Article CAS PubMed PubMed Central Google Scholar * McKinney, S. A.,
Joo, C. & Ha, T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. _Biophys. J._ 91, 1941–1951 (2006). Article CAS PubMed PubMed Central Google Scholar *
Dudko, O. K., Hummer, G. & Szabo, A. Intrinsic rates and activation free energies from single-molecule pulling experiments. _Phys. Rev. Lett._ 96, 108101 (2006). Article PubMed Google
Scholar * Bullerjahn, J. T., Sturm, S. & Kroy, K. Theory of rapid force spectroscopy. _Nat. Commun._ 5, 4463 (2014). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS We are deeply grateful to J. Fernandez and C. Badilla (Columbia University) for their pioneering work on technique development and protein engineering and for their legacy
in the field. We thank S. Board, J. Walker and P. Paracuellos for help in protein expression and purification. This work was supported in part by the Francis Crick Institute, which receives
its core funding from Cancer Research U.K. (CC0102), the U.K. Medical Research Council (CC0102) and the Wellcome Trust (CC0102). R.T.-R. is the recipient of a King’s Prize Fellowship. This
work was supported by the European Commission (Mechanocontrol, Grant Agreement 731957), BBSRC sLoLa (BB/V003518/1), Leverhulme Trust Research Leadership Award RL 2016-015, Wellcome Trust
Investigator Award 212218/Z/18/Z and Royal Society Wolfson Fellowship RSWF/R3/183006 to S.G.-M. AUTHOR INFORMATION Author notes * These authors contributed equally: Rafael Tapia-Rojo, Marc
Mora. AUTHORS AND AFFILIATIONS * Single Molecule Mechanobiology Laboratory, The Francis Crick Institute, London, UK Rafael Tapia-Rojo, Marc Mora & Sergi Garcia-Manyes * Department of
Physics, Randall Centre for Cell and Molecular Biophysics, Centre for the Physical Science of Life and London Centre for Nanotechnology, King’s College London, London, UK Rafael Tapia-Rojo,
Marc Mora & Sergi Garcia-Manyes Authors * Rafael Tapia-Rojo View author publications You can also search for this author inPubMed Google Scholar * Marc Mora View author publications You
can also search for this author inPubMed Google Scholar * Sergi Garcia-Manyes View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS R.T.-R, M.M.
and S.G.-M wrote the paper. CORRESPONDING AUTHORS Correspondence to Rafael Tapia-Rojo, Marc Mora or Sergi Garcia-Manyes. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Protocols_ thanks Tony Huang and the other, anonymous, reviewer(s) for their contribution to the peer review process of this
work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS KEY
REFERENCE USING THIS PROTOCOL Tapia-Rojo, R. et al. _Nat. Phys_. 19, 52–60 (2023): https://doi.org/10.1038/s41567-022-01808-4 EXTENDED DATA EXTENDED DATA FIG. 1 CALCULATING THE STIFFNESS OF
THE MAGNETIC TRAP. Stiffness of the magnetic trap created by the N52 magnets (voice-coil configuration) (A) and magnetic tape head (B). The magnetic trap stiffnesses can be simply calculated
as _dF/dz_, where _z_ is the distance between the gap (magnets or tape head) and the magnetic bead. Because of the nonlinearity of _F_(_z_), the stiffness changes over the control parameter
(magnet position or electric current), but in the operating regime of the trap this results in a very soft trap (~10−4 pN/nm), resulting in effective force clamp conditions (no appreciable
change in force over the range in which the bead moves). EXTENDED DATA FIG. 2 CALIBRATION OF THE TWEEZERS. Calibration of the voice coil-based (A) or tape head–based (B) magnetic tweezers
using the worm-like chain model for polymer elasticity (left) and comparison of the calibration using the worm-like chain (WLC) and freely jointed chain (FJC) (right). The FJC gives a lower
contour length (_ΔL_c = 16.3 nm) compared to the WLC (_ΔL_c = 18.6 nm). All error bars are s.d. EXTENDED DATA FIG. 3 TAPE HEAD AND MAGNETS. The magnetic tape head and voice-coil-mounted
permanent magnets with a magnification of the gap region. SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY DATA 1 Raw traces from talin R3IVVI pulled at 1 pN/s and protein L pulled
at 5 and 10 pN/s SUPPLEMENTARY DATA 2 Raw trace and fluctuation analysis of talin R3IVVI pulled at 8.5 pN SUPPLEMENTARY VIDEOS 1–3 1, how to pull on a protein by using single-molecule
magnetic tweezers; 2, how to assemble the fluid chambers; 3, how to calibrate the distance between the bottom glass cover slide and the magnets RIGHTS AND PERMISSIONS Springer Nature or its
licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Tapia-Rojo, R., Mora, M. & Garcia-Manyes, S. Single-molecule magnetic tweezers to probe the equilibrium dynamics of individual proteins at physiologically relevant forces and timescales.
_Nat Protoc_ 19, 1779–1806 (2024). https://doi.org/10.1038/s41596-024-00965-5 Download citation * Received: 26 April 2023 * Accepted: 18 December 2023 * Published: 11 March 2024 * Issue
Date: June 2024 * DOI: https://doi.org/10.1038/s41596-024-00965-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
