Cellular morphological trait dataset for extant coccolithophores from the atlantic ocean
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Calcification and biomass production by planktonic marine organisms influences the global carbon cycle and fuels marine ecosystems. The major calcifying plankton group
coccolithophores are highly diverse, comprising ca. 250–300 extant species. However, coccolithophore size (a key functional trait) and degree of calcification are poorly quantified, as most
of our understanding of this group comes from a small number of species. We generated a novel reference dataset of coccolithophore morphological traits, including cell-specific data for
coccosphere and cell size, coccolith size, number of coccoliths per cell, and cellular calcite content. This dataset includes observations from 1074 individual cells and represents 61
species from 25 genera spanning equatorial to temperate coccolithophore populations that were sampled during the Atlantic Meridional Transect (AMT) 14 cruise in 2004. This unique dataset can
be used to explore relationships between morphological traits (cell size and cell calcite) and environmental conditions, investigate species-specific and community contributions to pelagic
carbonate production, export and plankton biomass, and inform and validate coccolithophore representation in marine ecosystem and biogeochemical models. SIMILAR CONTENT BEING VIEWED BY
OTHERS CASCADE: DATASET OF EXTANT COCCOLITHOPHORE SIZE, CARBON CONTENT AND GLOBAL DISTRIBUTION Article Open access 24 August 2024 EOCENE EMERGENCE OF HIGHLY CALCIFYING COCCOLITHOPHORES
DESPITE DECLINING ATMOSPHERIC CO2 Article Open access 01 September 2022 MACROSCALE PATTERNS OF OCEANIC ZOOPLANKTON COMPOSITION AND SIZE STRUCTURE Article Open access 03 August 2021
BACKGROUND & SUMMARY Coccolithophores (Prymnesiophyceae) are abundant calcifying marine phytoplankton that are major contributors to the marine carbon cycle1,2 through their dual
production of organic carbon (biomass) and biosynthesis of their inorganic carbon (calcium carbonate, calcite) exoskeletal plates. Coccolithophore calcification and photosynthesis are highly
dynamic cellular processes that respond to changes in cell size, cell stoichiometry (elemental content), and the morphology and production rates of the calcite plates (coccoliths) that form
the exoskeleton (coccosphere) of each cell3,4 that can occur as cell physiology responds to fluctuating environmental conditions. At the community level, changes in population growth rates
and assemblage composition, including changes in the relative and absolute abundance of species present with different morphological traits and therefore degrees of calcification, influences
the contribution of coccolithophores to pelagic carbonate production and carbonate flux to the deep ocean5,6,7. Variability in surface ocean carbonate production2, of which coccolithophores
represent ca. 50–90%1,8,9, and carbonate export affects ocean alkalinity on shorter timescales and therefore the air-sea flux of carbon dioxide9,10,11,12. On longer timescales (Myr), the
production and sedimentation of biogenic carbonate (the counter carbonate pump) acts as a long-term CO2 sink13 that helps to regulate global temperatures14. Given the major role of
coccolithophores in these global processes and the sensitivity of coccolithophore physiology (including calcification) to environmental stressors15,16,17,18,19,20,21,22, understanding the
biogeochemical and ecological consequences of the variability in coccolithophore calcification and productivity and the drivers of this variability have been pressing research questions for
more than 40 years. Coccolithophore morphological traits (i.e., cell size, coccolith size and morphological features, number and arrangement of coccoliths around the cell) are a principal
determinant of coccolithophore calcification. There are approximately 250–300 (morpho)species of extant coccolithophores23 across two life cycle stages (diploid and haploid) that exhibit
extensive morphological diversity24, which is critical for understanding the contribution of different coccolithophore species and coccolithophores as a functional group to marine carbon
cycle dynamics. Despite this, coccolithophores are regularly regarded as a homogenous group and interspecies differences in size, calcification and physiological traits are often overlooked
in common analyses. For example, coccolithophores are usually parameterised as a single ‘calcifying phytoplankton’ class in many Earth system models25. This is often unavoidable due to both
analytical complexity and data availability, as most data for extant coccolithophore cellular biomass and calcite have been measured through the geochemical analysis of culture-derived
material for the relatively small number of species that have been isolated26. The species _Emiliania huxleyi_ has been the most extensively studied, with over 1000 experiments quantifying
biomass and calcite content in this species from a range of genetic strains and under a range of environmental conditions21. Limited data for the organic and inorganic carbon content of
other coccolithophore species are available for some species that are available in culture (e.g., _Gephyrocapsa_, _Coccolithus_, _Calcidiscus, Syracosphaera_, _Scyphosphaera_)27,28,29 but
are based on a comparatively small number of studies that represent only a handful of species or strains. Alternatively, morphometric traits can be used to estimate cellular-level calcite
content for a wide diversity of extant coccolithophore species. A morphometric approach to calculating cellular calcite content is based on estimating the volume of individual coccoliths and
the number of coccoliths per cell30,31. Morphometric-based calcite estimates generally agree well with calcite estimates derived through other approaches32,33,34,35 (see Technical
Validation). With due consideration for the distinct morphological characteristics for each species, morphometric-based cellular calcite estimates can be made across the full diversity of
extant and extinct species. The approach can be applied to coccoliths of any size or thickness and uses measurements taken on both entire, intact coccospheres and on individual coccoliths.
Morphometric-based cellular calcite estimates are also truly cellular, as opposed to the assay-based measurements that average population calcite (including the calcite of dead cells and
shed coccoliths) across population abundance and overestimate mean cell calcite as a result. Morphometric-based estimates of coccolith and cellular calcite content have been widely used to
estimate pelagic carbonate production and carbonate export fluxes (sometimes using a species’ mean coccolith size and the number of coccoliths per cell only)36,37,38,39,40,41,42,43,44,45,
and to quantify calcification responses to environmental and physiological change in both micropaleontological studies46,47,48 and laboratory experiments on _E. huxleyi_, _Coccolithus_,
_Calcidiscus_, and _Helicosphaera_4,49,50,51,52. For the remaining species of extant coccolithophores, very little is known concerning their species- or genus-specific biomass and calcite.
Here, we present a novel dataset of cellular-level extant coccolithophore morphological traits53, including coccosphere and cell size, coccolith size, number of coccoliths per cell, and
morphometric-based estimates of cellular calcite content. All data were measured from pelagic samples obtained from the Atlantic Ocean during the Atlantic Meridional Transect (AMT) cruise 14
(April-June 2004). The samples span several hydrographic provinces, including northern gyre, equatorial, subtropical gyre, and temperate waters54,55, thus capturing natural variations in
morphology that arise due to genetic and phenotypic diversity. This dataset provides some of the first estimates of cellular calcite diversity across a broad diversity of extant
coccolithophores from the Atlantic Ocean. The dataset presents 4712 morphometric measurements from 1074 individual coccospheres representing 25 extant coccolithophore genera and 61 species,
representing all major families except for the Coccolithales and 80% of common genera identified during AMT 1455. The dataset offers reference data for taxon-specific size and calcification
traits that can be used to investigate the contribution of coccolithophores to the global carbon cycle, spatial and temporal variability in coccolithophore calcite content, the links between
morphometric traits (such as coccolith size) and the physiochemical properties of seawater, and trait responses to climate variability. The dataset, supporting data, and the archived images
used to obtain the morphometric data are openly accessible through Zenodo53,56,57. METHODS FIELD SAMPLES Samples were collected during the Atlantic Meridional Transect 14 cruise (28 April
to 1 June 2004). The cruise track ran from the Falkland Islands to the UK covering a sea surface temperature range of 5 to 27 °C and a dissolved nitrate range of 1 to >10 µmol N L−1
54,58. The coccolithophore community during AMT-14 has previously been investigated over the euphotic zone at 19 stations55. For coccolithophore analysis, 1-2 L of a 20 L seawater sample
collected using a rosette sampler from between 5 and 13 water depths (55, 33, 14, 1, and 0.1% of surface irradiance plus additional water depths at some stations) was gently filtered onto 25
mm polycarbonate filters with a 0.45 µm pore size. Filters were dried at room temperature and stored until imaging. For the purposes of this morphological trait dataset, a selection of
samples were examined, covering a range of latitudes and water depths (Table 1) to ensure that morphometric data from a wide range of both common and less common extant coccolithophore
species could be measured. Coccolithophore morphometric data were collected from six stations at 55% surface irradiance (7 to 17 m water depth) and at a further seven water depths (30 m, 60
m, 90 m, 120 m, 140 m, 160 m and 200 m) at station CTD 34 in the southern subtropical gyre (Table 1). A further three samples identified as having higher abundances of generally less
abundant taxa (_Oolithotus_, _Calciosolenia_, _Gephyrocapsa_) were also examined (Table 1). IMAGING Archived images from the study of Poulton _et al_.55 were re-examined here for the
purposes of measuring key coccolith and coccosphere morphometric dimensions and estimating cellular calcite content. The sub-set of images examined for this study can be accessed through
Zenodo56. The scanning electron microscopy (SEM) methodology applied to generate the images is described in Poulton _et al_.55 and follows Charalampopoulou _et al_.59. Segments of each
filter were mounted on an aluminium stub and coated with gold. A SEM (LEO 1450VP Carl Zeiss combined with the software SmartSEM) at 5000x magnification was used to image each filter segment
across 3-4 pre-defined meander-shaped, semi-continuous transects (zero overlap between fields of view). For each sample (station, water depth; Table 1), ca. 600–700 fields of view with a
size 4.054 × 10−3 mm2 (1024 × 768 px, resolution of 13.92 px µm−1) were imaged, representing ca. 8–15 mL filtered seawater volume surveyed per sample. The number of intact coccospheres
observed across these images varied from 16 to 217 between samples (Table 1). EXTANT COCCOLITHOPHORE MORPHOMETRY For each sample, SEM images were surveyed sequentially until an intact
coccosphere was observed. Each intact coccosphere was first identified to species level where possible (and genus level if species could not be identified) following the taxonomy of
Nannotax3 (http://www.mikrotax.org/Nannotax3/) and supporting literature23,60,61 and forms a single data entry in the database comprising the following morphological measurements: coccolith
length, coccosphere diameter, and number of coccoliths per cell. All measurements in the dataset therefore represent morphometric measurements obtained from intact coccospheres only, i.e.,
we do not make any measurements on loose, individual coccoliths and coccolith size data represents measurements made on coccoliths retaining their original arrangement within intact
coccospheres. Measurements were made using the freeware ImageJ62 (v1.53a). Additional coccolith morphometrics were also measured when appropriate for each species, including coccolith width
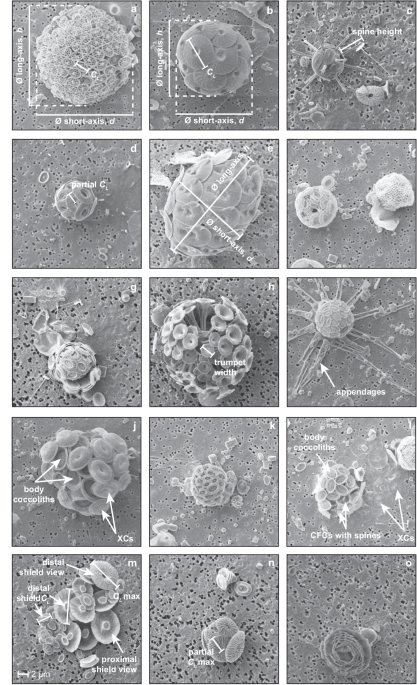
or the height of coccolith processes (e.g., spine height). The measurement procedure of these morphological features is described and discussed in detail below and an illustration of these
measurements on example specimens from a range of species encountered within the samples is shown in Fig. 1. Further taxonomic considerations that influence the accurate collection of
morphometric data are discussed in a following section. MEASUREMENT OF COCCOLITH LENGTH, COCCOLITH WIDTH AND THE SIZE OF SPECIES-SPECIFIC MORPHOLOGICAL FEATURES Coccolith length (_C__L_) was
measured on a single, flat-lying coccolith on the surface of the coccosphere where the outer rim of the coccolith distal shield was clearly visible (Fig. 1a,b). _C__L_ measurements went
precisely through the centre point of the coccolith. For species with larger central area processes (e.g., the spine of _Rhabdosphaera_, the trumpet-shaped processes of _Discosphaera_), the
height of one process was also measured (Fig. 1c). For some coccospheres, a completely flat-lying coccolith was not visible on the upper coccosphere surface or the distal shield edge(s) of
the most flat-lying coccolith were partially or fully concealed by overlapping adjacent coccoliths. In these cases, the length from central area mid-point to distal shield edge was measured
and doubled to approximate coccolith length (Fig. 1d). Based on measurements of 100 loose coccoliths on a range of species, this method introduced a mean error of −0.6% (ranging ca. ±8%)53.
Ideally, the measured flat-lying coccolith was representative of the size of all coccoliths forming the coccosphere. Coccolith width was similarly measured if necessary. MEASUREMENT OF
COCCOSPHERE DIAMETER A wide range of coccosphere shapes exist across extant coccolithophores. Species are most commonly spherical or near spherical (60–75% of entries on Nannotax323 are
tagged as ‘equant’) but prolate spheroid and ellipsoid-shaped coccospheres are also relatively common (e.g., _Helicosphaera_, Fig. 1e) and ovoid to obpyriform, fusiform, tubular and
bowl-shaped coccospheres are characteristic coccosphere shapes of certain species (for an overview of coccolith and coccosphere terminology, see Young _et al_.63). For easier comparison of
coccosphere size across such a diversity of coccosphere shapes, we therefore recorded coccosphere diameter as equivalent spherical diameter, ∅, which is back calculated from coccosphere
volume that has been calculated with a shape-specific equation for volume appropriate for the species in question. The size measurements required for the calculation of coccosphere volume
(and equivalent spherical diameter) therefore depend on the shape of the coccosphere. As such, each genus or within-genus morpho-group has been assigned a best-fit mathematic shape label
(Supplementary Table 1) that determines the volume calculation64 used to back calculate equivalent spherical diameter, as follows: $${Prolate\; sphere}({PS}){volume}\,({\mu m}^{3})=\frac{\pi
}{6}{d}^{2}h$$ (1) $${Cone}+{half\; sphere}({CHS}){volume}\,({\mu m}^{3})=\frac{\pi }{4}h{d}^{2}$$ (2) $${Double\; cone}({DC}){volume}\,({\mu m}^{3})=\frac{\pi }{12}h{d}^{2}$$ (3) where _d_
is the short-axis measurement (µm) and _h_ is the long-axis measurement (µm) of the coccosphere (see also Fig. 1). Many spherical species (particularly those with placolith-type coccoliths)
do not have precisely spherical coccosphere dimensions, as slight variability in coccosphere thickness can arise from the arrangement of overlapping coccoliths. As such, both long axis and
short axis measurements are always made (Fig. 1a,b,e) and the volume of spherical, sub-spherical and prolate spheroid coccospheres (Supplementary Table 1) were all calculated using the
equation for volume of a prolate sphere (Eq. 1). Although some coccosphere shapes are best described as ellipsoid, it was not possible to measure all axes necessary to calculate ellipsoid
volume from two-dimensional SEM images, so volume was calculated as prolate spheroid (PS) for species with more elongated shapes (Supplementary Table 1). COUNTING THE NUMBER OF COCCOLITHS
PER CELL In SEM images, only one surface of the coccosphere is visible (Fig. 1). To count the number of coccoliths per cell, therefore, the number of coccoliths on the visible surface of the
coccosphere was counted and this number was doubled to approximate the total number of coccoliths in the coccosphere (_C__N_). Some species have coccospheres comprised of a very large
number (>100) of very small (ca. 0.5 to 2 µm) coccoliths (e.g., the polycrater phase of _Alisphaera;_ Fig. 1f). For these coccospheres, the number of coccoliths per cell was counted as
accurately as possible but there is a degree of uncertainty in the counts (see Technical Validation). MORPHOMETRIC-BASED ESTIMATES OF CELLULAR PARTICULATE INORGANIC CARBON (PIC)
Morphometric-based estimates of cellular calcite (particulate inorganic carbon, PIC) depend on the number of coccoliths per cell and the PIC of each of those coccoliths. For each individual
coccosphere, we first estimated coccolith PIC using the method of Young and Ziveri30 based on coccolith size (usually using coccolith length) and a shape factor related to coccolith
cross-sectional shape, as follows: $${coccolith\; PIC}\left({pg}{{CaCO}}_{3}\right)={{C}_{L}}^{3}\times {K}_{s}\times 2.7$$ (4) Where _C__L_ is coccolith size (µm), _Ks_ is a species- or
genus-specific shape factor, and 2.7 is the mass of calcite (pg µm−3). Between species, _Ks_ values vary by around an order of magnitude (minimum of ca. 0.01 to maximum of ca. 0.2 in extant
species30). We used recommended species-specific _Ks_ values from the literature30,32,44,65,66 or adapted the _Ks_ values of a species with similar morphologies for species lacking a
published _Ks_ value. The specific coccolith length parameter used in the calculation (e.g., distal shield length or process height) and _Ks_ value used for each genus or morpho-species
group in the calculation of coccolith PIC are detailed in Supplementary Table 1. Cellular PIC was then calculated by multiplying coccolith PIC by the number of coccoliths per cell, as
follows: $${coccosphere\; calcite}\left({pg}\right)={coccolith\; PIC}\times {C}_{N}$$ (5) Where _C__N_ is the number of coccoliths per coccosphere/cell and coccolith PIC is calculated as in
Eq. 4. The relationship between coccolith morphology and coccolith calcite mass parameterised in Eq. 4, where cell-specific variability in coccolith calcite is driven principally by
variability in coccolith size, represents a simplification of true cellular variation in coccolith calcite mass. Caveats and uncertainties related to this methodological approach, including
variability in coccolith thickness, the use of species-specific shape factors, and measurement uncertainties, are discussed in detail in Young and Ziveri30 and in the Technical Validation.
INNER CELL SIZE ESTIMATION The organic biomass of phytoplankton cells is strongly related to cell volume67. In coccolithophores, the organic cell cytoplasm is covered by the calcite
coccosphere so it is useful to report both coccosphere size and cell size dimensions. The inner coccosphere size (approximating the size of the organic cell) cannot be measured directly from
SEM images, as the cell is obscured by the coccosphere (Fig. 1). We therefore estimated cell diameter as a function of coccosphere diameter, which can be precisely measured from SEM images
(described above), using a species- or genus-specific function that estimates the percentage of coccosphere volume (from coccosphere diameter) that is occupied by cell volume, as follows:
$${Equivalent}({spherical}){cell\; diameter}(\mu m)=3\,\sqrt{\frac{6\left(\gamma \times \left(\frac{4}{3}\pi {\left(\frac{{\rm{\varnothing }}}{2}\right)}^{3}\right)\right)}{\pi }}$$ (6)
where ∅ is coccosphere diameter (µm) and _y_ is the species- or genus-specific function relating coccosphere volume and cell volume (%). The percentage of coccosphere volume that is cell
volume (_y_) is estimated using measurements of coccosphere diameter and cell diameter (as internal coccosphere diameter) from light microscopy images4,68 from multiple specimens using data
from Sheward _et al_.4,69, Villiot _et al_.29 and newly collected data57. All values for _y_ are reported in Supplementary Table 1. Estimates of cell size from coccosphere size are strongly
influenced by coccolith thickness (or alternatively coccolith ‘height’ for spinose coccolith morphologies). For species with coccoliths possessing processes that extend a substantial
distance from the cell (e.g., many Rhabdosphaeraceae species; Fig. 1c,h), values of _y_ for estimating cell size from coccosphere size both including and excluding spine height are provided.
The majority of extant coccolithophore species have coccospheres formed of a single layer of abutting, overlapping and/or interlocking coccoliths. A notable exception to this is the species
_Emiliania huxleyi_, which is well-known for producing coccospheres with multiple layers70. Other species that produce multilayer or pseudo-multilayer coccospheres include _Florisphaera
profunda_ and _Umbellosphaera tenuis_. We have accounted for the presence of (pseudo-)multi-layered coccospheres when converting from coccosphere size to cell size (see also details under
_Emiliania_, _Florisphaera_ and _Umbellosphaera_ in the taxonomic-specific consideration below). SPECIES- AND GENUS-SPECIFIC CONSIDERATIONS NOELAERHABDACEAE EMILIANIA HUXLEYI There are two
additional factors influencing the calculation of cellular calcite in _Emiliania huxleyi_: variable degrees of coccolith calcite across the different morphotypes and the presence of
multi-layered coccospheres. Coccoliths of _E. huxleyi_ have been categorised into informal morphotype groupings23 based on visual assessment of morphological features, including perceived
degrees of calcification, e.g. ‘_E. huxleyi_ Type A overcalcified’ or morphotypes defined as having open central areas (e.g., ‘_E. huxleyi_ Type O’). Young and Ziveri30 have defined separate
_Ks_ values for ‘Type A group’ and ‘Type B group’ morphotypes of _E. huxleyi_ and within _E. huxleyi_, Poulton _et al_.40 and Charalampopoulou _et al_.43 estimated from field biometric
measurements on SEM images that variable coccolith morphologies led to a sixfold range in coccolith calcite. However, the calcite mass of coccoliths across different morphotypes and within
morphotypes sharing the same morphological features are very variable71,72,73,74. Descriptive terms such as ‘_E. huxleyi_ Type A overcalcified’ can therefore be misleading about differences
in calcite mass between morphotypes, for example, a ‘Type A overcalcified’ coccolith with a closed or nearly closed central area can have less calcite mass than a “regular” ‘Type A’
coccolith71. For the purposes of our dataset, we did not attempt to classify _E. huxleyi_ coccospheres into specific morphotypes or apply morphotype-specific shape factors in our calculation
of coccolith calcite. In previous studies on the coccolithophore community composition of AMT-1455, relative abundance counts also did not distinguish between different _E. huxleyi_
morphotypes so we cannot say which, if any, of the morphotypes is most abundant in our dataset. For simplicity, we have therefore used a single _Ks_ value for _E. huxleyi_ rather than
different values for each morphotype, recognising that this will be an underestimate in some instance and an overestimate in others72 (estimated as coccolith calcite ±19%). _E. huxleyi_
often makes coccospheres consisting of more than one layer of coccoliths, with as many as 3–5 layers observed in culture70,75,76 and field samples77,78,79. This occurs especially under
nutrient depleted conditions68, when cell division has slowed or ceased. For intact _E. huxleyi_ coccospheres imaged using SEM, the presence of any additional coccolith layers is not always
apparent, as the outermost coccolith layer may conceal underlying coccolith layers. Presence of multiple coccolith layers is often revealed when one or more of the coccoliths forming the
outer coccolith layer becomes dislodged or the outermost coccolith layer has sheared off (Fig. 1g). Estimating cell diameter from the coccosphere diameter of multi-layered coccospheres by
directly applying a fixed percentage conversion factor between coccosphere volume and cell volume (_y_ = 86% for _E. huxleyi_; Supplementary Table 1) would introduce error, cell volume would
be overestimated. For the purpose of estimating cell size using _y_ for multilayer coccospheres of _E. huxleyi_, we have therefore artificially reduced the coccosphere diameter measurement
to the diameter of an equivalent single-layer coccosphere using an estimate of mean _E. huxleyi_ coccolith thickness. For two-layered coccospheres, we subtracted 2x coccolith thickness (0.13
µm x2 = 0.26 µm) from measured coccosphere diameter to simulate reducing the coccosphere diameter by the one extra layer of coccoliths present, for three-layered coccospheres we reduced
coccosphere diameter by 4x coccolith thickness (0.13 µm x4 = 0.52 µm) to simulate reducing the coccosphere diameter by the two extra layers of coccoliths present, and so on. In SEM images,
intact coccospheres of _E. huxleyi_ sometimes have loose coccoliths lying directly next to the coccosphere (especially larger cells with multiple layers) and these were included in the
_C__N_ of the coccosphere to account for their calcite as they are presumably shed from the adjacent coccosphere. Taxonomic note: In recent years, several publications have presented
phylogenetic support for the inclusion of _E. huxleyi_ in the genus _Gephyrocapsa_80,81,82 and _Gephyrocapsa huxleyi_ is therefore increasingly used in publications. Here, we have continued
to use the genus name _Emiliania_ for _E. huxleyi_ for three reasons23: (1) _E. huxleyi_ and _Gephyrocapsa_ have morphological distinctions that strongly influence their cellular calcite
content, (2) distinguishing between _E. huxleyi_ and _Gephyrocapsa_ spp. is of practical use for biostratigraphic and paleoceanographic studies, and (3) _E. huxleyi_ as a species name is
widely recognised by non-experts so continued use of the genus _Emiliania_ is useful for the wider communication of research outputs. GEPHYROCAPSA No specific considerations necessary.
COCCOLITHACEAE No specific considerations were necessary for _Calcidiscus_, _Oolithotus_, or _Umbilicosphaera_. CALCIOSOLENIACEAE CALCIOSOLENIA This genus produces rhombic coccoliths. We are
not aware of any previously published _Ks_ value for _Calciosolenia_ and so have back calculated a _Ks_ value of 0.007 based on values for coccolith calcite (2.5 pg) and coccolith length (5
µm) published in Bollmann _et al_.83 based on circular polarizer birefringence-based measurements (n = 10) of coccolith thickness on specimens of Holocene age. Note that both the reported
mass estimate and the coccolith length are likely to be underestimated, as the rim of _Calciosolenia_ coccoliths is largely formed of vertically orientated crystal units (V-units) that are
not birefringent under either cross polarised or circular polarised light. Coccospheres of _Calciosolenia_ are strongly elongated and taper to a point at either end, giving them a shape that
is best described mathematically as a ‘double cone’64. Measuring the dimensions of _Calciosolenia_ coccospheres was sometimes challenging as the large coccospheres were often split across
more than one SEM image. In these instances, if appropriate, we estimated the mid-point of the coccosphere within the image that contained the largest portion of the coccosphere and used 2x
the measurement from this mid-point to the tip as the measure of length. For instances where the coccosphere was split over more than one SEM image, _C__N_ was counted from all SEM images
that contained parts of the coccosphere. RHABDOSPHAERACEAE ACANTHOICA Some coccoliths have well-developed spines, for example the polar coccoliths on _Acanthoica quattrospina_. The
additional calcite content of these spinose coccoliths has not been explicitly accounted for in our calculations of cell PIC. Spine lengths are variable, ca. 6–17 µm in some specimens, and
if we assume that the _Ks_ of these spinose coccoliths is broadly similar to that of _Rhabdosphaera stylifera_ (spine length for the calculation of calcite), the calcite of these spines
would be ca. 9–136 pg, compared to a coccolith calcite of ca. 1 pg for regular body coccoliths (although this is likely an overestimate as _Acanthoica_ spines have a less complex structure
and are more tapered than _Rhabdosphaera_). The coccosphere calcite of _Acanthoica_ specimens with spinose polar coccoliths is therefore underestimated. The average coccosphere thickness of
_Acanothoica_ is 0.7 µm and cell volume is on average 56% of coccosphere volume (n = 6) but can be smaller than this in some coccospheres (20–40% coccosphere volume). DISCOSPHAERA TUBIFERA
Coccolith calcite was calculated using trumpet width (Fig. 1h) following Young and Ziveri30 and _C__L_ measurements refer to trumpet width in this taxon unless otherwise mentioned. Trumpet
height was also measured so that coccosphere diameter including and excluding the trumpet height could be reported. Trumpet height can be variable across coccoliths on the same coccosphere
(e.g., Fig. 1h; maximum trumpet height can be up to three-times longer than minimum trumpet height84). Occasionally, the trumpet-like processes of the coccoliths were broken off from the
base plates. PALUSPHAERA AND RHABDOSPHAERA XIPHOS Coccospheres of _Palusphaera_ and _R. xiphos_ were difficult to distinguish at the resolution of the SEM images available. The primary
distinguishing characteristic is that _Palusphaera_ is monomorphic (all coccoliths have spines) but _Rhabdosphaera_ is dimorphic (some but not all coccoliths have spines) (see taxonomic
discussion in Archontikis and Young61). Coccospheres were distinguished where possible in the SEM images but were largely collapsed, which further inhibited confirmation of mono- or
dimorphism and made accurate _C__L_ and _C__N_ measurements extremely challenging. As direct cellular morphometrics could not be taken accurately, _Palusphaera_ and _Rhabdosphaera xiphos_
were not included in the database. RHABDOSPHAERA CLAVIGERA Two varieties of _R. clavigera_ are distinguished by how robust (var. _clavigera_) or delicate (var. _stylifera_) the spine
structure is, although there is evidence of intergrading between the two forms23,85. Here, we separate the two varieties as the more robust spine has a greater calcite content (reflected in
its higher _Ks_ value) compared to the more delicate form. Note that, similarly to _Discosphaera_, there is variation in spine length ca. 5–10% between coccoliths on the same coccosphere84.
_Rhabdosphaera_ coccospheres are dimorphic, formed of coccoliths with and without spines (Fig. 1c). For the calculation of coccosphere calcite, it was assumed that 50% of the coccoliths were
without spines (coccolith calcite for these coccoliths was calculated using _C__L_ and a _Ks_ value of 0.025, modified from _Acanthoica_30) and 50% of the coccoliths were with spines
(calcite was calculated using spine length and a fixed value of 5 µm was used if spine length could not be measured). It was usually possible to accurately measure coccosphere diameter both
including and excluding spine length and coccosphere size is reported as excluding spine length unless otherwise stated. SYRACOSPHAERACEAE CALCIOPAPPUS _Calciopappus_ coccospheres were
extremely rare in the samples investigated and were not observed as intact coccospheres. We therefore do not report any data for _Calciopappus_. MICHAELSARSIA _Michaelsarsia_ coccospheres
were rare in our samples (n = 5). Each coccosphere typically has ca. 8–12 appendages each formed of 3–4 coccoliths86 (Fig. 1i). To avoid underestimating _Michaelsarsia_ coccosphere calcite
by omitting the calcite in these appendages, we assigned a value of 12.5 pg calcite per appendage (informed by a typical length of 5.5 µm for each appendage segment, a _Ks_ value of 0.007
and assuming 4 segments per appendage). If the number of appendages is unknown, we assumed that 8 appendages were present (i.e., an addition of 100 pg calcite). This additional calcite was
then added to the calcite of the main body coccoliths, which were calculated using a _Ks_ value adapted from _Syracosphaera_. OPHIASTER The appendage coccoliths of _Ophiaster_ are also
elongated but with a more solid structure than those of _Michaelsarsia_ and each coccosphere usually has 4–7 long ‘arms’ of these osteoliths86. As the appendages of _Ophiaster_ are longer
and with a much greater number of component osteoliths, an approximation of the additional calcite represented by the appendages was estimated as follows: assuming that each of the
osteoliths has the same _Ks_ value as a _Florisphaera profunda_ nannolith (_Ks_ = 0.0332 based on the broadly similar shape) and each ‘arm’ contains on average 14 nannoliths of ca. 1.8 µm
length, this would equate to 0.47 pg calcite per nannolith, 6.6 pg calcite per ‘arm’ and 33 pg additional calcite per coccosphere in total (assuming on average 5 arms). This fixed estimate
of an additional 33 pg calcite for appendages was applied where appendages were clearly present (as many specimens are without appendages86). This estimate of appendage calcite is broadly
comparable to the calcite contained in the body coccoliths of many specimens (i.e., including a calcite estimate for the appendages approximately doubles cell calcite content calculated on
body coccoliths alone). SYRACOSPHAERA The genus _Syracosphaera_ is very diverse87 (ca. 40 currently recognised species23). There is substantial morphological diversity in this genus,
including coccolith size variability, different degrees of calcification, and a range of central area structures. However, four sub-groups of species with similar morphological
characteristics can be distinguished (Nannotax323 following Cros88, Young _et al_.60 and Kleijne and Cros87) and we used these morpho-groups as a starting point to simplify the choice of
shape factor for calculation of cellular calcite content in this genus. In addition, many _Syracosphaera_ species have circum-flagella coccoliths (CFCs) with relatively small spines and
exothecal coccoliths (XCs) that form a partial or complete outer layer to the coccosphere (Fig. 1j). This range of features make it impractical to determine new species-specific _Ks_ values
for the calculation of coccolith calcite for _Syracosphaera_. For the calculation of cellular calcite, we therefore proceeded as follows: * For _Syracosphaera_ species that have a
well-developed inner wall cycle and robust central area structures such as a boss or spine, we use the published _Ks_ value of Young and Ziveri30 for _Syracosphaera pulchra_ (_Ks_ = 0.03).
This includes species in the ‘_pulchra_ group’ and _Syracosphaera mediterranea_, which has a thick rim cycle and low central mound. For this morpho-group (e.g., Fig. 1j), cell volume was
assumed to be 65% of coccosphere volume (ranging between ca. 60 and 80% for species in the group). * For species without either of these features we use the _Ks_ values of Young and Ziveri30
for a ‘small’ _Syracosphaera_ species (_Ks_ = 0.015), even though the coccoliths in question may not be ‘small’. This includes ‘_borealis_ type’ species, ‘_nodosa_ group’ species (e.g.,
Fig. 1l), and _Syracosphaera maxima_. For this morpho-group, cell volume was assumed to be 75% of coccosphere volume. * For species that have well-developed rims but no central area bosses
or spines, we chose a relatively arbitrary ‘intermediate’ value of _Ks_ = 0.022. This includes species in the ‘_molischii_ group’ (other than ‘_borealis_ type’ species; e.g., Fig. 1k). For
this morpho-group, cell volume was assumed to be 75% of coccosphere volume. * For coccospheres that could not be confidently identified to species level, a _Ks_ value of 0.02 was used to
calculate coccosphere calcite. * The morphology of CFCs is often similar to that of the body coccoliths but with relatively small spines (Fig. 1l). We therefore used the same _Ks_ value for
CFCs as for body coccoliths. * There is a much broader morphological diversity across XCs, which makes it challenging to apply any ‘one size fits all’ approach to account for differences in
the calcite content of XCs in the calculation of cellular calcite across all _Syracosphaera_ species. The morphologies of XCs have previously been tentatively classified into 12 groups88
spanning disk-like coccoliths (e.g., _Syracosphaera anthos_, _Syracosphaera nodosa_, _Syracosphaera lamina_, _Syracosphaera nana_, _Syracosphaera bannockii_), undulating coccoliths that have
a distinct rim and central area (e.g., _Syracosphaera borealis_, _Syracosphaera molischii_, _Syracosphaera ossa_), dome-like/vaulted coccoliths (e.g., _Syracosphaera pulchra_), and
caneoliths with a murolith morphology (e.g., _Syracosphaera dilatata_, _Syracosphaera prolongata_, _Syracosphaera noroitica_). As XCs can be numerous (e.g., Fig. 1j,l), we attempted to
account broadly for the calcite of these XCs when present by counting the number of XCs present and using a _Ks_ value of 0.02 for all morphologies to calculate the additional calcite
contained in these coccoliths, recognising that this _Ks_ value is an underestimate in some instances and an overestimate in others. * An additional challenge presented by the presence of
XCs is that they partially or sometimes entirely obscure the visibility of body coccoliths (Fig. 1j). In these instances, a best estimate of _C__N_ was determined by either scaling up the
number of body coccoliths visible over an unobscured portion of the cell, doubling the _C__N_ of visible XCs when the size of XCs and body coccoliths are broadly comparable and the entire
coccosphere is covered in XCs, or estimating _C__N_ based on the range of _C__N_ typical for the species (this is noted in the raw data file when applied). * Many species of _Syracosphaera_
are known to have holococcolith stages. See the remarks on ‘Holococcolithophores’ for further details. HELICOSPHAERACEAE No specific considerations were necessary for _Helicosphaera_.
ALISPHAERACEAE _ALISPHAERA_ AND _ALISPHAERA_ POLYCRATER PHASE (POL) _Alisphaera_ is a genus with a very high _C__N_ per coccosphere and small coccoliths, especially in polycrater phase (Fig.
1f). Where _C__N_ could not be accurately counted and/or _C__L_ could not be accurately measured (particularly for polycrater phase coccospheres) from SEM images, the _C__N_ /_C__L_ range
of the species reported on Nannotax323 was used to guide our choice of a fixed _C__N_ of 400 and fixed _C__L_ of 0.8 µm for the calculation of cellular calcite. UMBELLOSPHAERACEAE
UMBELLOSPHAERA _Umbellosphaera_ coccoliths have umbelliform morphology, reminiscent of a flaring trumpet shape. Coccoliths forming the coccosphere have a range of distal shield sizes (as
seen easily from collapsed coccospheres; Fig. 1m) that leads _Umbellosphaera_ coccospheres to have a pseudo-multilayered structure that is reminiscent of a rainforest canopy structure, where
the distal shields of the largest coccoliths overlay the distal shields of smaller coccoliths (Fig. 1n) whilst the proximal shields sit adjacent on the cell surface. This presents
challenges for counting _C__N_ accurately and accurately measuring a ‘representative’ coccolith size in _Umbellosphaera_. For _C__N_, we therefore multiplied counted coccoliths by three
(rather than by two for regular, single-layer coccospheres) to better account for the ‘hidden’ layer of coccoliths underneath the visible outer layer. For intact coccospheres, _C__L_ and
_C__W_ of the largest coccolith distal shield were measured. For the coccolith size measure used in the calculation of cellular calcite, we also adjusted the maximum (measurable) distal
shield length to approximate an ‘average’ distal shield length that accommodated the variable lengths of _Umbellosphaera_ coccoliths that occur on the same coccosphere. For 20 collapsed
_Umbellosphaera_ coccospheres (e.g., Fig. 1m), we measured the distal shield length of every coccolith (_C__N_ of the collapsed coccospheres ranged from 10 to 43) and calculated coccolith
calcite and coccosphere calcite53 using the _Ks_ values of Young and Ziveri30 for _Umbellosphaera tenuis_ and _Umbellosphaera irregularis_ as appropriate, following Eq. 4 and Eq. 5. We then
back-calculated an ‘average’ _C__L_ corresponding to the calculated cellular calcite and _C__N_ of the cell and determined what percentage of the largest measured coccolith size (_C__L_ max;
Fig. 1m) this ‘average’ coccolith represented53, as follows: $$ \mbox{`} {average}\mbox{'}{C}_{L}({\mu m}^{3})=3\,\sqrt{\frac{{coccosphere\; calcite}}{{K}_{S}{\times C}_{N}\times
2.7}}$$ (7) For the 20 collapsed coccospheres measured, the ‘average’ coccolith size ranged between 55 and 81% of the largest measured coccolith53. For the calculation of _Umbellosphaera_
cellular calcite, we therefore decided to use a _C__L_ value of 0.7*_C__L_ max, where _C__L_ max is the coccolith length of the largest (measurable) coccolith on the intact coccosphere
(e.g., Fig. 1n). HOLOCOCCOLITHOPHORES AND NANNOLITHS HOLOCOCCOLITHOPHORES Coccolithophores have a haplo-diplontic life cycle, and typically produce heterococcoliths in the diploid life cycle
stage and holococcoliths (HOL) during the haploid life cycle stage (e.g., Fig. 1a). Holococcoliths are formed from small, simple rhombohedral crystals and are morphologically distinct from
heterococcoliths89. Holococcoliths do, however, occur in a range of different shapes and forms60. The calcite content of individual holococcoliths is currently unknown and we are not aware
of any published values for inorganic carbon per cell measured from cultured holococcolith-bearing strains from which coccolith calcite and therefore _Ks_ could be estimated. Here, we
therefore used an estimated _Ks_ value of 0.036 for all holococcolith-bearing coccospheres45. If it was not possible to directly count _C__N_ (as holococcoliths are often very small and
coccospheres have high _C__N_), _C__N_ was estimated by back-calculating _C__N_ using the surface area of the coccospheres and the surface area of the measured coccolith or an average _C__N_
of that species was used if available from another published source. We assumed that cell volume is 80% of coccosphere volume for all holococcolithophore coccospheres. NANNOLITH-BEARING
TAXA CERATOLITHUS _Ceratolithus_ produces three different lith morphologies depending on the life cycle stage, including distinctive horseshoe-shaped nannoliths (ceratoliths, CER). No intact
_Ceratolithus_ coccospheres were observed in any of our samples so they are not present in the database. FLORISPHAERA PROFUNDA The sheet-like, angular nannoliths of _Florisphaera_ are
arranged to form a coccosphere of strongly overlapping layers reminiscent of a globe artichoke (Fig. 1o). As a result, the coccosphere is very thick and, on SEM, it is not usually possible
to accurately determine how many layers of coccoliths are present. Light microscopy and SEM images indicate that ca. 6 to 12 layers of overlapping coccoliths can be present23,60,84 (Fig. 1o)
and that coccospheres may have up to ca. 220 nannoliths84,90. New LM measurements of corresponding coccosphere and internal cell diameter measurements from 20 _Florisphaera_ coccospheres57
indicate that cell size is typically 3–5 µm and cell volume is ca. 10% of coccosphere volume (ranging from 3% to 21%)57. We therefore estimate cell size from coccosphere size assuming that
cell volume is 10% of coccosphere volume. As precise estimates of _C__N_ were not attainable, we assumed that _Florisphaera_ coccospheres have a fixed _C__N_ of 145, broadly in line with the
average _C__N_ reported in the literature for coccospheres for _Florisphaera profunda_ var. _elongata_ and _F. profunda_ var. _profunda_84. The use of a fixed _C__N_ obviously introduces
significant uncertainty in the estimates of _Florisphaera profunda_ cellular calcite (assuming a _C__L_ of 2 µm and that the _C__N_ of _Florisphaera_ typically ranges between ca. 90 and 170,
the use of the fixed _C__N_ introduces an uncertainty of ca. 20–40%). DATA RECORDS The extant coccolithophore morphometric trait dataset described in this study can be freely downloaded at
zenodo53. This data record contains the main dataset “_1. Cellular morphometric trait dataset for extant coccolithophores from the Atlantic Ocean_” (Microsoft Excel file) and four supporting
files: “_2. Descriptor – Cellular morphometric trait dataset for extant coccolithophores from the Atlantic Ocean_” (Microsoft Word file), “_3. Coccolith size, coccolith calcite and cellular
calcite in collapsed Umbellosphaera coccospheres_” (Microsoft Excel file), “_4. Sensitivity of cellular calcite content to uncertainties in coccolith size, number of coccoliths per cell and
shape factors_” (Microsoft Excel file), and “_5. Partial coccolith length and coccolith length measurements_” (Microsoft Excel file). This section describes the content of these files. The
_Cellular morphometric trait dataset for extant coccolithophores from the Atlantic Ocean_ is formatted as a column-orientated table in Microsoft Excel format. Each row of the dataset
represents the raw morphometric data (number of coccoliths per cell, coccolith length, coccolith width, coccosphere diameter long axis, coccosphere diameter short axis) and calculated
morphological trait data (coccosphere volume, equivalent spherical coccosphere diameter, cellular calcite) obtained from a single intact coccosphere. Each entry includes ancillary
information, including sample identifier (station and water depth), SEM image number and taxonomic classification (family, genus, species). A column entitled ‘Additional information’ records
any additional details relevant to the accuracy of the measurements and calculated parameters, for instance stating the presence and amount of XCs additionally included in the cellular
calcite calculation or the presence of a multi-layered coccosphere where this has been accounted for in the estimation of cell size from coccosphere size. The contents of the four supporting
files associated with the _Cellular morphometric trait dataset_53 are as follows: File 2. _Descriptor_ describes the column headings contained in the _Cellular morphometric trait dataset_.
File 3. _Coccolith size, coccolith calcite and cellular calcite in collapsed Umbellosphaera coccospheres_ contains the coccolith size and coccolith calcite data measured from collapsed
coccospheres of _Umbellosphaera_ used to derive the adjustment of coccolith size used in the calculation of cellular calcite _in Umbellosphaera_. File 4. _Sensitivity of cellular calcite
content to uncertainties in coccolith size, number of coccoliths per cell and shape factors_ contains the data used to perform the sensitivity analysis of cellular calcite to variability in
morphometric parameters (see Technical Validation). File 5. _Partial coccolith length and coccolith length measurements_ contains coccolith measurements used to calculate the measurement
uncertainty associated with measuring partial _C__L_ on coccoliths where the rim of the distal shield is partly obscured by an overlapping coccolith. In addition to this data record
containing the _Cellular morphometric trait dataset for extant coccolithophores from the Atlantic Ocean_ dataset and supporting files53, two new further records56,57 associated with the
dataset are available: * The photo record “_Atlantic Meridional Transect (AMT) 14: scanning electron microscopy images of the coccolithophore community_” is freely available for download at
zenodo56 and contains the original SEM image files as tagged image file format (.tif) files. The identifier of each image file corresponds to the image number in the dataset _Cellular
morphometric trait dataset for extant coccolithophores from the Atlantic Ocean_53. * The dataset “_Extant coccolithophore cell size measurements_” (Microscoft Excel file) is freely available
for download at zenodo57 and contains newly collected measurements of coccosphere diameter and cell diameter on individual coccospheres used to derive species- and genus-specific values for
the percentage of coccosphere volume that is cell volume (_y_). An overview table of the genus-specific morphometric parameters used to estimate cellular calcite (e.g. taxon-specific shape
factors, size measurement used) and the range of estimated taxon-specific cellular calcite content that summarise the data contained in the _Cellular morphometric trait dataset for extant
coccolithophores from the Atlantic Ocean_53 is available as Supplementary Table 1. TECHNICAL VALIDATION TAXONOMIC COVERAGE OF THE CELLULAR MORPHOMETRIC TRAIT DATASET In total, 4712
morphometric measurements were measured on 1074 individual coccospheres representing 61 species from 25 genera (of the 31 most common genera that collectively constituted >95% total cell
numbers during AMT 1455) (Fig. 2). The number of measurements for each genus represented in the dataset largely reflects the relative abundance of different species in the samples examined,
as well as the structural stability of different coccosphere architectures (as some species coccospheres have a greater tendency to collapse, even under gentle filter pressure, and were
therefore not measured). Five genera (20%) have more than 50 coccospheres in the dataset and 14 genera (70%) have 10 or more coccospheres in the dataset (Fig. 2b). Some extant taxa are not
present in our dataset for the following reasons: (1) the species was not observed because it is generally not present in the temperate to equatorial latitudes of the oceanic Atlantic ocean
sampled during AMT-14 (e.g., _Coccolithus pelagicus_ that has a biogeographic distribution in the northern hemisphere high latitudes, the genus _Braarudosphaera_ that is known to thrive in
hyposaline conditions, the predominantly polar Family Papposphaeraceae, and the neritic genera in the Families Hymenomonadaceae and Pleurochrysidaceae); (2) the species is generally rare
(e.g., _Calyptrosphaera_, _Navilithus_, _Placorhombus_, and _Turrilithus_) or rare in AMT samples (140 of 171 species, or 82%, recorded during AMT-14 constituted <5% total cell numbers
and are defined as rare55); (3) The species was not observed as intact coccospheres in our samples because it has a structural susceptibility to collapse and therefore could not be
accurately measured (e.g., the deep photic species _Gladiolithus_). Additionally, we did not observe any _Scyphosphaera_ or _Pontosphaera_ coccospheres (intact or collapsed) in any of the
samples we examined for this study. _Scyphosphaera apsteinii_ was only recorded in two samples during AMT-14 and no species of the genus _Pontosphaera_ were reported in any samples55. SIZE
MEASUREMENT ERRORS USING MICROSCOPY (SEM AND LM) SEM has a theoretical resolution of ca. 0.003 to 0.05 µm dependent on the electron source, the accelerating voltage, and the working
distance. The optical resolution of LM is dependent on the objective used, the numerical aperture of the objective and the use of any immersion liquid. Coccolithophore researchers typically
use a 100x/1.30 oil-immersion objective, which has an optical resolution of 0.26 µm. Combined with the pixel resolution of the SEM images, all _C__L_ and ∅ measurements53 therefore have a
maximum error of ca. ±0.12 µm. The LM measurements of coccosphere and cell size used to calculate the percentage of coccosphere volume that is cell volume (_y_)57 should be assumed to have a
minimum error of ±0.26 µm. Note that the new size measurements taken here from SEM images53 and from LM images57 were not specifically controlled with a calibration standard (e.g.,
calibration microbeads)91,92. COMPARISON OF MORPHOMETRIC-BASED ESTIMATES OF COCCOLITH CALCITE WITH OTHER ESTIMATION METHODS Due to the very small size of coccoliths (ca. 1 to 20 µm) and
coccospheres (ca. 3 to 40 µm), the mass of individual coccoliths and coccospheres cannot be measured directly and so must be estimated using indirect approaches. There are several
established methods used to estimate the calcite mass of coccoliths and/or coccospheres, each with advantages and limitations that also depend on the characteristics of the material being
investigated (e.g., whether samples from culturing, plankton or sediment are used and whether coccolith calcite or coccosphere calcite is being measured). An extensive discussion of the
advantages and limitations of each method is beyond the scope of our study. We refer readers to the literature cited in the following paragraphs for specific technical developments,
particularly regarding birefringence-based methods for the quantification of coccolith calcite mass. Instead, we briefly summarise the main advantages and disadvantages of alternative
methods for estimating coccolith and cellular calcite content within the scope of comparing our estimated cellular calcite data with data derived from other methodologies. Estimates of
coccolith calcite based on morphological measurements30,93, as used here, can be performed using measurements from either light microscopy images (LM) or scanning electron microscopy (SEM),
as here. The high resolution of SEM images can resolve very small and lightly calcified details of coccoliths, often improving taxonomic classification of material relative to LM and
enabling high accuracy morphometric data to be collected across a wide range of coccolith sizes and morphologies. A disadvantage of SEM is that the two-dimensional nature of the images
allows only one hemisphere of the coccosphere to be viewed directly (Fig. 1) and therefore there must be a degree of estimation in the total number of coccoliths per cell. This is an
advantage of LM, where the focal depth can be adjusted to observe and count each coccolith in the coccosphere4,66,68. Alternatives to morphometric-based calcite estimates include
birefringence-based mass estimates83,94,95, which are often used for calcite mass estimates of individual coccoliths. Technical advances in the calibration of birefringence-based methods for
coccolith mass estimation have been made in recent years32,83,96,97,98,99, notably using circular polarized light rather than cross-polarised light and calibrating the calcite interference
colours to the Michel-Lévy chart with additional technical management of the microscope, light source and imaging settings32,83. Despite these technical advances, mass estimates based on
birefringence are limited by both the orientation of the crystal(s) in the coccolith and by coccolith thickness, which does have a detection limit: thickness limits imposed by optical
physics are 1.34 µm32,83 to a maximum of 1.58 µm32 after which greyscale values repeat themselves. Thickness limits as high as 1.70 µm have also been published99. Birefringence-based methods
work well for species with smaller or thinner coccoliths, such as _E. huxleyi_, but underestimate the calcite mass of coccoliths that exceed thicknesses of ca. 1.4 µm (e.g., larger
specimens of _Coccolithus_, _Helicosphaera_ and _Calcidiscus_)83. In addition to coccolith thickness limits, birefringence-based approaches systematically underestimate coccolith calcite
mass in species where some or all of the coccolith is formed of vertically-arranged crystal units (V-units) that are not birefringent under either cross polarised or circular polarised light
(e.g., _Coccolithus_ spp., _Calcidiscus_ spp., _Umbilicosphaera_ spp.)65,83,94,99. Birefringence is also unsuitable for coccolith mass estimation when the coccolith is part of an intact
coccosphere (as measured here), where overlapping coccoliths can increase the calcite brightness leading to significant calcite overestimation100. Another commonly used approach in culturing
studies is the analytical determination of mean calcite per cell through assays for total sample particulate carbon and particulate organic carbon (from an acidified sample), where total
sample calcite is the difference between the two values (the inorganic carbon) and cellular calcite is determined by dividing the value by the number of cells present on the filter (when
cells per millilitre and number of millilitres filtered are known). This approach does not capture intraspecific variability in cellular calcite and has the additional disadvantage that any
‘additional calcite’, e.g. from dead cells or shed coccoliths, is also averaged into the cellular calcite value and this can lead to the overestimation of mean cellular calcite52.
Assay-derived cellular calcite estimates are effectively restricted to _E. huxleyi_, _Gephyrocapsa_ spp., _Coccolithus_ spp., _Calcidiscus_ spp., _Syracosphaera pulchra_ and _Helicosphaera
carteri_, i.e., species that have been used in monospecific culturing experiments27. It is not possible to use assay-based analysis for the determination of taxon-specific cellular calcite
content from a mixed population sample (i.e., a plankton sample from the field), as the nature of this analysis will produce a meaningless average value spanning all cells, regardless of
their taxonomic affinity. This method is, however, well suited to estimating total organic and inorganic carbon content of a population. Morphometric-based calcite estimates have been shown
to compare well to estimates of coccolith calcite derived using other methodologies. Birefringence-based estimates of coccolith calcite are consistently in good agreement with
morphometric-based estimates of coccolith calcite for species within the coccolith size and thickness range for which this method is optimised, especially when samples sizes are large enough
to account for intraspecific variability65,83,94. In one study32, the calcite mass of coccoliths measured using both the same morphometric-based method as here and a birefringence-based
method on the same samples showed good agreement in Noelaerhabdaceae and Umbellosphaeraceae coccoliths. For _E. huxleyi_ and small placolith coccoliths, mean calcite mass based on _C__L_ and
the recommended Ks value of 0.0230 was 1.7 pg _versus_ 2.2 pg derived through a birefringence-based method32. For _Umbellosphaera_ spp. coccoliths, the morphometric-based estimate of mean
coccolith calcite mass was 7.1 pg compared to a birefringence-based estimate of 7.5 pg32. A recent direct comparison study33 also showed that coccolith mass estimates for four strains of _E.
huxleyi_ obtained from morphometric data using SEM (as here) agreed well with estimates obtained through birefringence measurements of coccolith thickness using bidirectional circular
polarisation97,99 with LM (mean calcite mass of Type A coccoliths was 2.09 pg based on morphometrics _versus_ 2.02 pg based on birefringence, mean calcite mass of Type BC coccoliths was 1.71
pg based on morphometrics _versus_ 1.82 pg based on birefringence; differences were not significant)33. For species in the orders Coccolithales (e.g., _Coccolithus, Calcidiscus_,
_Umbilicosphaera_) and Zygodiscales (e.g., _Helicosphaera_), Linge Johnsen and Bollmann32 showed that coccolith calcite mass estimates calculated using birefringence-based methods were lower
than morphometric-based approaches. They explain that the coccolith calcite mass of these species is systematically underestimated by birefringence-based methods because their coccolith
thickness exceeds the theoretical thickness limit of 1.34 µm32. We are not aware of other studies where multiple methodologies for coccolith mass estimates have been directly compared on the
same material for species with a broad range of coccolith thicknesses. Morphometric-based estimates of both coccolith and coccosphere calcite mass have also been shown to agree well with
coccolith and coccosphere calcite mass values determined through opto-electrochemical dissolution34,35. QUANTIFYING SOURCES OF UNCERTAINTY IN MORPHOMETRIC-BASED ESTIMATES OF CELLULAR CALCITE
The morphometric-based calcite estimation method used here30 incorporates a number of (necessary) assumptions and sources of uncertainty. We summarise relevant caveats that have been raised
previously in the literature30 and, based on our own observations, highlight additional methodological points that users of the dataset should be aware of: * We use species- or
genus-specific _Ks_ values based on published _Ks_ values where available. If no published _Ks_ for a species or genus are available, we have either adopted the existing _Ks_ value of a
species with a very similar morphology or adjusted the _Ks_ value of a species with similar morphology to account for the presence, absence or different degree of morphological features that
are likely to increase or decrease the original _Ks_ value. The value, source and justification for all _Ks_ values used to estimate coccolith PIC in the database are detailed in
Supplementary Table 1. * Within-species, _Ks_ values derived from individual coccolith measurements vary by ca. ±20%30. We note that the species-specific shape factors recommended by Young
and Ziveri30 are based on measurements from a small number of individual coccoliths that are therefore unlikely to fully represent the range of shape factor variability within each species.
For example, a study focusing on _E. huxleyi_72 showed strain-specific variability in _Ks_, ranging between 0.014 and 0.022 across seven morphotype A strains. A subsequent study on natural
_E. huxleyi_ populations71 emphasised that within-morphotype variability in coccolith mass can be substantial and that changes in coccolith size and coccolith thickness can be decoupled. *
Here, we assume that within-species coccolith shape and thickness remains constant even if coccolith size changes, although this is not always the case72. Coccolith thickness (and therefore
mass) can vary independently of coccolith length and thickness variability can also be restricted to certain areas of the coccolith, such as changes in central tube cycle thickness71,101,102
or number and thickness of coccolith elements. As variation in coccolith size has a much stronger influence on coccolith volume than coccolith shape30, we did not account for shape
variability with size in our coccosphere calcite calculations. It was also not possible to quantify variability in coccolith thickness across individual coccospheres in our samples and use
this information to further refine our estimates coccolith calcite mass because methods to determine coccolith thickness are based on the birefringence of calcite in LM and our data is
collected from SEM images. * Within a species, coccolith size can vary considerably as a function of growth environment and genotypic variability. Even within a single genetic clone,
coccolith size can vary by ca. 40% across individual cells as a function of variable environment and growth phase4,69. Many studies estimate species calcite using a mean species-specific
coccolith length value. As we measure coccosphere-specific _C__L_, we circumvent uncertainty that would be introduced by within-species size variability when using a mean _C__L_ for all
individual coccospheres of that species. * A more relevant source of uncertainty for our measurements is that coccolith size (or spine length or other morphometric parameters used for
calcite calculation) on a single coccosphere can also vary by up to ca. 30%85,103. This may occur through several mechanisms104: (1) cell size changes through the daily cell division cycle,
from smallest immediately following cell division to largest just prior to cell division103 and during G1 interphase of the cell division cycle105,106. As heterococcolith coccolithogensis
occurs intracellularly107,108,109, cell size at the time of coccolith formation can limit maximum coccolith dimensions. Coccoliths formed early in the daily cell cycle would therefore be
likely to be slightly smaller than those formed later on; (2) As coccolith size is responsive to environmental stressors22,52,110,111,112,113,114,115,116, coccoliths produced under stressful
conditions may have different sizes from those produced before exposure to the stressful condition. As approximately half of the coccoliths forming the coccosphere of the parent cell are
divided equally amongst two daughter cells on cell division, cells will inherit a diminishing proportion of coccoliths of a different size originally from the parent cell (grown under
different environmental conditions) over successive cell division cycles. It may therefore take several generations of coccolith production and growth before the coccosphere is composed of
solely post-exposure coccoliths; (3) Small differences in the duration of coccolithogenesis by the cell could feasibly result in slight differences in coccolith size; (4) Large coccolith
size variability on the same coccosphere is also a taxonomic feature of several species, which are highlighted in the genus descriptions in the Methods. We measure a single, approximately
flat-lying coccolith on the upper surface of the coccosphere that we assume is representative of the size of all other coccoliths forming the coccosphere. However, it is likely that some
coccoliths in the coccosphere are slightly smaller (and therefore have less calcite) and others are slightly larger (and therefore have more calcite). This is one of the larger sources of
uncertainty in our calculations of coccosphere calcite from a single coccolith measurement (see below). For example, Beuvier _et al_.103 determined a threefold range in coccolith calcite
(7–23 pg) within a coccosphere of _Gephyrocapsa oceanica_ due largely to differences in coccolith size of 20–30%. A large range in coccolith size within the same coccosphere is a
morphological feature of _Umbellosphaera_ (Fig. 1m) and our measurements on collapsed coccospheres reveal that the difference in size between the smallest and largest coccoliths forming the
coccosphere ranged from as little as 2.43 µm to as much as 8.27 µm, leading to a range in coccolith calcite within the same coccosphere of 2.4–28.4 pg coccolith−1 in _Umbellosphaera_53. For
many species, the arrangement of coccoliths in the coccosphere rarely offers multiple, flat-lying coccoliths on the upper surface of the coccosphere for measurement (where the edge of the
coccolith is not obscured by other coccoliths) or the _C__N_ is high to very high, making it unreasonable to measure a representative selection of coccoliths on each coccosphere. As
coccolith size variation is particularly notable in _Umbellosphaera_, we have specifically accommodated intra-coccosphere variation in _C__L_ in our estimates of cellular calcite, which is
described in the Methods. We investigated the sensitivity of cellular calcite estimated using morphometrics (Eqs. 4 and 5) to variability in coccolith size, number of coccoliths per cell,
and _Ks_ value to better quantify the magnitude of uncertainty introduced by the (necessary) assumptions made in our methodology (Fig. 3). For coccospheres of _Gephyrocapsa mullerae_,
_Umbilicosphaera hulburtiana_, _Syracosphaera_ spp., and _Discosphaera tubifera_ (representing a selection of different morphological types and degrees of calcification), we re-calculated
cellular calcite content by systematically varying _Ks_ and _C__L_ by ±5–30%. These ranges of variability are guided by the range of _Ks_ reported by Young and Ziveri30 and Linge Johnsen _et
al_.72 for _E. huxleyi_ and the range of _C__L_ on individual coccospheres reported for _Gephyrocapsa_103. To assess sensitivity to uncertainty in _C__N_ counts, we varied _C__N_ of each
coccosphere by ±2 coccoliths and ±5 coccoliths. Uncertainty in coccolith size (i.e., the measured coccolith is not representative of other coccoliths in the coccosphere) has by far the
largest impact in estimated cellular calcite, irrespective of species, altering estimated cellular calcite by a minimum of ca. ±15% (for 5% uncertainty in _C__L_) and decreasing cellular
calcite content by a maximum of 65% or increasing it by a maximum of 120% for a 30% uncertainty in _C__L_ (Fig. 3). The large impact of _C__L_ uncertainty on estimated cellular calcite
content arises because _C__L_ is cubed in the calculation of coccolith calcite (Eq. 4). We consider a _C__L_ uncertainty of 30%, whilst possible, to be an unreasonably large uncertainty that
would only occur if the largest or smallest coccolith out of all the coccoliths on the coccosphere was measured103. A more reasonable level of uncertainty in measured _C__L_ of ±10–15%
between coccoliths on the same coccosphere results in estimated cellular calcite content decreasing/increasing by ca. 30% (10% _C__L_ uncertainty) to decreasing by 39% or increasing by 52%
(15% _C__L_ uncertainty). Variability of _Ks_ across individual coccoliths of, for example, ±20%30 results in cellular calcite estimates directly changing by ±20%. Errors in counting the
number of coccoliths per cell can occur, and we estimated these to be ca. ±1–5 coccoliths per cell at most for the majority of coccospheres observed using SEM but conservatively as high as
10–40 coccoliths per cell counting error in species like the polycrater phase of _Alisphaera_ that have several hundred very small coccoliths (Fig. 1f). Coccolith counting errors affect
estimated cellular calcite proportionally to both the number of coccoliths per cell (i.e., the relative percentage counting error) and coccolith size (i.e., the calcite mass of each
miscounted coccolith), thus giving a range of sensitivity in our analysis (Fig. 3). For the four species investigated, _C__N_ ±2 resulted in a cellular calcite uncertainty of 2–22% and
_C__N_ ±5 resulted in a cellular calcite uncertainty of 5–55% for coccospheres with _C__N_ ranging between 9 and 96. _C__N_ counting errors are least likely in species with lower _C__N_
and/or larger _C__L_ and increase in likelihood in species whose coccospheres are comprised of very high numbers of coccoliths, although the typically small _C__L_ of these higher-_C__N_
species acts to minimise the overall impact of counting errors on cellular calcite estimations. Overall, it is reasonable to assume that slight differences in _Ks_ and _C__L_ between
coccoliths on the same coccosphere and minor _C__N_ counting errors introduces an uncertainty of ca. 5–40% on estimated cellular calcite. QUANTIFYING SOURCES OF UNCERTAINTY IN ESTIMATION OF
CELL SIZE FROM COCCOSPHERE SIZE The percentage of coccosphere volume that is cell volume (_y_) determined through LM varies between individual coccospheres29,57,69,117 but the intraspecific
range in _y_ is overall relatively low for many species. Based on 2850 measurements of coccosphere diameter and cell diameter (LM-based) for cultured _Calcidiscus_ and _Helicosphaera_69 and
_Coccolithus_ plankton samples68,117, the percentage of coccosphere volume that was cell volume was relatively consistent across hundreds of individual coccospheres, varying by only 12–22%
(10th to 90th percentiles of the data; cellular _y_ for _Helicosphaera_ = mean 51% ranging between 45% and 58%, cellular _y_ for _Calcidiscus_ = mean 42% ranging between 32% and 52%, and
cellular _y_ for _Coccolithus_ = mean 45% ranging between 37% and 52%). As these species all have ‘heavily calcified’ coccolith morphologies (i.e., coccoliths are comparatively thick and
solid structures) and coccospheres with strongly overlapping coccolith arrangements, we estimate that the variability in _y_ in species with thinner coccoliths and those that have a lesser
degree of or no overlapping coccolith arrangement (e.g., species bearing murolith- and planolith-type coccolith morphologies) is less than _y_ ±10–15%. The relationship between coccosphere
volume and cell volume is much more variable in _Florisphaera profunda_ (up to _y_ ±80%), _Umbellosphaera_ spp. (up to _y_ ±75%), _Oolithotus antillarum_ (up to _y_ ±37%), and _Acanthoica_
spp. (up to _y_ ±63%). These species have staggered, overlapping coccoliths arrangements that produce a very thick coccosphere relative to size of the cell and where additional
(pseudo-)layers can be added with no change in cell size. The value of _y_ for _Algirosphaera robusta_ (up to _y_ ±60%) depends on the variability in the height of central area processes,
the morphology of which makes it impossible to measure coccosphere diameter with and without the height of the processes. Additionally, coccospheres of _Umbilicosphaera sibogae_ contain
multiple cells within a single coccosphere, making it extremely challenging to quantify _y_ and its variability. For these specific taxa, the use of a fixed value for _y_ was unavoidable but
introduces uncertainty of ca. 30–60% in cell size estimates from coccosphere volume. USAGE NOTES This dataset53 is generated from 16 plankton samples obtained from the temperate to
equatorial Atlantic Ocean during 2004 and is therefore particularly well-suited for addressing research questions that focus on this region. As environmental conditions influence cell
physiology, phenotype expression, biogeography and species’ evolution, coccolithophore populations present in different ocean basins, latitudes and/or hydrographic regimes may exhibit
different ranges of morphological traits43,82,115,118,119 and species’ morphological traits have been shown to vary on a range of short-term (daily to seasonal) to longer-term (multi-annual
to millennial) timescales4,102,120,121,122,123,124,125,126. As such, users of the dataset should note that the range of morphometric traits in the dataset are not necessarily representative
of the full range of morphologies exhibited by each species globally and that the range of morphological traits present at specific temporal or spatial scales are dependent on the diversity
of ecotypes present in the population. This may especially be true for species represented by a limited number of entries in the dataset (Fig. 2; Supplementary Table 1). A simple indicator
of the range of morphometric data observed in the dataset relative to published literature is reported in Supplementary Table 1 for initial context. Coccolithophore taxonomy is under
constant refinement through morphological and, increasingly, genomic studies. We therefore strongly encourage users of the dataset to confirm the most up to date taxonomic identifier of each
species in the dataset before use, for example using the Nannotax3 website23 as this resource is constantly maintained, provides details of previous species synonyms, and provides extensive
references to primary literature. Users of the dataset may wish to use one of the several published cell volume to cell biomass relationships29,67,127 to further convert the cell size data
in the dataset into estimates of cellular organic carbon (biomass). Identical morphometric measurements to those presented here can also be made on intact fossil coccospheres preserved in
marine sediments66,68,128,129. Using the same morphometric-based method30, the cellular calcite of extinct calcareous nannoplankton species can be estimated and used to investigate evolution
in cellular calcite content through time31,66,68,130. CODE AVAILABILITY No custom code was used for the generation or processing of the datasets used in this study. REFERENCES * Ziveri, P.
_et al_. Pelagic calcium carbonate production and shallow dissolution in the North Pacific Ocean. _Nat Commun_ 14, 805 (2023). Article ADS CAS PubMed PubMed Central Google Scholar *
Daniels, C. J. _et al_. A global compilation of coccolithophore calcification rates. _Earth Syst Sci Data_ 10, 1859–1876 (2018). Article ADS Google Scholar * Sheward, R. M. Cycling carbon
with coccolithophores. _Nat Geosci_ 15, 758–759 (2022). Article ADS CAS Google Scholar * Sheward, R. M., Poulton, A. J., Gibbs, S. J., Daniels, C. J. & Bown, P. R. Physiology
regulates the relationship between coccosphere geometry and growth phase in coccolithophores. _Biogeosciences_ 14, 1493–1509 (2017). Article ADS CAS Google Scholar * Ziveri, P., de
Bernardi, B., Baumann, K. H., Stoll, H. M. & Mortyn, P. G. Sinking of coccolith carbonate and potential contribution to organic carbon ballasting in the deep ocean. _Deep Sea Res II Top
Stud Oceanogr_ 54, 659–675 (2007). Article ADS Google Scholar * Westbroek, P. _et al_. A model system approach to biological climate forcing. The example of Emiliania huxleyi. _Glob
Planet Change_ 8, 27–46 (1993). Article ADS Google Scholar * Suchéras-Marx, B. & Henderiks, J. Downsizing the pelagic carbonate factory: Impacts of calcareous nannoplankton evolution
on carbonate burial over the past 17 million years. _Glob Planet Change_ 123, 97–109 (2014). Article ADS Google Scholar * Broecker, W. & Clark, E. Ratio of coccolith CaCO3 to
foraminifera CaCO3 in late Holocene deep sea sediments. _Paleoceanography_ 24, PA3205 (2009). Article ADS Google Scholar * Milliman, J. Production and accumulation of calcium carbonate in
the ocean: Budget of a nonsteady state. _Global Biogeochem Cycles_ 7, 927–957 (1993). Article ADS CAS Google Scholar * Berelson, W. M. _et al_. Relating estimates of CaCO3 production,
export, and dissolution in the water column to measurements of CaCO3 rain into sediment traps and dissolution on the sea floor: A revised global carbonate budget. _Global Biogeochem Cycles_
21, GB1024 (2007). Article ADS Google Scholar * Archer, D. & Maier-Reimer, E. Effect of deep-sea sedimentary calcite preservation on atmospheric CO2 concentration. _Nature_ 367,
260–263 (1994). Article ADS CAS Google Scholar * Rost, B. & Riebesell, U. Coccolithophores and the biological pump: responses to environmental changes. in _Coccolithophores: from
molecular processes to global impact_ (eds. Thierstein, H. R. & Young, J. R.) vol. Springer 99–125 (Springer, Berlin, 2004). * Boudreau, B. P., Middelburg, J. J. & Luo, Y. The role
of calcification in carbonate compensation. _Nat Geosci_ 11, 894–900 (2018). Article ADS CAS Google Scholar * Ridgwell, A. & Zeebe, R. E. The role of the global carbonate cycle in
the regulation and evolution of the Earth system. _Earth Planet Sci Lett_ 234, 299–315 (2005). Article ADS CAS Google Scholar * Riebesell, U. _et al_. Reduced calcification of marine
plankton in response to increased atmospheric CO2. _Nature_ 407, 364–366 (2000). Article ADS CAS PubMed Google Scholar * Iglesias-Rodriguez, M. D. _et al_. Phytoplankton calcification
in a high-CO2 world. _Science_ 320, 336–40 (2008). Article ADS CAS PubMed Google Scholar * Lohbeck, K. T., Riebesell, U. & Reusch, T. B. H. Adaptive evolution of a key phytoplankton
species to ocean acidification. _Nat Geosci_ 5, 346–351 (2012). Article ADS CAS Google Scholar * Beaufort, L. _et al_. Sensitivity of coccolithophores to carbonate chemistry and ocean
acidification. _Nature_ 476, 80–3 (2011). Article CAS PubMed Google Scholar * O’Dea, S. A. _et al_. Coccolithophore calcification response to past ocean acidification and climate change.
_Nat Commun_ 5, 5363 (2014). Article ADS PubMed Google Scholar * Kroeker, K. J., Kordas, R. L., Crim, R. N. & Singh, G. G. Meta-analysis reveals negative yet variable effects of
ocean acidification on marine organisms. _Ecol Lett_ 13, 1419–1434 (2010). Article PubMed Google Scholar * Sheward, R. M., Liefer, J. D., Irwin, A. J. & Finkel, Z. V. Elemental
stoichiometry of the key calcifying marine phytoplankton _Emiliania huxleyi_ under ocean climate change: A meta-analysis. _Glob Chang_. _Biol_ 29, 4259–4278 (2023). CAS Google Scholar *
Gebühr, C., Sheward, R. M., Herrle, J. O. & Bollmann, J. Strain-specific morphological response of the dominant calcifying phytoplankton species _Emiliania huxleyi_ to salinity change.
_PLoS One_ 16, e0246745 (2021). Article PubMed PubMed Central Google Scholar * Young, J. R., Bown, P. R. & Lees, J. A. Nannotax3 website, http://www.mikrotax.org/Nannotax3/ (2023). *
Young, J. R., Geisen, M. & Probert, I. A review of selected aspects of coccolithophore biology with implications for paleobiodiversity estimation. _Micropaleontology_ 51, 267–288
(2005). Article Google Scholar * Séférian, R. _et al_. Tracking Improvement in Simulated Marine Biogeochemistry Between CMIP5 and CMIP6. _Curr Clim Change Rep_ 6, 95–119 (2020). Article
PubMed PubMed Central Google Scholar * Probert, I. & Houdan, A. The laboratory culture of coccolithophores. in _Coccolithophores: From Molecular Processes to Global Impact_ (ed
Thierstein, H. R. & Young, J. R.) 217–249 (2004). * Krumhardt, K. M., Lovenduski, N. S., Iglesias-rodriguez, M. D. & Kleypas, J. A. Coccolithophore growth and calcification in a
changing ocean. _Prog Oceanogr_ 159, 276–295 (2017). Article ADS Google Scholar * Gafar, N. A., Eyre, B. D. & Schulz, K. G. A comparison of species specific sensitivities to changing
light and carbonate chemistry in calcifying marine phytoplankton. _Sci Rep_ 9, 2486 (2019). Article ADS PubMed PubMed Central Google Scholar * Villiot, N., Poulton, A. J., Butcher, E.
T., Daniels, L. R. & Coggins, A. Allometry of carbon and nitrogen content and growth rate in a diverse range of coccolithophores. _J Plankton Res_ 43, 511–526 (2021). Article CAS
PubMed PubMed Central Google Scholar * Young, J. R. & Ziveri, P. Calculation of coccolith volume and it use in calibration of carbonate flux estimates. _Deep-Sea Res Pt II_ 47,
1679–1700 (2000). Article ADS Google Scholar * Henderiks, J. Coccolithophore size rules — Reconstructing ancient cell geometry and cellular calcite quota from fossil coccoliths. _Mar
Micropaleontol_ 67, 143–154 (2008). Article ADS Google Scholar * Linge Johnsen, S. A. & Bollmann, J. Segmentation, retardation and mass approximation of birefringent particles on a
standard light microscope. _J Microsc_ 280, 30–50 (2020). Article Google Scholar * Valença, C. R., Beaufort, L., Hallegraeff, G. M. & Müller, M. N. Technical note: A comparison of
methods for estimating coccolith mass. _Biogeosciences_ 21, 1601–1611 (2023). Article Google Scholar * Yang, M. _et al_. Opto‐electrochemical dissolution reveals coccolith calcium
carbonate content. _Angewandte Chemie_ 133, 21167–21174 (2021). Article ADS Google Scholar * Yang, M. _et al_. Single-entity coccolithophore electrochemistry shows size is no guide to the
degree of calcification. _Env Sci, Adv_ 1, 156–163 (2022). Article CAS Google Scholar * Hernández, A. S. R. _et al_. Coccolithophore biodiversity controls carbonate export in the
Southern Ocean. _Biogeosciences_ 17, 245–263 (2020). Article ADS Google Scholar * Baumann, K.-H., Boeckel, B. & Frenz, M. Coccolith contribution to South Atlantic carbonate
sedimentation. in _Coccolithophores: From Molecular Processes to Global Impact_ (ed Thierstein, H. R. & Young, J. R.) 367-402 (2004). * Broerse, A. T. C., Ziveri, P., van Hinte, J. E.
& Honjo, S. Coccolithophore export production, species composition, and coccolith-CaCO3 fluxes in the NE Atlantic (34° N 21° W and 48° N 21° W). _Deep-Sea Res Pt II_ 47, 1877–1905
(2000). Article ADS Google Scholar * Poulton, A. J. _et al_. Coccolithophore dynamics in non-bloom conditions during late summer in the central Iceland Basin (July-August 2007). 55,
1601–1613 (2010). * Poulton, A. J., Young, J. R., Bates, N. R. & Balch, W. M. Biometry of detached _Emiliania huxleyi_ coccoliths along the Patagonian Shelf. _Mar Ecol Prog Ser_ 443,
1–17 (2011). Article ADS CAS Google Scholar * Guerreiro, C. V. _et al_. Carbonate fluxes by coccolithophore species between NW Africa and the Caribbean: Implications for the biological
carbon pump. _Limnol Oceanogr_ 66, 3190–3208 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Triantaphyllou, M. V., Ziveri, P. & Tselepides, A. Coccolithophore
export production and response to seasonal surface water variability in the oligotrophic Cretan Sea (NE Mediterranean). _Micropaleontology_ 50, 127–144 (2004). Article Google Scholar *
Charalampopoulou, A. _et al_. Environmental drivers of coccolithophore abundance and calcification across Drake Passage (Southern Ocean). _Biogeosciences_ 13, 5917–5935 (2016). Article ADS
Google Scholar * Daniels, C. J., Tyrrell, T., Poulton, A. J. & Young, J. R. A mixed life-cycle stage bloom of _Syracosphaera bannockii_ (Borsetti and Cati, 1976) Cros _et al_. 2000
(Bay of Biscay, April 2010). _Journal of Nannoplankton Research_ 34, 31–35 (2014). Article Google Scholar * Daniels, C. J. _et al_. Species-specific calcite production reveals _Coccolithus
pelagicus_ as the key calcifier in the Arctic Ocean. _Mar Ecol Prog Ser_ 555, 29–47 (2016). Article ADS CAS Google Scholar * Agnini, C., De Bernardi, B. & Erba, E. Volume and
carbonate production estimates of early Palaeogene calcareous nannofossils. _Lethaia_ 50, 58–68 (2016). Article Google Scholar * Erba, E. & Tremolada, F. Nannofossil carbonate fluxes
during the Early Cretaceous: Phytoplankton response to nutrification episodes, atmospheric CO2, and anoxia. _Paleoceanography_ 19, PA1008 (2004). Article ADS Google Scholar *
Preiss-Daimler, I., Baumann, K.-H. & Henrich, R. Carbonate budget mass estimates for Neogene discoasters from the Equatorial Atlantic (Ceara Rise: ODP Site 927). _J Micropalaeontol_ 31,
169–178 (2012). Article ADS Google Scholar * Daniels, C. J., Sheward, R. M. & Poulton, A. J. Biogeochemical implications of comparative growth rates of _Emiliania huxleyi_ and
_Coccolithus_ species. _Biogeosciences_ 11, 6915–6925 (2014). Article ADS Google Scholar * Langer, G. _et al_. Distinct physiological responses of _Coccolithus braarudii_ life cycle
phases to light intensity and nutrient availability. _Eur J Phycol_ 58, 58–71 (2023). Article CAS Google Scholar * Gerecht, A. C., Šupraha, L., Edvardsen, B., Langer, G. & Henderiks,
J. Phosphorus availability modifies carbon production in _Coccolithus pelagicus_ (Haptophyta). _J Exp Mar Biol Ecol_ 472, 24–31 (2015). Article CAS Google Scholar * Rosas-Navarro, A.,
Langer, G. & Ziveri, P. Temperature effects on sinking velocity of different _Emiliania huxleyi_ strains. _PLoS One_ 13, e0194386 (2018). Article PubMed PubMed Central Google Scholar
* Sheward, R. M. & Poulton, A. J. Cellular morphological trait dataset for extant coccolithophores from the Atlantic Ocean. _zenodo_ https://doi.org/10.5281/zenodo.11483788 (2024). *
Aiken, J. _et al_. The Atlantic Meridional Transect: Overview and synthesis of data. _Prog Oceanogr_ 45, 257–312 (2000). Article ADS Google Scholar * Poulton, A. J., Holligan, P. M.,
Charalampopoulou, A. & Adey, T. R. Coccolithophore ecology in the tropical and subtropical Atlantic Ocean: New perspectives from the Atlantic meridional transect (AMT) programme. _Prog
Oceanogr_ 158, 150–170 (2017). Article ADS Google Scholar * Poulton, A. J. & Sheward, R. M. Scanning electron microscopy images of the coccolithophore community during Atlantic
Meridional Transect (AMT) 14. _zenodo_ https://doi.org/10.5281/zenodo.10571820 (2024). * Young, J. R. Extant coccolithophore size measurements. _zenodo_
https://doi.org/10.5281/zenodo.10572754 (2024). * Robinson, C. _et al_. The Atlantic Meridional Transect (AMT) Programme: A contextual view 1995-2005. _Deep-Sea Res II_ 53, 1485–1515 (2006).
ADS Google Scholar * Charalampopoulou, A., Poulton, A. J., Tyrrell, T. & Lucas, M. I. Irradiance and pH affect coccolithophore community composition on a transect between the North
Sea and the Arctic Ocean. _Mar Ecol Prog Ser_ 431, 25–43 (2011). Article ADS CAS Google Scholar * Young, J. _et al_. A guide to extant coccolithophore taxonomy. _Journal of Nannoplankton
Research, Special Issue_ (2003). * Archontikis, O. A. & Young, J. R. A reappraisal of the taxonomy and biodiversity of the extant coccolithophore genus _Palusphaera_ (Rhabdosphaeraceae,
Prymnesiophyceae). _Phycologia_ 60, 589–602 (2021). Article CAS Google Scholar * Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis.
_Nat Methods_ 9, 671–675 (2012). Article CAS PubMed PubMed Central Google Scholar * Young, J. R. _et al_. Guidelines for coccolith and calcareous nannofossil terminology.
_Palaeontology_ 40, 875–912 (1997). Google Scholar * Sun, J. & Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. _J Plankton Res_ 25, 1331–1346
(2003). Article Google Scholar * Fuertes, M.-Á., Flores, J.-A. & Sierro, F. J. The use of circularly polarized light for biometry, identification and estimation of mass of coccoliths.
_Mar Micropaleontol_ 113, 44–55 (2014). Article ADS Google Scholar * Gibbs, S. J., Sheward, R. M., Bown, P. R., Poulton, A. J. & Alvarez, S. A. Warm plankton soup and red herrings:
Calcareous nannoplankton cellular communities and the Palaeocene-Eocene Thermal Maximum. _Philos Trans R Soc A_ 376, 20170075 (2018). Article ADS Google Scholar * Menden-Deuer, S. &
Lessard, E. J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. _Limnol Oceanogr_ 45, 569–579 (2000). Article ADS CAS Google Scholar * Gibbs, S.
J. _et al_. Species-specific growth response of coccolithophores to Palaeocene–Eocene environmental change. _Nat Geosci_ 6, 218–222 (2013). Article ADS CAS Google Scholar * Sheward, R.
M., Poulton, A. J., Gibbs, S. J., Daniels, C. J. & Bown, P. R. Coccosphere geometry measurements from culture experiments on the coccolithophore species _Calcidiscus leptoporus_,
_Calcidiscus quadriperforatus_ and _Helicosphaera carteri_. _Pangaea_, https://doi.org/10.1594/PANGAEA.865403 (2016). * Hoffmann, R. _et al_. Insight into _Emiliania huxleyi_ coccospheres by
focused ion beam sectioning. _Biogeosciences_ 12, 825–834 (2015). Article ADS Google Scholar * Linge Johnsen, S. A. & Bollmann, J. Coccolith mass and morphology of different
Emiliania huxleyi morphotypes: A critical examination using Canary Islands material. _PLoS One_ 15, e0230569 (2020). Article CAS PubMed PubMed Central Google Scholar * Linge Johnsen, S.
A., Bollmann, J., Gebuehr, C. & Herrle, J. O. Relationship between coccolith length and thickness in the coccolithophore species _Emiliania huxleyi_ and _Gephyrocapsa oceanica_. _PLoS
One_ 14, e0220725 (2019). Article CAS PubMed PubMed Central Google Scholar * Young, J. R. & Westbroek, P. Genotypic variation in the coccolithophorid species _Emiliania huxleyi_.
_Mar Micropaleontol_ 18, 5–23 (1991). Article ADS Google Scholar * Hagino, K. _et al_. New evidence for morphological and genetic variation in the cosmopolitan coccolithophore _Emiliania
huxleyi_ (Prymnesiophyceae) from the COX1b-ATP4 genes. _J Phycol_ 47, 1164–1176 (2011). Article CAS PubMed Google Scholar * Balch, W. M., Kilpatrick, K. A., Holligan, P. M. & Cucci,
T. Coccolith production and detachment by _Emiliania huxleyi_ (Prymnesiophyceae). _J Phycol_ 29, 566–575 (1993). Article Google Scholar * Paasche, E. Roles of nitrogen and phosphorus in
coccolith formation in _Emiliania huxleyi_ (Prymnesiophyceae). _Eur J Phycol_ 33, 33–42 (1998). Article Google Scholar * Boeckel, B. & Baumann, K.-H. Vertical and lateral variations in
coccolithophore community structure across the subtropical frontal zone in the South Atlantic Ocean. _Mar Micropaleontol_ 67, 255–273 (2008). Article ADS Google Scholar * Van Der Wal,
P., Kempers, R. S. & Veldhuis, M. J. W. Production and downward flux of organic matter and calcite in a North Sea bloom of the coccolithophore _Emiliania huxleyi_. _Mar Ecol Prog Ser_
126, 247–265 (1995). Article ADS Google Scholar * Malinverno, E., Triantaphyllou, M. V. & Dimiza, M. D. Coccolithophore assemblage distribution along a temperate to polar gradient in
the West Pacific sector of the Southern Ocean (January 2005). _Micropaleontology_ 61, 489–506 (2015). Article Google Scholar * Bendif, E. M. _et al_. Repeated species radiations in the
recent evolution of the key marine phytoplankton lineage _Gephyrocapsa_. _Nat Commun_ 10, 4234 (2019). Article ADS PubMed PubMed Central Google Scholar * Filatov, D. A., Bendif, E. M.,
Archontikis, O. A., Hagino, K. & Rickaby, R. E. M. The mode of speciation during a recent radiation in open-ocean phytoplankton. _Current Biology_ 31, 5439–5449.e5 (2021). Article CAS
PubMed Google Scholar * Bendif, E. M. _et al_. Rapid diversification underlying the global dominance of a cosmopolitan phytoplankton. _ISME Journal_ 17, 630–640 (2023). Article PubMed
PubMed Central Google Scholar * Bollmann, J. Technical Note: Weight approximation of coccoliths using a circular polarizer and interference colour derived retardation estimates - (The CPR
Method). _Biogeosciences_ 11, 1899–1910 (2014). Article ADS Google Scholar * Kahn, A. C. M. Morphometric variability in the extant coccolithophores: implications for the fossil record.
_Rutgers Graduate School_, https://rucore.libraries.rutgers.edu/rutgers-lib/23445/ (2007). * Kahn, A. & Aubry, M. Intraspecific morphotypic variability in the family Rhabdosphaeraceae.
52, 317–342 (2006). * Young, J. R., Andruleit, H. & Probert, I. Coccolith function and morphogenesis: Insights From appendage-bearing coccolithophores of the family Syracosphaeraceae
(Haptophyta). _J Phycol_ 45, 213–226 (2009). Article PubMed Google Scholar * Kleijne, A. & Cros, L. Ten new extant species of the coccolithophore _Syracosphaera_ and a revised
classification scheme for the genus. _Micropaleontology_ 55, 425–462 (2009). Article Google Scholar * Cros, L. Variety of exothecal coccoliths of _Syracosphaera_. _J Nannoplankton Res_ 22,
41–51 (2000). Article Google Scholar * Young, J., Davis, S., Bown, P. & Mann, S. Coccolith ultrastructure and biomineralisation. _J Struct Biol_ 126, 195–215 (1999). Article CAS
PubMed Google Scholar * Yang, T.-N. & Wei, K.-Y. How many coccoliths are there in a coccosphere of the extant coccolithophorids? — a compilation. _J Nannoplankton Res_ 25, 7–15 (2003).
Article Google Scholar * Nederbragt, A. J., Francus, P., Bollmann, J. & Soreghan, M. J. Image calibration, filtering, and processing. in _Image Analysis, Sediments and
Paleoenvironments_ (ed. Francus, P.) 35-58 (Kluwer Academic Publishers, Dordrecht, The Netherlands, 2004). * Lamoureux, S. F. & Bollmann, J. Image Acquisition. in _Image Analysis,
Sediments and Paleoenvironments_ (ed. Francus, P.) 11-34 (Kluwer Academic Publishers, Dordrecht, The Netherlands, 2004). * Beaufort, L. & Heussner, S. Coccolithophorids on the
continental slope of the Bay of Biscay - Production, transport and contribution to mass fluxes. _Deep-Sea Res II_ 46, 2147–2174 (1999). ADS Google Scholar * Beaufort, L. Weight estimates
of coccoliths using the optical properties (birefringence) of calcite. _Micropaleontology_ 51, 289–297 (2005). Article Google Scholar * Cubillos, J. C., Henderiks, J., Beaufort, L.,
Howard, W. R. & Hallegraeff, G. M. Reconstructing calcification in ancient coccolithophores: Individual coccolith weight and morphology of _Coccolithus pelagicus_ (sensu lato). _Mar
Micropaleontol_ 92–93, 29–39 (2012). Article ADS Google Scholar * Ridgwell, A. _et al_. From laboratory manipulations to Earth system models: scaling calcification impacts of ocean
acidification. _Biogeosciences_ 6, 2611–2623 (2009). Article ADS CAS Google Scholar * Beaufort, L., Barbarin, N. & Gally, Y. Optical measurements to determine the thickness of
calcite crystals and the mass of thin carbonate particles such as coccoliths. _Nat Protoc_ 9, 633–42 (2014). Article CAS PubMed Google Scholar * González-Lemos, S., Guitián, J., Fuertes,
M. Á., Flores, J. A. & Stoll, H. M. Technical note: An empirical method for absolute calibration of coccolith thickness. _Biogeosciences_ 15, 1079–1091 (2018). Article ADS Google
Scholar * Beaufort, L., Gally, Y., Suchéras-Marx, B., Ferrand, P. & Duboisset, J. Technical note: A universal method for measuring the thickness of microscopic calcite crystals, based
on bidirectional circular polarization. _Biogeosciences_ 18, 775–785 (2021). Article ADS Google Scholar * Jin, X. & Liu, C. Estimating coccolithophore PIC:POC based on coccosphere and
coccolith geometry. _J Geophys Res Biogeosci_ 128, e2022JG007355 (2023). Article ADS CAS Google Scholar * D’Amario, B., Ziveri, P., Grelaud, M. & Oviedo, A. _Emiliania huxleyi_
coccolith calcite mass modulation by morphological changes and ecology in the Mediterranean Sea. _PLoS One_ 13, e0201161 (2018). Article PubMed PubMed Central Google Scholar *
Triantaphyllou, M. _et al_. Seasonal variation in _Emiliania huxleyi_ coccolith morphology and calcification in the Aegean Sea (Eastern Mediterranean). _Geobios_ 43, 99–110 (2010). Article
MathSciNet Google Scholar * Beuvier, T. _et al_. X-ray nanotomography of coccolithophores reveals that coccolith mass and segment number correlate with grid size. _Nat Commun_ 10, 751
(2019). Article ADS CAS PubMed PubMed Central Google Scholar * Suchéras-Marx, B. _et al_. Coccolith size rules – What controls the size of coccoliths during coccolithogenesis? _Mar
Micropaleontol_ 170, 102080 (2022). Article ADS Google Scholar * Müller, M. N., Antia, A. N. & LaRoche, J. Influence of cell cycle phase on calcification in the coccolithophore
_Emiliania huxleyi_. _Limnol Oceanogr_ 53, 506–512 (2008). Article ADS Google Scholar * Walker, C. E. _et al_. The requirement for calcification differs between ecologically important
coccolithophore species. _New Phytologist_ 220, 147–162 (2018). Article CAS PubMed Google Scholar * Drescher, B., Dillaman, R. M. & Taylor, A. R. Coccolithogenesis in _Scyphosphaera
apsteinii_ (Prymnesiophyceae). _J Phycol_ 48, 1343–1361 (2012). Article PubMed Google Scholar * Taylor, A. R., Russell, M. A., Harper, G. M., Collins, T. F. T. & Brownlee, C. Dynamics
of formation and secretion of heterococcoliths by _Coccolithus pelagicus_ ssp. _braarudii_. _Eur J Phycol_ 42, 125–136 (2007). Article Google Scholar * Van De Locht, R. _et al_.
Ultrastructure and crystallography of nanoscale calcite building blocks in _Rhabdosphaera clavigera_ coccolith spines. _Cryst Growth Des_ 14, 1710–1718 (2014). Article Google Scholar * de
Bodt, C., Van Oostende, N., Harlay, J., Sabbe, K. & Chou, L. Individual and interacting effects of pCO2 and temperature on _Emiliania huxleyi_ calcification: study of the calcite
production, the coccolith morphology and the coccosphere size. _Biogeosciences_ 7, 1401–1412 (2010). Article ADS Google Scholar * Bollmann, J., Klaas, C. & Brand, L. E. Morphological
and physiological characteristics of _Gephyrocapsa oceanica_ var. _typica_ Kamptner 1943 in culture experiments: Evidence for genotypic variability. _Protist_ 161, 78–90 (2010). Article
PubMed Google Scholar * Perrin, L., Probert, I., Langer, G. & Aloisi, G. Growth of the coccolithophore _Emiliania huxleyi_ in light- and nutrient-limited batch reactors: Relevance for
the BIOSOPE deep ecological niche of coccolithophores. _Biogeosciences_ 13, 5983–6001 (2016). Article ADS CAS Google Scholar * Faucher, G., Riebesell, U. & Thomas Bach, L. Can
morphological features of coccolithophores serve as a reliable proxy to reconstruct environmental conditions of the past? _Climate of the Past_ 16, 1007–1025 (2020). Article ADS Google
Scholar * Müller, M. N. _et al_. Influence of CO2 and nitrogen limitation on the coccolith volume of _Emiliania huxleyi_ (Haptophyta). _Biogeosciences_ 9, 4155–4167 (2012). Article ADS
Google Scholar * Bollmann, J. & Herrle, J. O. Morphological variation of _Emiliania huxleyi_ and sea surface salinity. _Earth Planet Sci Lett_ 255, 273–288 (2007). Article ADS CAS
Google Scholar * von Dassow, P. _et al_. Overcalcified forms of the coccolithophore _Emiliania huxleyi_ in high CO2 waters are not pre-adapted to ocean acidification. _Biogeosciences_ 15,
1515–1534 (2018). Article ADS Google Scholar * Sheward, R. M., Daniels, C. J. & Gibbs, S. J. Growth rates and biometric measurements of coccolithophores (_Coccolithus pelagicus_,
_Coccolithus braarudii_, _Emiliania huxleyi_) during experiments. _Pangaea_, https://doi.org/10.1594/PANGAEA.836841 (2014). * Bollmann, J. & Klaas, C. Morphological variation of
_Gephyrocapsa oceanica_ Kamptner 1943 in plankton samples: Implications for ecologic and taxonomic interpretations. _Protist_ 159, 369–381 (2008). Article PubMed Google Scholar * Renaud,
S., Ziveri, P. & Broerse, A. T. C. Geographical and seasonal differences in morphology and dynamics of the coccolithophore _Calcidiscus leptoporus_. _Mar Micropaleontol_ 46, 363–385
(2002). Article ADS Google Scholar * Kottmeier, D. M., Terbrüggen, A., Wolf-Gladrow, D. A. & Thoms, S. Diel variations in cell division and biomass production of _Emiliania
huxleyi_—Consequences for the calculation of physiological cell parameters. _Limnol Oceanogr_ 65, 1781–1800 (2020). Article ADS CAS Google Scholar * Knappertsbusch, M., Cortes, M. Y.
& Thierstein, H. R. Morphologic variability of the coccolithophorid _Calcidiscus leptoporus_ in the plankton, surface sediments and from the Early Pleistocene. _Mar Micropaleontol_ 30,
293–317 (1997). Article ADS Google Scholar * Renaud, S. & Klaas, C. Seasonal variations in the morphology of the coccolithophore _Calcidiscus leptoporus_ off Bermuda (N. Atlantic). _J
Plankton Res_ 23, 779–795 (2001). Article Google Scholar * Herrmann, S., Weller, A. F., Henderiks, J. & Thierstein, H. R. Global coccolith size variability in Holocene deep-sea
sediments. _Mar Micropaleontol_ 82–83, 1–12 (2012). Article ADS Google Scholar * Young, J. Size variation of Neogene _Reticulofenestra_ coccoliths from Indian Ocean DSDP Cores. _J
Micropalaeontol_ 9, 71–85 (1990). Article ADS Google Scholar * Henderiks, J. & Renaud, S. Coccolith size increase of _Calcidiscus leptoporus_ offshore Morocco during the Last Glacial
Maximum: an expression of enhanced glacial productivity? _Journal of Nannoplankton_. _Research_ 26, 1–12 (2004). Google Scholar * Meier, K. J. S., Beaufort, L., Heussner, S. & Ziveri,
P. The role of ocean acidification in _Emiliania huxleyi_ coccolith thinning in the Mediterranean Sea. _Biogeosciences_ 11, 2857–2869 (2014). Article ADS CAS Google Scholar * Montagnes,
D. J. S., Berges, J. A., Harrison, P. J. & Taylor, F. J. R. Estimating carbon, nitrogen, protein, and chlorophyll _a_ from volume in marine phytoplankton. _Limnol Oceanogr_ 39, 1044–1060
(1994). Article ADS CAS Google Scholar * Bown, P. R., Gibbs, S. J., Sheward, R., O’Dea, S. A. & Higgins, D. Searching for cells: the potential of fossil coccospheres in
coccolithophore research. _Journal of Nannoplankton Research_ 34, 5–21 (2014). Article Google Scholar * Sheward, R. M., Gibbs, S. J., Higgins, D., Bown, P. R. & Herrle, J. O.
Coccosphere geometry dataset for Eocene and Oligocene calcareous nannoplankton. _zenodo_ https://doi.org/10.5281/zenodo.11473074 (2024). * Henderiks, J. & Pagani, M. Refining ancient
carbon dioxide estimates: Significance of coccolithophore cell size for alkenone-based _p_CO2 records. _Paleoceanography_ 22, PA3202 (2007). Article ADS Google Scholar Download references
ACKNOWLEDGEMENTS The authors acknowledge the support of the scientists and crew associated with the AMT 14 cruise, especially Patrick Holligan who pioneered the AMT coccolithophore research
and Tim Adey and Anastasia Charalampopoulou who performed the original coccosphere abundance counts and taxonomic identification. AMT 14 was supported by the UK Natural Environment Research
Council (NERC) through the Atlantic Meridional Transect consortium (NER/O/S/2001/00680). This is contribution number 404 of the AMT programme. The authors also acknowledge the contribution
of Aimee Coggins to the initial assessments of coccosphere thickness and cell volume in extant coccolithophores as part of her MSci thesis (University of Southampton, 2015). This research is
funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number 447581699 to R.M.S. A.J.P., J.dV. and F.F.M. acknowledge funding from the UKRI NERC (Cocco
Trait, NE/X001261/1). AJP is also supported by co-funding from the Horizon Europe Funding programme and UK Research and Innovation (OceanICU, agreement number 10054454). J.O.H. and R.M.S.
received funding through Project A4 of the VeWA consortium (_Past Warm Periods as Natural Analogues of our high-CO__2_ _Climate Future_) by the LOEWE programme of the Hessen Ministry of
Higher Education, Research and the Arts, Germany. FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Geosciences,
Goethe-University Frankfurt, Frankfurt am Main, Germany Rosie M. Sheward & Jens O. Herrle * The Lyell Centre for Earth and Marine Science, Heriot-Watt University, Edinburgh, UK Alex J.
Poulton * Department of Earth Sciences, University College London, London, UK Jeremy R. Young * BRIDGE, School of Geographical Sciences, University of Bristol, Bristol, UK Joost de Vries
& Fanny M. Monteiro * Biodiversity and Climate Research Centre (BIK-F), Frankfurt am Main, Germany Jens O. Herrle Authors * Rosie M. Sheward View author publications You can also search
for this author inPubMed Google Scholar * Alex J. Poulton View author publications You can also search for this author inPubMed Google Scholar * Jeremy R. Young View author publications You
can also search for this author inPubMed Google Scholar * Joost de Vries View author publications You can also search for this author inPubMed Google Scholar * Fanny M. Monteiro View author
publications You can also search for this author inPubMed Google Scholar * Jens O. Herrle View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
The data collection and study design were conceived by R.M.S. and A.J.P. All morphometric data collection was conducted by R.M.S. with methodological consultation throughout with A.J.P.
J.R.Y. contributed new morphometric data used to define the _y_ variable and estimate cell diameter from coccosphere diameter. The manuscript was written by R.M.S. with contributions from
A.J.P., J.R.Y., J.dV., F.M.M. and J.O.H. CORRESPONDING AUTHOR Correspondence to Rosie M. Sheward. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY TABLE 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Sheward, R.M., Poulton, A.J., Young, J.R. _et al._ Cellular morphological trait dataset for extant coccolithophores from the Atlantic Ocean. _Sci Data_ 11, 720 (2024).
https://doi.org/10.1038/s41597-024-03544-1 Download citation * Received: 30 January 2024 * Accepted: 17 June 2024 * Published: 02 July 2024 * DOI: https://doi.org/10.1038/s41597-024-03544-1
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
