Brain-derived neurotrophic factor is regulated via myd88/nf-κb signaling in experimental streptococcus pneumoniae meningitis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _Streptococcus pneumoniae_ meningitis is an intractable disease of the central nervous system (CNS). Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophic family
and found to participate in the immune inflammatory response. In this study, we investigated if activation of the classical inflammatory signaling pathway, myeloid differentiation factor 88
(MyD88)/nuclear factor-kappa B (NF-κB), regulates BDNF expression in experimental _S. pneumoniae_ meningitis. MyD88 knockout (_myd88_−/−) mice and wild-type littermates were infected
intracisternally with _S. pneumoniae_ suspension. Twenty-four hours after inoculation, histopathology of brains was evaluated. Cytokine and chemokine in brains and spleens was analyzed using
ELISA. NF-κB activation was evaluated using EMSA. Cortical and hippocampal BDNF was assessed using RT-PCR and ELISA, respectively. BDNF promoter activity was evaluated using ChIP-PCR.
_myd88_−/− mice showed an obviously weakened inflammatory host response. This diminished inflammation was consistent with worse clinical parameters, neuron injury, and apoptosis. Deficiency
in MyD88 was associated with decreased BDNF expression. Furthermore, we identified a valid κB-binding site in the BDNF promoter, consistent with activation of NF-κB induced by inflammation.
To sum up, MyD88/NF-κB signaling has a crucial role in up-regulating BDNF, which might provide potential therapeutic targets for _S. pneumoniae_ meningitis. SIMILAR CONTENT BEING VIEWED BY
OTHERS CSF1R SIGNALING IS A REGULATOR OF PATHOGENESIS IN PROGRESSIVE MS Article Open access 23 October 2020 _MYCOPLASMA FERMENTANS_ INFECTION INDUCES HUMAN NECROTIC NEURONAL CELL DEATH VIA
IFITM3-MEDIATED AMYLOID-Β (1–42) DEPOSITION Article Open access 26 April 2023 NEUROINFLAMMATION INDUCES SYNAPTIC SCALING THROUGH IL-1Β-MEDIATED ACTIVATION OF THE TRANSCRIPTIONAL REPRESSOR
REST/NRSF Article Open access 15 February 2021 INTRODUCTION _Streptococcus pneumoniae_ meningitis is an invasive and often intractable disease of the central nervous system (CNS). Despite
effective antibiotics and application of vaccinations, such infection is still associated with an unacceptably high morbidity and mortality1. The main limitation to advance in prevention and
treatment of the disease is incomplete knowledge of its pathogenesis and pathophysiology. Generally, the host immune response, such as the activation of macrophages, production of cytokines
and chemokines, and migration of leukocytes, is believed to be the first line of defense in response to bacterial invasion during the process of _S. pneumoniae_ meningitis2. Toll-like
receptors (TLRs), which are widely expressed in central resident macrophages, sense antigens from microorganisms, leading to the recruitment of myeloid differentiation factor 88 (MyD88) and
the activation of downstream signaling pathways3, 4. MyD88 is crucial for the induction of a full innate inflammation response to most TLRs ligands, with the exception of TLR35. Furthermore,
the MyD88-dependent pathway elicits nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) activation, which drives robust gene expression of cytokines and
pro-inflammatory mediators6. However, increasing evidence has demonstrated that activation of NF-κB can lead to uncontrolled expression of those pro-inflammatory mediators, which contributes
to the pathogenesis of disease processes7. Innate immune response is now widely recognized as a double-edged blade possessing both protective and damaging properties8. There is now solid
evidence that intense inflammatory host response causes important damage to the brain, thus inducing unfavorable outcomes of meningitis9, 10. Brain-derived neurotrophic factor (BDNF) is a
member of the neurotrophic family and is widely expressed in the adult brain. In CNS, multiple cell types express BDNF including neurons and glia11. BDNF promotes neuronal survival,
maturation, and growth by binding to its high-affinity tropomyosin-related kinase receptor, type B (TrkB)12, 13. Dysfunction in the regulation of BDNF is associated with numerous disorders
of CNS, including Alzheimer’s disease (AD), multiple sclerosis (MS), depression, and unacceptable outcomes of bacterial meningitis14,15,16,17. Our previous study showed that increased
expression of BDNF following the acute _S. pneumonia_ meningitis was alleviated after antibiotic treatment18. Furthermore, Barichello _et al_.19 demonstrated that down-regulated BDNF
expression in the hippocampus was associated with cognition and memory deficiency in experimental _S. pneumoniae_ meningitis. Interestingly, administration of exogenous BDNF increased the
neuronal population in both the cortex and hippocampus, and reversed brain damage20. These findings indicate that regulatory expression of BDNF may be a part of the host inflammatory
response in _S. pneumoniae_ meningitis. However, the underlying regulatory mechanism is still not clear. A recent report has shown that TLR agonists up-regulate nerve growth factor (NGF) in
human intervertebral discs by activating and translocating NF-κB into the nucleus21. A tissue engineering study showed that hyaluronic acid-based hydrogels could attenuate inflammatory
receptor activity in an interleukin (IL)-1β-induced inflammation model of nucleus pulposus cells, with down-regulation of NGF and BDNF22. Pro-inflammatory factors including endotoxins,
cytokines, and oxidative stress have been reported to up-regulate BDNF in immune cells _in vitro_. In particular, tumor necrosis factor-alpha (TNF-α) has been demonstrated to increase BDNF
expression via the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway in primary astrocytes23. In this study, we aimed to investigate if activation
of the classical inflammatory signaling pathway, namely the MyD88/NF-κB signaling pathway, regulates BDNF expression in experimental _S. pneumoniae_ meningitis. RESULTS EFFECT OF MYD88
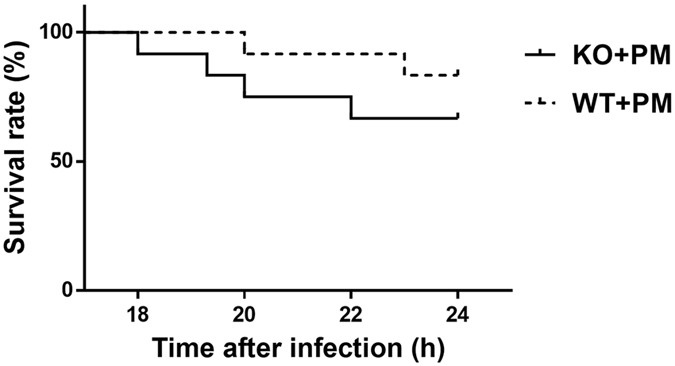
DEFICIENCY ON CHARACTERISTICS OF THE MENINGITIS AND HISTOPATHOLOGY As a result of disease progression following inoculation with _S. pneumoniae_, all infected mice began to exhibit clinical
symptoms of meningitis around 18 h after intracisternal injection, with loss of weight, hypothermia, lags in response and lethargy. Furthermore, the mortality rate was high during the acute
disease phase, between 18 and 24 h after infection (Fig. 1). Of the 12 infected wild-type mice, 2 (16.70%) died within 24 h after inoculation, while 4 of 12 (33.3%) infected _myd88_−/− mice
died during the same period. The survival rate of infected _myd88_−/− mice tended to be lower than infected wild-type mice; however, there was no significant variance (p > 0.05; log rank
test). Data from two-way ANOVA indicated significant interactions between the variables (MyD88 and meningitis) on weight loss [F (1,30) = 8.687, p < 0.01] and clinical scores [F (1,36) =
5.345, p = 0.027]. In addition, infected _myd88_−/− mice lost more weight and had markedly enhanced clinical scores for severity of the disease compared to the infected wild-type mice (Table
1). None of the control mice induced with pyrogen-free saline showed symptoms of infection within the same observation period. All of the infected mice had positive bacterial cultures of
the cerebellar homogenates but no pneumococci grew from the brains of uninfected mice. Representative examples of H&E-stained brain sections of each group are shown in Fig. 2A. Infected
wild-type mice showed vast inflammatory exudate in the subarachnoid cavity and ventricle, with hemorrhage-like spots in the parenchyma. However, the brains of infected _myd88_−/− mice showed
obviously weak inflammatory response in the subarachnoid space. This finding corroborated with the dramatic weak antibacterial properties of _myd88_−/− mice to intracerebral _S. pneumoniae_
infection. In addition, neuronal injury in hippocampus was analyzed by using Nissl staining. As shown in Fig. 2B, neuron loss was more remarkable in the hippocampus of infected _myd88_−/−
mice than in infected wild-type mice, with a more severely incomplete structure of neurons induced by intracerebral _S. pneumoniae_ infection. Furthermore, hippocampal apoptosis was
investigated by TUNEL staining. Two-way ANOVA indicated significant interactions between the variables (MyD88 and meningitis) on hippocampal apoptosis [F (1,12) = 8.089, p = 0.015]. _S.
pneumoniae_ infection caused obvious apoptosis in the hippocampal dentate gyrus as compared with the control groups (Fig. 2C), and the number of TUNEL-positive cells was significantly higher
in infected _myd88_−/− mice than in infected wild-type mice (Fig. 2D). EFFECT OF MYD88 DEFICIENCY ON INFLAMMATORY MEDIATOR PRODUCTION IN BRAINS AND SPLEENS At 24 h after infection, the
expression of cytokines and chemokines (i.e., TNF-α, IL-1β, IL-6, and IL-10) was evaluated by using ELISA. Two-way ANOVA indicated interactions significant between the variables (MyD88 and
meningitis) on these inflammatory mediator productions (except expression of IL-10 in cortex). Data from two-way ANOVA for interaction on TNF-α in cortex and spleens is [F (1,22) = 29.185, p
< 0.01] and [F (1,27) = 38.852, p < 0.01], respectively. Interactions between the variables on IL-1β is [F (1,20) = 11.845, p < 0.01] and [F (1,24) = 36.218, p < 0.01],
respectively in cortex and spleens. Data of interactions on IL-6 is [F (1,20) = 115.165, p < 0.01] and [F (1,28) = 13.383, p < 0.01]. In the cortex, a two-way ANOVA of IL-10 expression
indicated that there was no significant effect of the MyD88 group [F (1,18) = 2.244, p = 0.151], and there was no significant effect of the meningitis group [F (1,18) = 0.669, p = 0.424]
with no significant interaction [F (1,18) = 0.149, p = 0.704]. However, in the spleens, interactions were identified between MyD88 and meningitis [F (1,25) = 35.366, p < 0.01].
_Streptococcus pneumoniae_ infection led to massive cytokine and chemokine increase in both the cerebral cortex and spleen homogenates of wild-type mice (Fig. 3, except expression of IL-10
in cortex). In contrast, the expression of TNF-α, IL1-β, IL-6, and IL-10 was significantly attenuated in infected _myd88_−/− mice at the same time point, reflecting impaired immune
activation. MYD88 IS A MAJOR CONTRIBUTOR TO ACTIVATION OF NF-ΚB FOLLOWING _S. PNEUMONIAE_ ADMINISTRATION To further determine the role played by MyD88 in _S. pneumoniae_-induced activation
of NF-κB, EMSA was performed in nuclear extracts from brains derived from both _myd88_−/− and wild-type mice. NF-κB binding activation was significantly enhanced in infected wild-type mice
compared with wild-type control mice (Fig. 4). However, this bacteria-induced NF-κB binding activation was markedly attenuated in _myd88_−/− mice. These data are in agreement with published
studies24, 25 and indicate that MyD88 is required for _S. pneumoniae_-induced NF-κB binding activation in the CNS. MYD88 IS REQUIRED FOR _S. PNEUMONIAE_-MEDIATED INCREASE IN BDNF EXPRESSION
Our previous studies have demonstrated that BDNF increases both in the cortex and hippocampus during the acute phase of _S. pneumoniae_ meningitis, but decreases after antibiotics
treatment18. In the present study, we further explored if MyD88 activation could induce the expression of BDNF in _S. pneumoniae_ meningitis. At 24 h following inoculation, cortical cortex
and hippocampus homogenates were collected and analyzed for expression of BDNF at the transcriptional and translational level. Data from two-way ANOVA indicated significant interactions
between MyD88 and meningitis on BDNF expression. For BDNF mRNA [F (1,14) = 64.356, p < 0.01] and [F (1,20) = 9.731, p < 0.01], respectively in cortex and hippocampus. For BDNF protein
[F (1,21) = 48.726, p < 0.01] and [F (1,17) = 6.902, p = 0.018], respectively in cortex and hippocampus. Infected wild-type mice promoted a strong trend of increased BDNF mRNA expression
compared to saline controls (Fig. 5). Importantly, this bacteria-induced BDNF mRNA elevation was markedly attenuated in the cortical cortex and hippocampus isolated from _myd88_−/− mice.
Next, we analyzed the protein level of BDNF in the cortical cortex and hippocampus. Similarly, we found that increases in levels of BDNF protein observed at 24 h following _S. pneumoniae_
administration were absent in _myd88_−/− mice (Fig. 6). NF-ΚB DIRECTLY ACTS WITH BDNF PROMOTER EXPRESSION IN BV-2 STRAIN As shown in Fig. 7, the binding site of NF-κB to the BDNF promoter
was found by ChIP-PCR assay. Mouse GAPDH prime sets amplified mouse gapdh gene only in Input and RNA pol II group, examining validity of ChIP-PCR experiment (Fig. 7A). Then, one pair of
primers could amplify the ChIP products to mouse bdnf gene (about 300 bp) in NF-κB group (Fig. 7B). Thus, there is a consensus NF-κB binding site present in the BDNF promoter region.
DISCUSSION The present results demonstrate that _S. pneumoniae_ infection drives robust gene expression of cytokines and chemokines in the CNS and peripheral immune organs, along with BDNF
gene and protein expression in the cortex and hippocampus. The activity of MyD88/NF-κB signaling is not only crucial to innate immune response, but it is also required for BDNF expression.
These results identify a new insight into the regulatory mechanism mediating BDNF expression during _S. pneumoniae_ meningitis, thus contributing to a deep understanding of pathogenesis and
pathophysiology of this disease. _Streptococcus pneumoniae_ is still the most common cause of community-acquired meningitis in developing countries26. Current viewpoints are that when
bacteria enter the CNS, innate immune response is activated initially to limit bacteria diffusion as well as eliminate its components27. Although the inflammatory response can exert its
defensive role, it can also induce neurotoxic effects, which are associated with cell death and neurological sequelae8, 28. The inefficiency of the host immune response is assumed to be
associated with higher mortality of meningitis. Modulating inflammatory response and reducing side effects associated with excessive immune response have long been a hot-spot therapeutic
target during bacterial meningitis. In the past decades, numerous researchers have tried different adjuvant treatments involving dexamethasone, IL-1β receptor antagonist, and mood-stabilizer
lithium, among others, to improve the outcomes of _S. pneumoniae_ meningitis. However, the clinical efficacy remains barely satisfactory29,30,31. Better knowledge of the pathogenesis and
pathophysiology of this disease may be the sally port. Our previous results have demonstrated that BDNF increases during acute stage of _S. pneumoniae_ meningitis but decreases with time18,
32. Similarly, BDNF concentration in the peripheral blood and injured brain increases in humans and animals suffering from trauma and ischemic insult33, 34. These prior studies indicated
that besides its normal physiological functions, increased expression of BDNF in the CNS after a variety of insults indicates a neuro-restorative and neuroprotective role for this
neurotrophic factor. Clinically, BDNF has been suggested to increase neurogenesis of neural stem cells (NSCs) and has been potentially to reduce neurological sequelae associated with
meningitis and focal cerebral ischemia32, 35. It is estimated that there is not only the destruction of inflammatory factors but also a protective effect of neurotrophic factors. The two
systems combine to the pathophysiologic process of _S. pneumoniae_ meningitis. MyD88 has been identified as TLRs adapter molecule that plays a crucial role in initiating inflammatory host
immune responses to bacterial challenge mainly via TLRs engagement28. As it behaves dually in host immune defense, it is now widely recognized as a doubled-edged blade. MyD88-deficient mice
have been demonstrated to be highly susceptible to intracerebral infection with _Escherichia coli_ strain K136 and have a high mortality and severe bacteremia in infectious diseases37, 38.
In present study, we showed that absence of MyD88 decreased the resistance of mice to _S. pneumoniae_ meningitis, including more weight loss and worse clinical manifestations, with
diminishing neutrophil infiltration and subarachnoid hemorrhage, attenuated production of cytokines (TNF-α, IL-1β, and IL-6) and anti-inflammatory factor (IL-10) in both CNS and peripheral
regions. Although significant difference in mortality between two infectious groups has not been observed in our study, there was a tendency that infected _myd88_−/− mice had a lower
survival rate than infected wild-type mice. We speculate that the mortality difference would reach the level of statistical significance if we enlarge the number of animals. These results
are in accordance with previous studies regarding the key role for MyD88 in immune defense but also neurons injure in both Gram-positive and Gram-negative bacterial meningitis37, 38.
Moreover, the population of survival neurons significantly decreased and apoptosis body increased in hippocampus of infected _myd88_−/− mice compared with infected wild-type mice. Here, we
also found that MyD88 underlies the ability for immune response to enhance neurotrophic expression, as _myd88_−/− mice express significantly lower BDNF production after infected with
bacteria when compared with infected wild-type mice. Accordingly, this signaling adaptor molecule not only has a critical role in producing inflammatory factors during _S. pneumoniae_
meningitis, but also is essential to induce neuroprotective agent. Therefore, this signal point may potentially exert the ability to protect from neuronal injury and death via balancing
extent of inflammatory response and expression of neurotrophic elements. In addition to emphasizing the ability of MyD88 in up-regulation of BDNF during _S. pneumoniae_ meningitis, the
present study also verified the effect of the downstream transcription factor NF-κB involved in the process. The activation of MyD88 activates downstream signal pathways, thus inducing a
variety of transcription factors to translocate to the nucleus. NF-κB is one of the most major transcription factors, acting as a gate for immune response. As expected, our EMSA results
confirmed previous findings that MyD88 activation increased NF-κB activation and induced p65 translocation. Potential roles for NF-κB have long been suggested in inflammation and immune
response39 and recently in neuron survival. For example, blockade of NF-κB activity by pharmacological inhibitors attenuates CNS complications and provides protective effects for brain in
bacterial meningitis40, 41. A recent study found that activation of NF-κB increases expression of NGF in disc cells during disc degeneration21. Therefore, we presume that NF-κB may be
required for MyD88 to increase BDNF expression. To confirm this speculation, we performed ChIP-PCR to search for probable NF-κB binding sites with the promoter region of murine BDNF.
Excitingly, this search revealed a consensus NF-κB binding site present in the BDNF promoter region, confirming a direct proof for this transcription factor in regulating BDNF. Therefore, we
hypothesize that there is target point of NF-κB to both inflammation-related genes and neurotrophic elements. In conclusion, the present findings suggested that _S. pneumoniae_ meningitis
induces neurotoxic cytokines and chemokines, but also neuroprotective elements. The activity of MyD88/NF-κB signaling induced innate immune response, and this pathway is also required for
BDNF expression. However, how to modulate this key signal pathway to balance the effect of excessive inflammatory injury and neuroprotective survival need further investigation. BDNF
undoubtedly plays a neuroprotective effect during the process of _S. pneumoniae_ meningitis. Better understanding of how BDNF is regulated can be helpful to find a therapeutic target in
bacterial meningitis. METHODS MOUSE STRAINS AND EXPERIMENTAL DESIGN Six-week old MyD88 knockout (_myd88_−/−) mice that have been backcrossed with C57BL/6 mice for over 10 generations, as
well as wild-type littermates, were purchased from Jackson Laboratory (Bar Harbor, ME). We divided the mice into the following groups: (1) _myd88_−/− mice injected intracisternally with _S.
pneumoniae_ suspension (n = 12); (2) _myd88_−/− mice injected intracisternally with sterile saline (n = 8); (3) wild-type mice injected intracisternally with _S. pneumoniae_ suspension (n =
12); and (4) wild-type mice injected intracisternally with sterile saline (n = 8). The animal experiments were approved by the Animal Ethical and Welfare Committee of Xinhua Hospital
Affiliated to Shanghai Jiaotong University School of Medicine. All methods were performed in accordance with the relevant guidelines and regulations. We made efforts to minimize the number
of animals used and their suffering. INFECTING ORGANISMS We used the serotype III _S. pneumoniae_ strain, which was obtained from ATCC. To obtain the appropriate concentrations of bacteria,
serotype III _S. pneumoniae_ was cultured on a blood-agar plate for 18 h and then inoculated into Vital Aer Broth overnight at 37 °C in air with 5% CO2 for another 18 h to reach the
logarithmic phase. The bacteria were then centrifuged and washed twice with sterile saline. We used a nephelometer to achieve a concentration of approximately 1 × 104 colony forming units
(cfu)/ml by re-suspending the bacteria with sterile saline. ANIMAL MODEL OF MENINGITIS All mice (n = 40) were anesthetized with 1–1.5 ml/100 g 5% chloral hydrate by intraperitoneal
injection. A 10-μl volume containing either 1 × 104 cfu/ml _S. pneumoniae_ or sterile saline was injected intracisternally into each mouse. At 24 h after infection with _S. pneumoniae_ or
saline, the health status of the mice were assessed by weighting and by a clinical score: 0 = no apparent behavioral abnormality; 1 = moderate lethargy (apparent decrease of spontaneous
activity); 2 = severe lethargy (rare spontaneous movements, but walking after stimulation by the investigator); 3 = unable to walk; and 4 = dead42. After clinical evaluation, mice from all
groups were sacrificed at 24 h after inoculation to harvest brains and spleens. In order to document the development of bacterial meningitis, cerebellum homogenates were plated and cultured
on blood-agar plates. HISTOPATHOLOGICAL EVALUATION OF MURINE BRAIN SECTIONS At 24 h after inoculation, all surviving animals were anesthetized and perfused through the left ventricular with
50 ml normal saline. Then, the animals were decapitated, the brains were removed, followed by segmentation of the brains into 2 hemispheres. The left hemispheres were fixed in 4%
paraformaldehyde overnight at 4 °C, embedded in paraffin, and cut into 4-μm-thick coronal sections through the hippocampus. The sections were stained with hematoxylin & eosin (H&E)
(Beyotime Biotechnology, China) and cresyl violet (Sigma-Aldrich, USA) according to a standard protocol. Apoptosis-like cell death was determined by terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) immunofluorescence staining using the _In-Situ_ Cell Death Detection Kit (Roche, USA) according to the manufacturer’s instructions. QUANTIFICATION OF CYTOKINE
AND CHEMOKINE SECRETION Brains and spleens were sonicated in PBS buffer containing a proteinase inhibitor cocktail and centrifuged at 12000 g for 15 min to remove cellular debris. The
concentrations of TNF-α, IL-1β, IL-6, and IL-10 in supernatant were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, USA). Cytokine and chemokine
levels in brain homogenates were normalized to total brain weight and reported as picograms per milliliter. ELECTROPHORETIC MOBILITY SHIFT ASSAY FOR NF-ΚB ACTIVATION Nuclear extract
preparation and electrophoretic mobility shift assay (EMSA) were performed using the EMSA kit (Thermofisher, USA) as described previously, with some modifications34. Briefly, nuclear extract
(20 ug) was incubated with binding buffer and nonspecific oligonucleotides for 15 min, and then incubated with NF-κB biotin-labeled oligonucleotide probes (forward: 5′-AGT TGA GGG GAC TTT
CCC AGG C-3′; reverse: 5′-G CCT GGG AAA GTC CCC TCA ACT-3′) for another 15 min. For super shift assays, NF-κB antibody was added along with the binding buffer. Subsequently, samples were
separated by electrophoresis in a 5.5% polyacrylamide gel with 0.25 × Tris-borate-EDTA buffer. The retarded bands were detected by chemiluminescence. ANALYSES OF BDNF AT TRANSCRIPTIONAL AND
TRANSLATIONAL LEVEL Reverse transcription polymerase chain reaction (RT-PCR) was performed as previously described43. Briefly, total RNA from brain tissues (cerebral cortex and hippocampus)
were extracted using Trizol reagent (TaKaRa, Japan) and isolated using chloroform following the manufacturer’s instructions. RNA was converted to cDNA by using the Primer-Script One-Step
RT-PCR kit (TaKaRa). Real-time PCR was performed using the ABI7500 system (Applied Biosystems, Carlsbad, CA) using the SYBR Premix Dimmer Eraser kit (TaKaRa) to amplify the cDNA template.
The forward and reverse strand primers that were used to amplify the mRNA encoding mouse BDNF were ATTAGCGAGTGGGTCACAGC and TCAGTTGGCCTTTGGATACC, respectively. The primers were synthesized
by Shanghai Sangon Biological Engineering Technology Company Limited. BDNF gene expression in each sample was normalized to β-actin expression. The relative expression level of mRNAs was
calculated by the 2−ΔΔCt method. To assay BDNF synthesis, BDNF ELISA kit (Cusabio, China) was used to quantify protein concentration in cerebral cortex and hippocampus according to the
manufacturer’s instruction. CHROMATIN IMMUNOPRECIPITATION ASSAY Chromatin immunoprecipitation (ChIP) were performed with the BV-2 cell strain (a standard microglia cell stain). Cellular
protein-DNA complexes were cross-linked, isolated and fragmented. Immunoprecipitation was performed with NF-κB antibody (Millipore, USA) to enrich specific chromatin fragments. RNA pol II
antibody (Santa Cruz, USA) was added as positive control, and goat IgG was used as negative control. Antibody complexes were collected and washed, then reversed the cross-links. The amounts
of immunoprecipitated DNA were calculated by comparison to input DNA. The ChIP products were also analyzed by PCR. The primer sets used to amplify mouse _bdnf_ gene are listed in
supplementary data. STATISTICAL ANALYSIS The Shapiro-Wilk test and Levene test were used to distinguish between parametric and nonparametric values. Differences among groups were analyzed
using two-way ANOVA (MyD88 and meningitis) for parametric data, followed by Tukey’s post-hoc test; otherwise, the Mann-Whitney test was used, the intergroup comparisons were performed using
Wilcoxon’s tests. If not stated otherwise, the data was reported as the mean ± SD. Survival rates were compared by the log rank test. Differences were considered significant at p < 0.05.
All graphs were generated using Graph Pad Prism 5.0. Statistical analyses were performed using SPSS software version 18.0. REFERENCES * Backhaus, E. _et al_. Epidemiology of invasive
pneumococcal infections: manifestations, incidence and case fatality rate correlated to age, gender and risk factors. _BMC Infect Dis_ 16, 367, doi:10.1186/s12879-016-1648-2 (2016). Article
PubMed PubMed Central Google Scholar * Scheld, W. M., Koedel, U., Nathan, B. & Pfister, H. W. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. _J Infect
Dis_ 186 Suppl 2, S225–233, doi:JID020578 (2002). * Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. _Nat Immunol_ 11,
373–384, doi:10.1038/ni.1863 (2010). Article CAS PubMed Google Scholar * Carpentier, P. A., Duncan, D. S. & Miller, S. D. Glial toll-like receptor signaling in central nervous system
infection and autoimmunity. _Brain Behav Immun_ 22, 140–147, doi:S0889-1591(07)00211-5 (2008). * Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. _Cell_ 140,
805–820, doi:10.1016/j.cell.2010.01.022 (2010). Article CAS PubMed Google Scholar * Mitchell, J. A., Paul-Clark, M. J., Clarke, G. W., McMaster, S. K. & Cartwright, N. Critical role
of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. _J Endocrinol_ 193, 323–330, doi:193/3/323 (2007). * Bondeson, J., Foxwell, B., Brennan,
F. & Feldmann, M. Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares
anti-inflammatory mediators. _Proc Natl Acad Sci USA_ 96, 5668–5673 (1999). Article ADS CAS PubMed PubMed Central Google Scholar * Sellner, J., Tauber, M. G. & Leib, S. L.
Pathogenesis and pathophysiology of bacterial CNS infections. _Handb Clin Neurol_ 96, 1–16, doi:10.1016/S0072-9752(09)96001-8 (2010). Article PubMed Google Scholar * Meli, D. N.,
Christen, S., Leib, S. L. & Tauber, M. G. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. _Curr Opin Infect Dis_ 15, 253–257 (2002). Article
PubMed Google Scholar * Kim, K. S. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. _Nat Rev Neurosci_ 4, 376–385, doi:10.1038/nrn1103 (2003). Article CAS
PubMed Google Scholar * Riley, C. P., Cope, T. C. & Buck, C. R. CNS neurotrophins are biologically active and expressed by multiple cell types. _J Mol Histol_ 35, 771–783,
doi:10.1007/s10735-004-0778-9 (2004). Article CAS PubMed Google Scholar * McAllister, A. K., Katz, L. C. & Lo, D. C. Neurotrophins and synaptic plasticity. _Annu Rev Neurosci_ 22,
295–318, doi:10.1146/annurev.neuro.22.1.295 (1999). Article CAS PubMed Google Scholar * Binder, D. K. & Scharfman, H. E. Brain-derived neurotrophic factor. _Growth Factors_ 22,
123–131 (2004). Article CAS PubMed PubMed Central Google Scholar * Tanila, H. The role of BDNF in Alzheimer’s disease. _Neurobiol Dis_, doi:S0969-9961(16)30102-4 (2016). * Makar, T. K.
_et al_. TrkB agonist, 7,8-dihydroxyflavone, reduces the clinical and pathological severity of a murine model of multiple sclerosis. _J Neuroimmunol_ 292, 9–20,
doi:10.1016/j.jneuroim.2016.01.002 (2016). Article CAS PubMed Google Scholar * Kozisek, M. E., Middlemas, D. & Bylund, D. B. Brain-derived neurotrophic factor and its receptor
tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. _Pharmacol Ther_ 117, 30–51, doi:S0163-7258(07)00152-0 (2008). * Li, L. _et al_. [Expression of
brain-derived neurotrophic factor at acute inflammatory injury of the brain]. _Zhejiang Da Xue Xue Bao Yi Xue Ban_ 32, 433–436 (2003). CAS PubMed Google Scholar * Li, L., Shui, Q. X.
& Zhao, Z. Y. Regulation of brain-derived neurotrophic factor (BDNF) expression following antibiotic treatment of experimental bacterial meningitis. _J Child Neurol_ 18, 828–834 (2003).
Article PubMed Google Scholar * Barichello, T. _et al_. Correlation between behavioral deficits and decreased brain-derived neurotrophic [correction of neurotrofic] factor in neonatal
meningitis. _J Neuroimmunol_ 223, 73–76, doi:10.1016/j.jneuroim.2010.04.004 (2010). Article CAS PubMed Google Scholar * Li, L., Shui, Q. X., Liang, K. & Ren, H. Brain-derived
neurotrophic factor rescues neurons from bacterial meningitis. _Pediatr Neurol_ 36, 324–329, doi:S0887-8994(07)00042-2 (2007). * Krock, E. _et al_. Nerve Growth Factor Is Regulated by
Toll-Like Receptor 2 in Human Intervertebral Discs. _J Biol Chem_ 291, 3541–3551, doi:10.1074/jbc.M115.675900 (2016). Article CAS PubMed Google Scholar * Isa, I. L. _et al_. Hyaluronic
Acid Based Hydrogels Attenuate Inflammatory Receptors and Neurotrophins in Interleukin-1beta Induced Inflammation Model of Nucleus Pulposus Cells. _Biomacromolecules_ 16, 1714–1725,
doi:10.1021/acs.biomac.5b00168 (2015). Article CAS PubMed Google Scholar * Saha, R. N., Liu, X. & Pahan, K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the
neuroprotective role of cytokine. _J Neuroimmune Pharmacol_ 1, 212–222, doi:10.1007/s11481-006-9020-8 (2006). Article PubMed PubMed Central Google Scholar * Gaikwad, S. M., Thakur, B.,
Sakpal, A., Singh, R. K. & Ray, P. Differential activation of NF-kappaB signaling is associated with platinum and taxane resistance in MyD88 deficient epithelial ovarian cancer cells.
_Int J Biochem Cell Biol_ 61, 90–102, doi:10.1016/j.biocel.2015.02.001 (2015). Article CAS PubMed Google Scholar * Wu, Y. _et al_. Inhibiting the TLR4-MyD88 signalling cascade by genetic
or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. _Br J Pharmacol_ 165, 1319–1329, doi:10.1111/j.1476-5381.2011.01572.x (2012). Article CAS
PubMed PubMed Central Google Scholar * Kuti, B. P., Bello, E. O., Jegede, T. O. & Olubosede, O. Epidemiological, clinical and prognostic profile of childhood acute bacterial
meningitis in a resource poor setting. _J Neurosci Rural Pract_ 6, 549–557, doi:10.4103/0976-3147.165424 (2015). Article PubMed PubMed Central Google Scholar * Barichello, T. _et al_.
Targets for adjunctive therapy in pneumococcal meningitis. _J Neuroimmunol_ 278, 262–270, doi:10.1016/j.jneuroim.2014.11.015 (2015). Article CAS PubMed Google Scholar * Gerber, J. &
Nau, R. Mechanisms of injury in bacterial meningitis. _Curr Opin Neurol_ 23, 312–318, doi:10.1097/WCO.0b013e32833950dd (2010). Article PubMed Google Scholar * Blaser, C., Wittwer, M.,
Grandgirard, D. & Leib, S. L. Adjunctive dexamethasone affects the expression of genes related to inflammation, neurogenesis and apoptosis in infant rat pneumococcal meningitis. _PLoS
One_ 6, e17840, doi:10.1371/journal.pone.0017840 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Barichello, T. _et al_. Interleukin-1beta Receptor Antagonism Prevents
Cognitive Impairment Following Experimental Bacterial Meningitis. _Curr Neurovasc Res_ 12, 253–261, doi:CNR-EPUB-67917 (2015). * Liechti, F. D. _et al_. The mood-stabilizer lithium prevents
hippocampal apoptosis and improves spatial memory in experimental meningitis. _PLoS One_ 9, e113607, doi:10.1371/journal.pone.0113607 (2014). Article ADS PubMed PubMed Central Google
Scholar * Lian, D. _et al_. Exogenous BDNF increases neurogenesis in the hippocampus in experimental Streptococcus pneumoniae meningitis. _J Neuroimmunol_ 294, 46–55,
doi:10.1016/j.jneuroim.2016.03.014 (2016). Article CAS PubMed Google Scholar * Bucker, J. _et al_. Brain-derived neurotrophic factor and inflammatory markers in school-aged children with
early trauma. _Acta Psychiatr Scand_ 131, 360–368, doi:10.1111/acps.12358 (2015). Article CAS PubMed Google Scholar * Jiang, Y. _et al_. Intranasal brain-derived neurotrophic factor
protects brain from ischemic insult via modulating local inflammation in rats. _Neuroscience_ 172, 398–405, doi:10.1016/j.neuroscience.2010.10.054 (2011). Article CAS PubMed Google
Scholar * Lu, H. _et al_. Neuroprotective Effects of Brain-Derived Neurotrophic Factor and Noggin-Modified Bone Mesenchymal Stem Cells in Focal Cerebral Ischemia in Rats. _J Stroke
Cerebrovasc Dis_ 25, 410–418, doi:10.1016/j.jstrokecerebrovasdis.2015.10.013 (2016). Article ADS PubMed Google Scholar * Ribes, S. _et al_. Resistance of the brain to Escherichia coli K1
infection depends on MyD88 signaling and the contribution of neutrophils and monocytes. _Infect Immun_ 81, 1810–1819, doi:10.1128/IAI.01349-12 (2013). Article CAS PubMed PubMed Central
Google Scholar * Koedel, U. _et al_. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. _Brain_ 127, 1437–1445, doi:10.1093/brain/awh171
(2004). Article PubMed Google Scholar * Liu, S. & Kielian, T. MyD88 is pivotal for immune recognition of Citrobacter koseri and astrocyte activation during CNS infection. _J
Neuroinflammation_ 8, 35, doi:10.1186/1742-2094-8-35 (2011). Article PubMed PubMed Central Google Scholar * Rothwarf, D. M. & Karin, M. The NF-kappa B activation pathway: a paradigm
in information transfer from membrane to nucleus. _Sci STKE_ 1999, RE1, doi:10.1126/stke.1999.5.re1 (1999). CAS PubMed Google Scholar * Koedel, U., Bayerlein, I., Paul, R., Sporer, B.
& Pfister, H. W. Pharmacologic interference with NF-kappaB activation attenuates central nervous system complications in experimental Pneumococcal meningitis. _J Infect Dis_ 182,
1437–1445, doi:JID000589 (2000). * Selvaraj, S. K. & Prasadarao, N. V. Escherichia coli K1 inhibits proinflammatory cytokine induction in monocytes by preventing NF-kappaB activation. _J
Leukoc Biol_ 78, 544–554, doi:jlb.0904516 (2005). * Kuhn, P. L. & Wrathall, J. R. A mouse model of graded contusive spinal cord injury. _J Neurotrauma_ 15, 125–140,
doi:10.1089/neu.1998.15.125 (1998). Article CAS PubMed Google Scholar * Krock, E. _et al_. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through
nociceptive factors. _J Cell Mol Med_ 18, 1213–1225, doi:10.1111/jcmm.12268 (2014). Article ADS CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work
was supported by National Natural Science Foundation of China [grant numbers 81571180 and 81271337]. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatric Neurology, Xinhua
Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, 200092, P.R. China Danfeng Xu, Di Lian, Zhijie Zhang & Ling Li * Department of Clinical Laboratory,
Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, 200092, P.R. China Ying Liu * Department of Pathology, Xinhua Hospital Affiliated to Shanghai
Jiaotong University School of Medicine, Shanghai, 200092, P.R. China Jiaming Sun Authors * Danfeng Xu View author publications You can also search for this author inPubMed Google Scholar *
Di Lian View author publications You can also search for this author inPubMed Google Scholar * Zhijie Zhang View author publications You can also search for this author inPubMed Google
Scholar * Ying Liu View author publications You can also search for this author inPubMed Google Scholar * Jiaming Sun View author publications You can also search for this author inPubMed
Google Scholar * Ling Li View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Danfeng Xu wrote the main manuscript text. Di Lian performed the
_in vivo_ experiment of the study. Zhijie Zhang helped with the _in vitro_ experiment. Ying Liu cultured the infectious organisms for the study. Jiaming Sun performed the histopathological
experiments of the study. Prof. Ling Li provided necessary guidance on the performance of all the experiment. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Ling
Li. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xu, D., Lian, D., Zhang, Z. _et al._ Brain-derived
neurotrophic factor is regulated via MyD88/NF-κB signaling in experimental _Streptococcus pneumoniae_ meningitis. _Sci Rep_ 7, 3545 (2017). https://doi.org/10.1038/s41598-017-03861-z
Download citation * Received: 03 February 2017 * Accepted: 04 May 2017 * Published: 14 June 2017 * DOI: https://doi.org/10.1038/s41598-017-03861-z SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
