Integrated analysis of tobacco mirna and mrna expression profiles under pvy infection provids insight into tobacco-pvy interactions
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _Potato virus Y_ (PVY) is a globally and economically important pathogen of potato, tobacco, tomato and other staple crops and caused significant yield losses and reductions in
quality.To explore the molecular PVY-host interactions, we analysed changes in the miRNA and mRNA profiles of tobacco in response to PVY infection. A total of 81 differentially expressed
miRNAs belonging to 29 families and 8133 mRNAs were identified. The Gene Ontology (GO) enrichment analyses showed that genes encoding the DNA/RNA binding, catalytic activity and signalling
molecules were all significantly enriched. Moreover, 88 miRNA-mRNA interaction pairs were identified through a combined analysis of the two datasets. We also found evidence showing that the
virus-derived siRNAs (vsiRNAs) from the PVY genome target tobacco translationally controlled tumor protein (_NtTCTP_) mRNA and mediate plant resistance to PVY. Together, our findings
revealed that both miRNA and mRNA expression patterns can be changed in response to PVY infection and novel vsiRNA-plant interactions that may regulate plant resistance to PVY. Both provide
fresh insights into the virus-plant interactions. SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFICATION OF SELF- AND PATHOGEN-TARGETED MIRNAS FROM RESISTANT AND SUSCEPTIBLE _THEOBROMA CACAO_
VARIETY TO BLACK POD DISEASE Article Open access 08 February 2024 SUPPRESSION OF RICE MIR168 IMPROVES YIELD, FLOWERING TIME AND IMMUNITY Article 15 February 2021 IDENTIFICATION AND ANALYSIS
OF MIRNAS IN IR56 RICE IN RESPONSE TO BPH INFESTATIONS OF DIFFERENT VIRULENCE LEVELS Article Open access 05 November 2020 INTRODUCTION _Potato virus Y_ (PVY) is an economically important
pathogen of many crops, with many of its strains differing from one another both in their genomic sequences and in the symptoms they produce in their hosts1,2,3. The PVY genome is a
positive-sense, single-stranded RNA molecule consisting of approximately 9700 nucleotides. The PVY genome contains two open reading frames (ORFs). The first ORF is translated into a single
polyprotein and then processed into individual mature proteins by the viral proteases. The second—and shorter—ORF is translated as the P3N–PIPO fusion protein which is embedded within the P3
cistron of the polyprotein4. Small RNAs are a group of regulatory molecules that play important roles in diverse biological processes, namely in development, genome maintenance and
integrity, and in the adaptive responses to biotic and abiotic stress in most of the eukaryotes. Small RNAs are of two major types: microRNAs (miRNAs) and short interfering RNAs (siRNAs),
both function by suppressing the expression of target genes at the transcriptional and/or post-transcriptional level via specific base-pairing with their targets5. As a key compontent of the
eukaryotic gene regulatory networks, miRNA has attracted increasing attention with respect to its biogenesis and mechanisms of miRNA-mediated gene regulation6,7,8. It was reported that some
animal cellular miRNAs play important roles in the proess of development and the response to pathogens and stresses6, 7. Many animal viruses can down-regulate the expression level of host
miRNAs9,10,11,12,13. Deep sequencing has also identified a few new miRNAs induced only in virus infected-cells14, 15. In _Arabidopsis_, the activation of antiviral RNAi is accompanied by the
production of an abundant class of endogenous siRNAs mapped to the exon regions of more than 1,000 host genes and rRNA. These virus-activated siRNAs are predominantly 21 nucleotides in
length, with an approximately equal ratio of sense and antisense strands, and they may confer broad-spectrum antiviral activity16. As a part of small RNA, virus-derived small interfering RNA
(vsiRNA), is abundant during the viral infection in plants. Although double-strand replication intermediates (RIs) could form in the process of virus multiplication, the dsRNA-like
secondary structures of single stranded viral RNAs were those that most likely contributed to vsiRNA biogenesis17,18,19,20. Analogous to that of endogenous small RNA, the biogenesis of
vsiRNA requires not only Dicer-like (DCL) —especially DCL4 which processes the viral dsRNA transcript into primary vsiRNA21, 22—but also (RNA- dependent RNA polymerase (RDR) and Argonaute
(AGO) which produced secondary vsiRNA through amplification23,24,25. Biogenesis of vsiRNA during virus infection indicates that vsiRNA may function in many regulation pathways. For instance,
vsiRNA could be recruited by diverse AGOs to form RNA-induced silencing complex (RISC) and target viral genome molecules (including viral RNA and viral DNA) through post-transcriptional
gene silencing (PTGS)21, 26, and vsiRNA might also target and down-regulate host transcripts that largely determine the virus symptoms in the host27,28,29,30. To date, the responses of
plants to PVY infection have until now been studied at different levels, ranging from morphological to biochemical, and from proteomic to transcriptomic and metabolic31,32,33,34,35.
Nevertheless, our knowledge of how plants respond to PVY infection remains rather limited. Hence, further investigation is warranted to fully explore the plant-virus interaction dynamics
behind the appearance of disease symptoms and the plant resistance processes. To acquire a better understanding of how the transcriptome changes in response to viral infection in tobacco, we
used high-throughput sequencing technology to simultaneously analyse the miRNA and mRNA expression profiles in virus-infected tobacco plants. We combined these two datasets and identified
miRNA-mRNA interactions under PVY infection. We also found that the vsiRNAs from PVY target tobacco _NtTCTP_ mRNA and mideate plant resistance to PVY infection.These integrated
high-throughput expression data provide a new and valuable resource for examining global genome expression changes in response to PVY infection. This may contribute to viral symptom
development and thereby provide new insights into plant-virus interactions. RESULTS CONSTRUCTION AND DEEP SEQUENCING OF SMALL RNA AND MRNA LIBRARIES To profile the global small RNA and mRNA
changes via deep sequencing, the total RNAs were isolated from the PVY-inoculated (three biological replicates: A1, A2, A3) and mock-inoculated (three biological replicates: B1, B2, B3)
tobacco plants, and used to construct small RNA and transcriptome libraries. All the data generated by this deep sequencing exercise were uploaded to the SRA database (Accession number:
SRP090053). Small RNA sequencing generated approximately 1 million raw reads from each library. After the 5′ and 3′ adaptors were identified and removed from the raw reads, those reads with
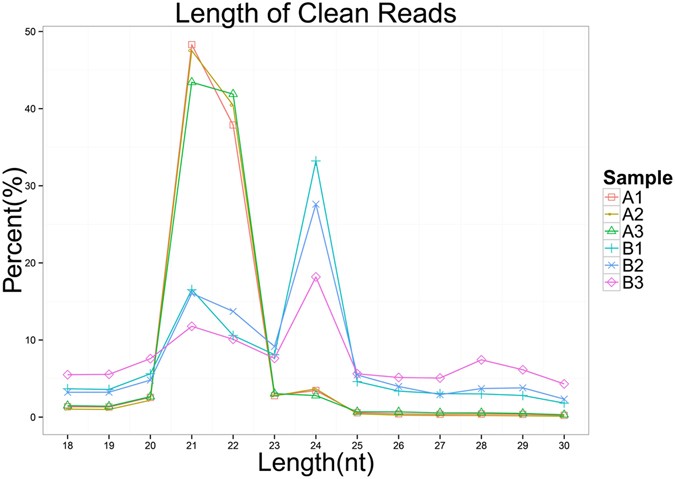
a sequence length of 18 to 30 nt were selected for further analysis (Table 1). The distribution of small RNAs among the different categories is summarized in Fig.1. In the PVY-infected
plants, 21 nt and 22 nt are the most frequent sizes. By contrast, in the mock-inoculated plants, the most frequent small RNAs were 21 nt and 24 nt in length. More than 75% of the small RNA
sequences that had a perfect match to the tobacco genome sequence (https://solgenomics.net) were obtained from each library. In the small RNA libraries of the PVY-infected tobacco plants, a
similar percentage of high-quality read and unigene were found well matched to the PVY genome. Approximately 7G of clean reads were obtained from each mRNA library. The mapping rate of all
three PVY-infected libraries (A1, A2, A3) was 77–78%, while it was slightly higher for the mock-inoculated libraries (B1, B2, B3) at 84–89%. Meanwhile, the multi-map rate of the samples was
less than 0.1% (Table 2). The gene expression density of all the samples showed a high similarity: there were few differential genes, and these usually would not change the overall gene
distribution (Fig. 2A). Both the PVY-infected samples and mock-inoculated samples showed good correlations based on the Hierarchical cluster analysis (Fig. 2C). All the data showed good
agreement among the replicates. Therefore, the data derived to form high quality small RNA and mRNA libraries were deemed robust for further analysis. PVY INFECTION SELECTIVELY ALTERED THE
EXPRESSION OF TOBACCO MIRNAS To speculate on how PVY affects the expression of miRNAs, we compared the total reads of the tobacco miRNAs among the six libraries using pooled data from the
three independent biological replicates. The analysis of the miRNA data showed that 322 miRNAs were detected in the PVY-infected and mock-inoculated tobacco plants (Supplementary Table S1),
However, only 81 miRNAs changed their expression level significantly in the PVY-infected tobacco, of which 24 miRNAs were down-regulated and 57 miRNAs were up-regulated (Supplementary Fig.
S1A, Supplementary Table S2). This suggested that some miRNA, or the locus encoding the miRNA precursor, could respond to the PVY infection. To verify the accuracy of the miRNA alterations
by data calculation, several characteristic miRNAs, such as mi156g and mi168a, were selected for confirmation by the northern blot method. These results showed that the differences in the
heat map were consistent with those of the northern blot (Supplementary Fig. S1B,C). To obtain those genes that possibly regulated by the differentially expressed miRNAs, targets of the
miRNAs were predicted by using psRobot software (Supplementary Table S3). To confirm the predicted results, we sequenced a degradome library constructed using the total RNA isolated from the
leaf of PVY-infeted tobacco plants. Over 9.9 million of raw reads were thus generated (Accession number: SAMN06844094). The degradome sequencing datasupported the predicted results for the
miRNA targets (Supplementary Table S4). GO enrichment analysis of the target genes of significantly differentially-expressed miRNAs (_P_ _adj_ < 0.05 and |log2(fold_change)| ≥ 1) among
the paired group samples was performed. The ensuing GO categorization of the predicted targets of the differentially expressed miRNAs showed that these genes were involved in a broad range
of biological processes related to cellular responses to various stimuli; namely, the positive regulation of cellular processes, the negative regulation of growth, the nucleic acid binding
and the transcription factors requied for signal transduction (Fig. 3A,B,C). GLOBAL MRNA EXPRESSION PROFILES OF TOBACCO IN RESPONSE TO PVY INFECTION To further study the tobacco gene
expression profiles responsive to PVY infection, the total RNA samples used for small RNA sequencing were subjected to transcriptome sequencing. Analysing this deep sequencing revealed that
a total of 8133 genes were significantly altered by PVY infection, of which 3790 were up-regulated and 4343 were down-regulated (Fig. 2B, Supplementary Table S5). To confirm the RNA
sequencing data, we selected 10 up-regulated genes, 2 down-regulated genes and 1 gene that was not changed via qRT-PCR to check their expression patterns compared with RNA-seq data, the
qRT-PCR results were consistent with the deep sequencing data (Supplementary Fig. S2). To understand the putative roles of the significantly altered genes, GO analyses were conducted to
discover their relevance to diverse biological processes, molecular functions and cellular components. Genes in PVY-infected plant were found to be enriched in metabolic and stress response
processes (Fig. 4A,B,C). COMBINED ANALYSIS OF MIRNA AND MRNA EXPRESSION NETWORK UNDER PVY INFECTION To explore the miRNA and mRNA expression networks in the PVY-infected tobacco plants, data
on the miRNA and mRNA expression profiles were combined for a further correlation analysis. It is well known that miRNAs play pivotal roles in regulating mRNA expression. Correlation
analysis of the 81 differentially-expressed miRNAs and the 8133 differentially-expressed mRNAs identified a total of 88 interaction pairs of miRNA and its corresponding targets mRNA
(Supplementary Table S6). For each such pair, a one-to-one correspondence between up-regulated miRNA (or down-regulated miRNA) and down-regulated target mRNA (or up-regulated target mRNA)
was not necessarily expected. So, to directly demonstrate the relationship between differentially-expressed miRNAs and mRNAs, we then classified these relationships into two categories
depending on their regulation mode: either as positive or negative, respectively, for up-regulated (down-regulated) miRNAs versus up-regulated (down-regulated) target genes (Fig. 5A,
Supplementary Table S7) or up-regulated (down-regulated) miRNAs versus down-regulated (up-regulated) target genes (Fig. 5B, Supplementary Table S8). The results showed that in addition to
the repression caused by miRNA, other mechanisms such as ceRNA regulation might be involved. These data collectively suggested that the interactions between mRNA and miRNA are very
complicated in the tobacco plant. PVY-DERIVED SIRNA TARGETS THE _NTTCTP_ MRNA AND MIDEATE PLANT RESISTANCE TO PVY INFECTION To further investigate the plant response to PVY infection, we
analysed the PVY-derived siRNAs and their relationships with the tobacco mRNAs. Computer-assisted analyses identified several vsiRNAs derived from the PVY genome that were complementary to
the mRNA sequence of the _NtTCTP_ gene (_Nicotiana tabacum_ Translationally Controlled Tumor Protein, mRNA_68091) ORF (Fig. 6A). The most abundant vsRNA that targets _NtTCTP_ was quantified
by Northern blotting (Supplementary Fig. S3). Subsequent analysis of the degradome sequencing data also confirmed that the _NtTCTP_ was excised in the PVY-infected plants, thus suggesting
that vsiRNAs from PVY may down-regulate _NtTCTP_ expression such that _NtTCTP_ may also have an important role in plant-PVY interactions. To test this hypothesis, we conducted a PVY
infection assay with two NtTCTP-overexpressing lines (NtTCTP-OE: O2 and O7) and two NtTCTP_-_silencing lines (NtTCTP-RNAi: Ri16 and Ri20) as part of a further analysis36. Seedlings of O2,
O7, Ri16, Ri20 and the wild type (variety _Xanthi_) were challenged with PVY at the four-leaf stage of development. After 2 weeks, PVY-induced symptoms in the systemic leaves of the wild
type and in the two NtTCTP-overexpressing lines showed greater sensitivity when compared with the wild type seedlings. In contrast, the two NtTCTP-silencing lines showed high resistance to
PVY infection and they did not display any obvious symptoms (Fig.6B,C). ELISA consistently detected high PVY tires in the wild type and NtTCTP-overexpressing lines, whereas PVY was hardly
detected in the two NtTCTP-silencing lines (Fig. 6D). These results suggest that PVY-plant interaction involved _NtTCTP_ which acted as a susceptibility factor to promote the PVY infection.
A prior study reported that NtTCTP interacts and stabilizes the ethylene receptor NtHK1 to reduce the plant response to ethylene and to promote plant growth through accelerated cell
proliferation36. To see whether NtHK1 also participated in the tobacco plant reponse to the PVY infection, we tested several NtHK1-overexpressing (NtHK1-OE:16-4) and NtHK1_-_silencing
(NtHK1-RNAi:1–8) lines. However, no apparent differences were found among the NtHK1-overexpressing, the NtHK1_-_silencing and the wild type plants after inoculation with PVY. All of those
plants showed typical symptoms and their incidence rate and virus content were almost the same across the three lines (data not shown), which together suggested that NtHK1 had no function in
the PVY infection of tobacco. Nonetheless, these results did demonstrate that the involvement of _NtTCTP_ in the PVY-plant interaction did not occur via the ethylene pathway. DISCUSSION
High throughput sequencing approaches have become powerful tools for analysing global gene expression profiles and for identifying low-abundance novel miRNAs unidentifiable by traditional
cloning and sequencing techniques37,38,39. Global expression profiling analysis of miRNAs and mRNAs in the same samples can provide a unique opportunity to enhance our understanding of
potential miRNA regulatory mechanisms in host-infection by virus. RNA-seq analyses have been done for PVY-infected potato plants40, with these studies finding that several genes were
expressed differently between the susceptible and resistant varieties. By way of comparison, our sequencing results provide a detailed view of miRNAs and mRNAs expression in tobacco leaf in
response to PVY infection, thus adding new information to better understand the virus-host interactions as well as offering novel insights into the impact of viral infection on host small
RNA and mRNA exprsssion. In general, miRNA accumulation will lead to the down-regulation of corresponding mRNA targets, and vice-versa. After an integrated analysis of the
differentially-expressed miRNAs and mRNAs, we found several important regulatory miRNAs likely involved in virus infection. For example, miRNA6019a was able to target a “disease resistance
protein” (mRNA_90605), which is considered as one of TIR-NBS-LRR family resistance genes. In the PVY infection process, miRNA6019a was down-regulated (Supplementary Fig. S1B,C) while the
amount of its target mRNA 90605 increased correspondingly (Supplementary Table S5). This coordinated activity suggests that miRNA-regulated resistance might be promoted during PVY infection.
When studying vsiRNA, how it performs key roles in antiviral resistance and in host transcripts regulation are the core questions. For example, it was reported that a siRNA derived from CMV
satellite RNA could target and silence CHLI, a chlorophyll biosynthetic gene, to induce the symptoms of yellowing in virus-infected plants27, 28. Moreover, a vsiRNA derived from RSV (_Rice
stripe virus_) targets _eIF4A_ mRNA in tobacco and down-regulates its expression, resulting in a phenotype of leaf-twisting, deficient flowers and stunting30. In the present study, we
identified several vsiRNAs derived from the PVY genome which could target the host gene _NtTCTP_. The PVY infection assay on the _NtTCTP-_silencing and over-expressing transgenic lines
showed that silencing _NtTCTP_ suppressed the PVY infection, whereas the over-expression of _NtTCTP_ increased plant susceptibility to the PVY infection. Therefore, it is plausible _NtTCTP_
encodes a host factor that is essential to the PVY infection process, not unlike for other host factors recently reviewed41. TCTP is a highly conserved protein present in all eukaryotes. Its
mammalian homologs are perhaps the best studied due to their role in cancer development. TCTP is an important component of the TOR (target of rapamycin) signalling pathway, the major
regulator of cell growth in both animals and fungi. Though many studies have revealed that TCTP is involved in cell cycle progression, cell growth, stress protection, maintenance of genomic
integrity and apoptosis42, its molecular function remains elusive. Recently, TCTP was suggested as an important host factor in the _Pepper yellow mosaic virus_ (PepYMV) infection of tomato
and _Nicotiana benthamiana_ plants. This particular virus interferes with the subcellular localization of this protein, probably due to the involvement of TCTP at some crucial stage of the
infection process43. Tobacco TCTP (_NtTCTP_) encodes a small ER-located protein containing 168 amino acids, and the transcripts of _NtTCTP_ were more abundant in roots than in the other
plant organs36. Finally, our research found that tobacco _NtTCTP_ was a target of vsiRNA from the PVY genome and that the silencing of _NtTCTP_ mediated resistance against PVY. As our primal
expectation, the content of PVYN should accumulate increasingly in NtTCTP-silencing lines because vsiRNA derived from PVY targeted to _NtTCTP_, but the opposite results showed that _NtTCTP_
was required for PVY infection. Why does the virus generate the vsiRNA which has adverse effect on itself? We speculated that targeting of _NtTCTP_ by vsiRNA is a host strategy resistant
against PVY infection, but the function of these vsiRNA could be suppressed to some extend by some else mechanism. It might be an episode of host-virus competition series. Further research
is planned towards determining the functional role of TCTP in viral infection, and to test whether this gene can be manipulated against PVY, PepYMV and related viruses. CONCLUSION We
described the miRNA and mRNA expression profiles in virus-infected tobacco plants by using high-throughput sequencing technology. Combining these two datasets we identified an network
consisting of 88 miRNA-mRNA interactions. In so doing, we further found that vsiRNA from PVY target tobacco _NtTCTP_ mRNA to mediate plant resistance to PVY infection. The integrated
high-throughput expression datasets we obtained provides a valuable resource to examine global genome expression changes in plant responses to PVY infection, which should also contribute to
viral symptom development. This study thus offers new insights into the pathogenicity mechanisms of PVY and associated plant resistance mechanisms. MATERIALS AND METHODS PLANT GROWTH
CONDITIONS AND VIRUS INFECTION Seeds of tobacco (_Nicotiana tabacum_) were surface sterilized with a 3% sodium hypochlorite solution, rinsed five times with distilled water, immersed in
distilled water for two days, and then allowed to germinate for another 2 days at 37 °C. Seedlings were grown in a medium of half-strength growth nutrients under a 16-h light (28 °C)/8-h
dark (25 °C) photoperiod. The PVYN (tobacco veinal-necrotic strain) was maintained in potato in green houses at 25 ± 3 °C, 60 ± 5% relative humidity under natural sunlight. Seedlings were
challenged with PVY at the four-leaf stage. Two weeks later, when the virus induced symptoms appeared in the systemic leaves of the PVY-infected plants, leaves from the PVY-infected plants
(three biological replicates: A1, A2, A3) as well as the mock-inoculated plants (three biological replicates: B1, B2, B3) were collected and immediately frozen in liquid nitrogen until
subsequent use. RNA ISOLATION AND SEQUENCING Total RNA was extracted using the phenol-chloroform method.All samples were assessed for integrity and population size using the Agilent 2100
Bioanalyzer. The concentration and purity of each RNA sample was measured using the Nanodrop spectrophoto meter (Thermo Scientific). Small RNA and mRNA library preparation and sequencing
were performed using Small RNA Sample Preparation Kit (Illumina, RS-200-0048) and NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (#E7530L, NEB, USA) following the manufacturer’s
recommendations. ANALYSIS OF MIRNA AND MRNA SEQUENCING DATA The filtered sequence of all the samples were mapped to tobacco genome (http://solgenomics.net/) by bowtie144, 45 with –v –a
-best, that is, align reads to tobacco genome without any mismatch (because of the short length of miRNA) and the all best alignments were retained for follow-up analysis. The mapped sRNA
sequence would be used for the known miRNA, Rfam, repeat and some other annotation. Tobacco miRNA from miRBase 21 was used for known miRNA reference sequence. Rfam 12.0 was used for ncRNA
annotation. The unannotated sRNA was used for novel miRNA prediction by miRDeep-P software.To get the differential miRNA for both known miRNA and novel miRNA, DESeq software was used. MiRNAs
which satisfied FDR ≤ 0.05 and log2|fold change| > = 1 were considered as significantly differential expressed miRNAs (DEM) between the two groups.Target genes of the DEMs were
predicted by software psRobot. Libraries of RNA were sequenced on the Illumina Hiseq 2500 platform and sequencing reads that contained polyA/T and adapters were discarded, and reads with low
quality and high Ns were pre-filtered before mapping, too. Filtered reads were mapped to the reference Tobacco genome sequence (http://solgenomics.net/) by using Tophat with the default
parameters.HTseq is applied to generate gene counts. Then gene expression difference was analyzed by DEseq, genes with |log2FoldChange| ≥ 1 and p-value < 0.05 were determined to be
statistically significant. Function and pathway enrichment were analyzed depending on the Gene Ontology database (http://geneontology.org/). The GO term with a q-value lower than 0.05 is
determined to be enriched significantly. QUANTITATIVE REVERSE TRANSCRIPTION REAL-TIME PCR ANALYSIS Quantitative reverse transcription real-time PCR (qRT-PCR) was performed using a SYBR
Real-time PCR Detection System (MJ Research, Waltham, MA, USA) following the manufacturer’s instructions. Each reaction was prepared in a total volume of 20 µl containing 10 µl SYBR Green
Mix (Takara), 1.5 µl of diluted cDNA (corresponding to 1.5 ng of reverse-transcribed total RNA) and 0.2 µl of each primer (200 nM working concentration). The reactions were subjected to an
initial denaturation step of 95 °C for 10 s, followed by 35 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 10 s. Each sample was prepared in triplicate. Each sample was prepared in
triplicate.The qRT-PCR primers used in this study were list in Supplementary Table S9. NORTHERN BLOT HYBRIDIZATION MiRNA northern blot hybridization was performed as described46 by Guo _et
al_. with 30 micrograms of sRNA loaded for each sample. DNA oligonucleotides complementary to miRNA sequences were end-labeled with r-32P-ATP (5000 Ci mmol−1) using T4 polynucleotide kinase
(NEB, Beijing, China) as the probe. Membranes were hybridized for 16 h at 42 °C and were briefly air-dried and were exposed to X- ray film at −80 °C. TARGET PREDICTION OF THE DIFFERENTIALLY
EXPRESSED MIRNAS (DEMS) AND DEGRADOME SEQUENCING To predict the target genes of the DEMs, a PsRobot was used. This tool identifies a particular set of sRNAs with stem-loop shaped precursors
(such as microRNAs and short hairpin RNAs), as well as their target genes or transcripts, especially in plants. It predicts their targets using a modified Smith–Waterman algorithm. This
program was performed with –ts 2 –gl 10 setting. That means when aligned with the reference genes, those DEMs at position after 10 was allowed at most one gap or bulge, target penalty score
should be lower than 2. Mismatches, gaps or bulges are evaluated with a penalty of plus 1, while the G:U pairs are evaluated with a penalty of plus 0.547. The degradome library was
constructed as previously described48 by using the RNA of the A1 treatment as the core material. Firstly, the RNA fragments with poly (A) tail were isolated from the total RNA by using the
Oligo texm RNA mini kit (Qiagen), Secondly, a 5′ RNA adapter with a _Mme_ I restriction site at its 3′ end was added to the 5′ ends of the isolated poly(A) RNAs. Thirdly, reverse
transcription PCR using oligod (T) as the primer was performed and the PCR products were purified and digested with _Mme_ I. After ligating a double-stranded DNA adapter to the 3′ end of the
digested products, the ligated products were further purified and amplified, and then sequenced using the Illumina GAII platform. REFERENCES * Gray, S. _et al_. Potato virus Y: An evolving
concern for potato crops in the United States andCanada. _Plant Disease_ 94, 1384–1397 (2010). Article Google Scholar * Karasev, A. V. & Gray, S. M. Continuous and emerging challenges
of _potato virus Y_ in potato. _Annual Review of Phytopathology_ 51, 571–586 (2013). Article CAS PubMed Google Scholar * Nie, X., Singh, R. P. & Singh, M. Molecular and pathological
characterization of N:O isolates of the _Potato virus Y_ from Manitoba, Canada. _Canadian Journal of Plant Pathology_ 26, 573–583 (2004). Article CAS Google Scholar * Ivanov, K. I. _et
al_. Molecular insights into the function of the viral RNA silencing suppressor HCPro. _Plant Journal for Cell & Molecular Biology_ 85, n/a–n/a (2015). Google Scholar * Zamore, P. D.
& Haley, B. Ribo-gnome: The Big World of Small RNAs. _Science_ 309, 1519–1524 (2005). Article ADS CAS PubMed Google Scholar * Bartel, D. P. M. R. N. A. genomics, biogenesis,
mechanism, and function. _Cell_ 116, 281–297 (2004). Article CAS PubMed Google Scholar * Mallory, A. C. & Vaucheret, H. Erratum: Functions of microRNAs and related small RNAs in
plants. _Nature Genetics_ 38(Suppl), e471–e471 (2006). Google Scholar * Willmann, M. R. & Poethig, R. S. Conservation and evolution of miRNA regulatory programs in plant development.
_Current Opinion in Plant Biology_ 10, 503–511 (2007). Article CAS PubMed PubMed Central Google Scholar * Sinclair, S. J., Murphy, K. J. & Birch, C. D. Molecular characterization of
quinolinate _phosphoribosyltransferase_ (QPRTase) in Nicotiana. _Plant Molecular Biology_ 44, 603–617 (2000). Article CAS PubMed Google Scholar * Sinclair, S. J., Johnson, R. &
Hamill, J. D. Analysis of wound-induced gene expression in Nicotiana species with contrasting alkaloid profiles. _Functional Plant Biology_ 31, 721–729 (2004). Article CAS Google Scholar
* Deboer, K. D., Lye, J. C. & Aitken, C. D. The A622 gene in _Nicotiana glauca_ (tree tobacco): evidence for a functional role in pyridine alkaloid synthesis. _Plant Molecular Biology_
69, 299–312 (2009). Article CAS PubMed Google Scholar * Frazier, T. P., Xie, F., Freistaedter, A., Burklew, C. E. & Zhang, B. Identification and characterization of microRNAs and
their target genes in tobacco (_Nicotiana tabacum_). _Planta_ 232, 1289–1308 (2010). Article CAS PubMed Google Scholar * Kim, H. J., Baek, K. H., Lee, B. W., Choi, D. & Hur, C. G. In
silico identification and characterization of microRNAs and their putative target genes in Solanaceae plants. _Genome_ 54, 91–98 (2011). Article CAS PubMed Google Scholar * Chen, H. M.
& Weigel, D. 22-Nucleotide RNAs trigger secondary siRNA biogenesis in plants. _Proceedings of the National Academy of Sciences_ 107, 15269–15274 (2010). Article ADS CAS Google Scholar
* Howell, M. D. _et al_. Genome-wide analysis of the RNA-DEPENDENT RNA POLYMERASE6/DICER-LIKE4 pathway in _Arabidopsis_ reveals dependency on miRNA- and tasiRNA-directed targeting. _Plant
Cell_ 19, 926–942 (2007). Article CAS PubMed PubMed Central Google Scholar * Cao, M. _et al_. Virus infection triggers widespread silencing of host genes by a distinct class of
endogenous siRNAs in _Arabidopsis_. _Proceedings of the National Academy of Sciences_ 111, 14613–14618 (2014). * Donaire, L. _et al_. Deep-sequencing of plant viral small RNAs reveals
effective and widespread targeting of viral genomes. _Virology_ 392, 203 (2009). Article CAS PubMed Google Scholar * Szittya, G. _et al_. Structural and Functional Analysis of Viral
siRNAs. _Plos Pathogens_ 6, e1000838 (2010). Article PubMed PubMed Central Google Scholar * Wang, X. B. _et al_. RNAi-mediated viral immunity requires amplification of virus-derived
siRNAs in Arabidopsis thaliana. _Proceedings of the National Academy of Sciences_ 107, 484–489 (2010). Article ADS CAS Google Scholar * Zhang, C., Wu, Z., Li, Y. & Wu, J. Biogenesis,
Function, and Applications of Virus-Derived Small RNAs in Plants. _Frontiers in Microbiology_ 6, 50–69 (2015). Google Scholar * Fusaro, A. F. _et al_. RNA interference-inducing hairpin
RNAs in plants act through the viral defence pathway. _Embo Reports_ 7, 1168–1175 (2006). Article CAS PubMed PubMed Central Google Scholar * Garcia-Ruiz, H. _et al_. Arabidopsis
RNA-Dependent RNA Polymerases and Dicer-Like Proteins in Antiviral Defense and Small Interfering RNA Biogenesis during Turnip Mosaic Virus Infection. _Plant Cell_ 22, 481 (2010). Article
CAS PubMed PubMed Central Google Scholar * Voinnet, O. Use, tolerance and avoidance of amplified RNA silencing by plants. _Trends in Plant Science_ 13, 317–328 (2008). Article CAS
PubMed Google Scholar * Mallory, A. C. & Vaucheret, H. ARGONAUTE 1 homeostasis invokes the coordinate action of the microRNA and siRNA pathways. _Embo Reports_ 10, 521 (2009). Article
CAS PubMed PubMed Central Google Scholar * Mallory, A. & Vaucheret, H. Form, function, and regulation of ARGONAUTE proteins. _The Plant Cell_ 22, 3879–3889 (2010). Article CAS
PubMed PubMed Central Google Scholar * Moissiard, G. & Voinnet, O. RNA silencing of host transcripts by cauliflower mosaic virus requires coordinated action of the four Arabidopsis
Dicer-like proteins. _Proceedings of the National Academy of Sciences of the United States of America_ 103, 19593–19598 (2006). Article ADS CAS PubMed PubMed Central Google Scholar *
Shimura, H. _et al_. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. _Plos Pathogens_ 7,
202–221 (2011). Article Google Scholar * Smith, N. A., Eamens, A. L. & Wang, M. B. Viral Small interfering RNAs target host genes to mediate disease symptoms in plants. _Plos
Pathogens_ 7, e1002022 (2011). Article CAS PubMed PubMed Central Google Scholar * Navarro, B. _et al_. Small RNAs containing the pathogenic determinant of a chloroplast‐replicating
viroid guide the degradation of a host mRNA as predicted by RNA silencing. _Plant Journal for Cell & Molecular Biology_ 70, 991–1003 (2012). Article CAS Google Scholar * Shi, B. _et
al_. Identification and regulation of host genes related to _rice stripe virus_ symptom production. _New Phytologist_ 209, 95–102 (2016). Article Google Scholar * Kogovšek, P. &
Ravnikar, M. Physiology of the Potato_–Potato Virus Y_ Interaction. _ProgressinBotany_, 101–133 (2013). * Goyer, A., Hamlin, L., Crosslin, J. M., Buchanan, A. & Chang, J. H. RNA-Seq
analysis of resistant and susceptible potato varieties during the early stages of _potato virus Y_ infection. _BMC Genomics_ 16, 1–13 (2015). Article CAS Google Scholar * Baebler, Š. _et
al_. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Yinfection in potato. _Journal of Experimental Botany_ 65, 1095–1109
(2014). Article CAS PubMed PubMed Central Google Scholar * Stare, T., Ramšak, Ž., Blejec, A., Stare, K. & Turnšek, N. Bimodal dynamics of primarymetabolism-related responses in
tolerant potato-_Potato virus Y_ interaction. _BMC Genomics_ 1763, 290–295 (2015). Google Scholar * Kogovšek, P. _et al_. Primary Metabolism, Phenylpropanoids and Antioxidant Pathways Are
Regulated in Potato as a Response to _Potato virus Y_ Infection. _Plos One_ 11, e0146135 (2015). Article Google Scholar * Tao, J. J. _et al_. Tobacco translationally controlled tumor
protein interacts with ethylene receptor tobacco histidine kinase1 and enhances plant growth through promotion of cell proliferation. _Plant Physiology_ 169, 96–114 (2015). Article CAS
PubMed PubMed Central Google Scholar * Hsieh, L. C. _et al_. Uncovering small RNA-mediated responses to phosphate deficiency in _Arabidopsis_ by deep sequencing. _Plant Physiology_ 151,
2120–2132 (2009). Article PubMed PubMed Central Google Scholar * Lelandaisbrière, C. _et al_. Genome-wide _Medicago truncatula_ small RNA analysis revealed novel microRNAs and isoforms
differentially regulated in roots and nodules. _Plant Cell_ 21, 2780–2796 (2009). Article Google Scholar * Zhang, J., Xu, Y., Huan, Q. & Kang, C. Deep sequencing of Brachypodium small
RNAs at the global genome level identifies microRNAs involved in cold stress response. _BMC Genomics_ 10, 449 (2009). Article PubMed PubMed Central Google Scholar * Goyer, A., Hamlin,
L., Crosslin, J. M., Buchanan, A. & Chang, J. H. RNA-Seq analysis of resistant and susceptible potato varieties during the early stages of potato virus Y infection. _BMC Genomics_ 16,
472 (2015). Article PubMed PubMed Central Google Scholar * Wang, A. M. Dissecting the molecular network of virus-plant interactions: The complex roles of host factors. _Annual Review of
Phytopathology_ 53, 45–66 (2015). Article CAS PubMed Google Scholar * Bommer, U. A. Cellular function and regulation of the translationally controlled tumour protein TCTP. _Open Allergy
Journal_ 5, 19–32 (2012). Article CAS Google Scholar * Bruckner, F. P. _et al_. Translationally controlled tumor protein (TCTP) from tomato and _Nicotiana benthamiana_ is necessary for
successful infection by A Potyvirus. _Molecular Plant Pathology_ (2016). * Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA
sequences to the human genome. _Genome Biology_ 10, 1–10 (2009). Article Google Scholar * Yang, X., Zhang, H. & Li, L. Global analysis of gene-level microRNA expression in
_Arabidopsis_ using deep sequencing data. _Genomics_ 98, 40–46 (2011). Article CAS PubMed Google Scholar * Guo, Y. _et al_. Identification and characterization of miRNAome in tobacco
(_Nicotiana tabacum_) by deep sequencing combined with microarray. _Gene_ 501, 24 (2012). Article CAS PubMed Google Scholar * Schwab, R. _et al_. Specific effects of microRNAs on the
plant transcriptome. _Developmental Cell_ 8, 277–284 (2005). Article Google Scholar * German, M. A., Luo, S., Schroth, G., Meyers, B. C. & Green, P. J. Construction of parallel
analysis of RNA Ends (PARE) libraries for the study of cleaved miRNA targets and the RNA degradome. _Nature Protocol_ 4, 356–362 (2009). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank Prof. Xiangdong Li (Shandong Agricultural University, China) and members of our lab for helpful discussions. We also would like to acknowledge Prof. Jinsong Zhang
(State Key Lab of Plant Genomics, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing all the transgenic lines of tobacco TCTP and HK1 genes. This
work was financially supported by the Natural Science Foundation of China (31360431), Guizhou tobacco company project (Grant No. 2015-04), the Natural Science Foundation of Guizhou province
of China (qiankehe J[2014] 2117, 2118), the Doctorate staff Foundation at the Guizhou Normal University (000-030111037024). AUTHOR INFORMATION Author notes * Yushuang Guo, Meng-ao Jia and
Yumei Yang contributed equally to this work. AUTHORS AND AFFILIATIONS * Key Laboratory of Molecular Genetics, China National Tobacco Corporation, Guizhou Institute of Tobacco Science,
Guiyang, Guizhou, 550083, P. R. China Yushuang Guo, Meng-ao Jia, Jie Zhang, Qiang Fu & Jiehong Zhao * Annoroad Gene Technology (Beijing) Co., Ltd, Beijing, 101100, P. R. China Yumei
Yang, Jie Yang & Tao Liu * Department of Agronomy, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang, 310058, P. R. China Imran Haider Shamsi * College of
Agriculture and Food Science, Zhejiang Agriculture and Forestry University, Hangzhou, Zhejiang, 311300, P. R. China Linlin Zhan & Jianyu Cai * School of Life and Environmental science,
Hangzhou Normal University, Hangzhou, Zhejiang, 311121, P. R. China Xiaofei Cheng Authors * Yushuang Guo View author publications You can also search for this author inPubMed Google Scholar
* Meng-ao Jia View author publications You can also search for this author inPubMed Google Scholar * Yumei Yang View author publications You can also search for this author inPubMed Google
Scholar * Linlin Zhan View author publications You can also search for this author inPubMed Google Scholar * Xiaofei Cheng View author publications You can also search for this author
inPubMed Google Scholar * Jianyu Cai View author publications You can also search for this author inPubMed Google Scholar * Jie Zhang View author publications You can also search for this
author inPubMed Google Scholar * Jie Yang View author publications You can also search for this author inPubMed Google Scholar * Tao Liu View author publications You can also search for this
author inPubMed Google Scholar * Qiang Fu View author publications You can also search for this author inPubMed Google Scholar * Jiehong Zhao View author publications You can also search
for this author inPubMed Google Scholar * Imran Haider Shamsi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.A.J. and I.H.S. conceived
and designed the experiments, Y.S.G., M.A.J., Y.M.Y., L.C.L., L.L.Z., X.F.C., J.Y.C. and J.Z. conducted the experiments and analysed the data, Y.S.G., M.A.J, J.Y., T.L., Q.F. I.H.S and
J.H.Z. wrote the paper. All authors reviewed the manuscript. CORRESPONDING AUTHORS Correspondence to Meng-ao Jia or Imran Haider Shamsi. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPORTING INFORMATION TABLE_S1 TABLE_S2 TABLE_S3 TABLE_S4 TABLE_S5 TABLE_S6 TABLE_S7 TABLE_S8 TABLE_S9 RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Guo, Y., Jia, Ma., Yang, Y.
_et al._ Integrated analysis of tobacco miRNA and mRNA expression profiles under PVY infection provids insight into tobacco-PVY interactions. _Sci Rep_ 7, 4895 (2017).
https://doi.org/10.1038/s41598-017-05155-w Download citation * Received: 07 March 2017 * Accepted: 24 May 2017 * Published: 07 July 2017 * DOI: https://doi.org/10.1038/s41598-017-05155-w
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
