Ice slurry ingestion reduces human brain temperature measured using non-invasive magnetic resonance spectroscopy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We previously reported that ice slurry ingestion reduced forehead skin temperature, thereby potentially reducing brain temperature (Tbrain). Therefore, in the current study, we
investigated the effect of ice slurry ingestion on Tbrain using proton magnetic resonance spectroscopy, which is a robust, non-invasive method. Eight male participants ingested 7.5 g/kg of
either a thermoneutral drink (37 °C; CON) or ice slurry (−1 °C; ICE) for about 5 min following a 15-min baseline period. Then, participants remained at rest for 30 min. As physiological
indices, Tbrain, rectal temperature (Tre), mean skin temperature, nude body mass, and urine specific gravity were measured. Subjective thermal sensation (TS) and thermal comfort (TC) were
measured before and after the experiment. Tbrain and Tre significantly reduced after ingestion of ICE compared with after ingestion of CON, and there was a significant correlation between
Tbrain and Tre. The other physiological indices were not significantly different between beverage conditions. TS and TC were significantly lower with ICE than with CON (_p_ < 0.05). These
results indicate that ice slurry ingestion can cool the brain, as well as the body’s core. SIMILAR CONTENT BEING VIEWED BY OTHERS A FEASIBILITY STUDY ON USING FNIRS BRAIN SIGNALS TO
RECOGNIZE PERSONAL THERMAL SENSATION AND THERMAL COMFORT CONDITIONS Article 25 October 2023 CORTICAL ACTIVITY DURING PAINFUL AND NON-PAINFUL STIMULATION OVER FOUR LOWER LIMB BODY SITES: A
FUNCTIONAL NEAR-INFRARED SPECTROSCOPY STUDY Article Open access 11 February 2025 ENHANCED CONDUCTIVE BODY HEAT LOSS DURING SLEEP INCREASES SLOW-WAVE SLEEP AND CALMS THE HEART Article Open
access 26 February 2024 INTRODUCTION Ice slurry ingestion, which comprises an icy mixture that is consumed like a beverage1, has been recently reported to improve endurance exercise capacity
in the heat. Siegel _et al_.1 were the first to investigate the effect of ice slurry ingestion on endurance exercise capacity in the heat, and reported that when participants ingested ice
slurry (−1 °C) or cold water (4 °C) before exercise, their rectal temperature (Tre) was significantly reduced, and time to exhaustion improved by 19%. Thereafter, various studies have
investigated the effect of ice slurry ingestion in different types of exercise2,3, with varied timing of ingestion (e.g., during or after exercise4,5,6), as well as on the central nervous
system (e.g., voluntary contraction7,8). These studies suggest that ice slurry ingestion has an ergogenic effect. Two mechanisms are proposed as contributing to this effect: (1) increased
heat storage capacity through a reduction in core temperature and (2) a sensory effect, such as a reduction in ratings of perceived exertion or an improvement in thermal comfort (i.e.,
impairment of central fatigue). Another possible factor for the ergogenic effect of ice slurry ingestion in preventing central fatigue is a reduction in brain temperature (Tbrain). Vanden
Hoek _et al_.9 compared the core temperatures in swine after central catheter infusions of 50 mL/kg of saline ice slurry and 50 mL/kg of chilled saline, and reported that ice slurry
significantly reduced the animals’ Tbrain. In humans, Siegel _et al_.10 suggested that oral ingestion of ice slurries possibly resulted in conductive cooling of the facial skin and brain. To
verify this hypothesis, we previously investigated the effects of ice slurry ingestion on forehead skin temperature in the heat, and found a significant (vs. 37 °C, _p_ < 0.01; vs. 4 °C,
_p_ < 0.001) reduction in temperature, suggesting a potential reduction in Tbrain through conductive cooling of the facial skin and brain with ice slurry ingestion11. However, our study
used an indirect index (i.e., forehead skin temperature). In addition, to the best of our knowledge, no studies have directly observed an alteration in Tbrain after ice slurry ingestion.
Presently, magnetic resonance spectroscopy (MRS) is utilized as a non-invasive method to measure Tbrain12,13,14. Experimental studies in phantoms used water-N-acetyl aspartate (NAA)
solutions as models and revealed a reduction in the difference of NMR frequencies between NAA and water15,16. Moreover, experimental models15,16,17,18,19,20 showed a close correlation
between temperature that was measured using MRS and those measured using implanted probes. However, this method has only been applied in clinical settings and not in the field of exercise
physiology. Therefore, the aim of this study was to investigate the effect of ice slurry ingestion on Tbrain using MRS. Given the nature of MRS, we conducted the experiment with participants
at rest in a temperate condition. METHODS PARTICIPANTS Of the 10 healthy men recruited for this study, two were excluded because spectra measurement was difficult (i.e., the participants
could not remain still). Therefore, eight healthy men (mean age, 26.9 ± 4.6 y; mean height, 1.71 ± 0.05 m; mean body mass, 67.19 ± 7.80 kg; mean body mass index, 22.9 ± 2.5 kg/m2)
participaTed in this study. Our study was approved by the Ethics in Human Research Committee of the Japan Institute of Sports Sciences (no. 024), and was performed in accordance with the
Declaration of Helsinki. All participants signed an informed consent form prior to participation. EXPERIMENTAL DESIGN Our experiment included two separate conditions: ingesting a
thermoneutral sports beverage (37 °C; CON) and ingesting ice slurry (−1 °C; ICE). Throughout the study period, participants were asked to maintain their normal lifestyle activities,
including their physical activity and nutritional habits. Participants were asked to ingest 500 mL of water 2 h before the experimental trials. Two trials were conducted on 2 consecutive
days in a counterbalanced order and at the same time of day to eliminate any potential effects from circadian variations. Upon arrival in our laboratory, participants’ urine samples were
collected, and nude body mass was obtained. Then, a rectal temperature probe was self-inserted, and skin thermistors were attached. Subsequently, subjects put on t-shirts and shorts.
Participants entered a room that was maintained at 23 °C with 45% relative humidity (mean room temperature, 23.0 ± 0.2 °C; mean relative humidity, 43.8 ± 1.5%), and were asked to lie supine
on the magnetic resonance imaging (MRI) table. The head/neck coil was then attached. After a 15-min rest (baseline period), the participants ingested 7.5 g/kg of either CON or ICE ad libitum
over about 5 min (CON, 3.9 ± 1.1 min; ICE, 4.7 ± 1.7 min). After ingestion, participants remained at rest for an additional 30 min. BEVERAGE COMPONENTS AND PROTOCOL Both beverages were
conventional sports drinks (Pocari Sweat; Otsuka Pharmaceutical, Tokyo, Japan) containing 6.2 g of carbohydrates and 49 mg of sodium and 20 mg of potassium per 100 mL as electrolytes. CON
was heated in a thermostatic chamber (TR-2A; AS ONE, Osaka, Japan) and ICE was made using a slurry machine (Big Biz1; FMI, Osaka, Japan). Given the difficulty of consuming beverages while
lying supine, participants used a bottle (Floe Bottle; Teknicool Ltd., Auckland, New Zealand) during the ingestion period. The beverage volumes were calculated and prepared by the research
staff, who then filled two bottles, each with half of the beverage volume, with the respective beverage (CON or ICE). Before the baseline period, the participants were instructed to pick up
one of the two bottles and ingest its contents. Both bottles were then positioned near the participant’s hand immediately before the ingestion period. The participants were instructed to
ingest the beverage from either bottle while occasionally mixing the contents of the bottle. The bottles were collected after the contents were consumed. Despite these instructions, some ice
remained in the bottle because of its structure; thus, the actual total volume of ingested ICE was lower than 7.5 g/kg. Individual variations were noted for the remaining ice volume, and
the mean value for these four participants was 37.50 ± 48.32 g. MRI AND MRS MRI and MRS were performed using a 3-Tesla MRI scanner (Magnetom Skyra; Siemens Healthcare, Erlangen, Germany) and
64-channel head/neck coil. Three-dimensional (3D) T1-weighted images (magnetisation prepared rapid acquisition with gradient echo) were acquired using the following parameters: repetition
time (TR), 1,900 ms; echo time (TE), 2.29 ms; inversion time, 850 ms; flip angle (FA), 8°; matrix, 356 × 256; field of view, 240 mm; and slice thickness, 1 mm1. Hydrogen MRS was performed
with point-resolved spectroscopy localisation. Acquisition parameters were as follows: TR, 10,000 ms; TE, 135 ms; FA, 90°; data size, 2048; spectral width, 1,200 Hz; and excitation, 1. The
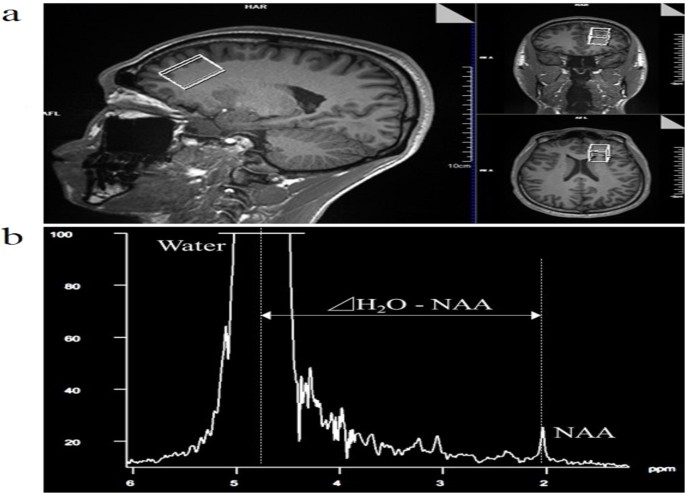
voxel of interest (VOI) was set in the frontal cortex, which has been shown to correlate with cognitive function21, using the 3D-T1 image (Fig. 1a). The VOI size was 20 × 30 × 20 mm. The
magnetic resonance spectrum showed choline-containing compounds at 3.2 ppm, creatine phosphate at 3.0 ppm, and NAA at 2.0 ppm. Tbrain was calculated from the chemical shift between NAA and
water using the equation (1) proposed by Cady _et al_.12: $${{\rm{T}}}_{{\rm{brain}}}={\rm{286}}\mathrm{.9}\,-[{\mathrm{94}\times ({\rm{\Delta }}{\rm{H}}}_{2}{\rm{O}}-\mathrm{NAA})],$$ (1)
where Δ was the difference between NAA and the water chemical shift (Fig. 1b). We used NAA peak because it is easy to observe and has been used in many earlier studies12,13,14,15,16,17,18.
MEASUREMENTS As physiological indices, Tbrain, Tre, mean skin temperature (Tsk), nude body mass, and urine specific gravity were measured. Tbrain was measured every 30 s as described above
and was averaged every 1 min. The change in Tbrain (ΔTbrain) was calculated using the following equation (2): $${\rm{\Delta
}}{{\rm{T}}}_{{\rm{brain}}}=\mathrm{mean}\,\,{{\rm{T}}}_{{\rm{brain}}}\,{\rm{after}}\,\,{\rm{beverage}}\,\mathrm{ingestion}\,-{\rm{mean}}\,{{\rm{T}}}_{{\rm{brain}}}\,{\rm{at}}\,\mathrm{Pre},$$
(2) where Pre was defined as the mean Tbrain of the 15-min baseline period (before beverage ingestion) and after beverage ingestion comprised the 30-min rest period, including the Post
measurement. The Post measurement was defined as the end of beverage ingestion (10 min after Pre). Tre was measured with a thermistor (LT-ST08-21; Nikkiso-Therm Co., Ltd., Tokyo, Japan) that
was inserted 12 cm from the anal sphincter with a disposable rubber sheath (11Y24; Nikkiso-Therm Co., Ltd.). The change in Tre (ΔTre) was calculated using the following equation (3):
$${\rm{\Delta
}}{{\rm{T}}}_{{\rm{r}}{\rm{e}}}={\rm{l}}{\rm{o}}{\rm{w}}{\rm{e}}{\rm{s}}{\rm{t}}\,{\rm{r}}{\rm{e}}{\rm{c}}{\rm{o}}{\rm{r}}{\rm{d}}{\rm{e}}{\rm{d}}\,\,{{\rm{T}}}_{{\rm{r}}{\rm{e}}}\,-{{\rm{T}}}_{{\rm{r}}{\rm{e}}}\,{\rm{a}}{\rm{t}}\,{\rm{P}}{\rm{r}}{\rm{e}},$$
(3) where the time when Tre was maximally reduced was defined as that in ICE (23.3 ± 6.9 min), and Pre was defined as the recorded value immediately prior to beverage ingestion (the end of
the 15-min baseline period). Skin temperature was measured at three sites (chest, upper arm, and thigh) using thermistors (LT-ST08-12; Nikkiso-Therm Co., Ltd.) that were secured with
micropore tape. All thermistors were connected to a data collection device (LT-8A; Gram Corporation, Saitama, Japan), and temperatures were recorded every 30 s. Tsk was calculated using the
equation (4) by Roberts22: $${{\rm{T}}}_{{\rm{sk}}}=({\rm{0.43}}\,\times \,{\rm{chest}}\,{\rm{temperature}})+({\rm{0.25}}\,\times \,{\rm{arm}}\,{\rm{temperature}})+({\rm{0.32}}\,\times
\,{\rm{thigh}}\,{\rm{temperature}}).$$ (4) Nude body mass was measured using a weighing machine (HW-100KGV; A and D, Tokyo, Japan) to the nearest 10 g, and urine specific gravity was
determined using a digital urine specific gravity scale (PAL-09S; Atago, Tokyo, Japan) before and after the experiment. Subjective thermal sensation (TS) and thermal comfort (TC) were
measured before and after the experiment using the scale derived by Gagge23 (0 = very cold to 8 = very hot) and the modified Bedford24 scale (1 = very uncomfortable to 7 = very comfortable),
respectively. TS and TC of the entire body and head were measured. STATISTICAL ANALYSIS Results are presented as mean ± standard deviation. Statistical comparisons of the results for
Tbrain, Tre, Tsk, TS, TC, body mass, and urine specific gravity were performed using a two-factor (condition × time) analysis of variance with repeated measures. When a significant main
effect was identified, the differences were compared using _t_-tests (Tbrain, Tre, Tsk, TS, and TC between conditions, and body mass and urine specific gravity before and after the
experiment). Pearson’s correlation coefficients were calculated to assess possible correlations between ΔTbrain and ΔTre. All statistical analyses were performed using SPSS Statistics 17.0
software package (SPSS, Inc., Chicago, IL, USA). Statistical significance was accepted at _p_ < 0.05. All physiological measurements after beverage ingestion are presented in 5-min
increments from Post to 30 min (+30) (Fig. 2). DATA AVAILABILITY The datasets generated during and/or analysed in the present study are available from the corresponding author on reasonable
request. RESULTS PHYSIOLOGICAL MEASUREMENTS Changes in temperature over time are presented in Fig. 3. Specifically, changes in Tbrain during the experiment and ΔTbrain are shown in Fig. 3a
and d, respectively. ICE had a significantly greater Tbrain reduction than CON at Post, +10, and +15 (_p_ < 0.05 for all). Moreover, ΔTbrain was significantly greater in the ICE condition
than in the CON condition (_p_ < 0.05). The changes in Tre during the experiment and ΔTre are shown in Fig. 3b and e, respectively. Tre exhibited a significantly greater reduction with
ICE than with CON from +5 to +25 (_p_ < 0.05 for all). Additionally, compared to Pre, Tre was significantly lower from Post to +25 (_p_ < 0.01 for all). Between conditions, ΔTre was
significantly greater with ICE than with CON (_p_ < 0.001). Further, ΔTbrain was significantly correlated with ΔTre (r = 0.60, _p_ < 0.05). Changes in Tsk are shown in Fig. 3c. Tsk
significantly increased at +20 and +25 in the CON condition and at Post and +5 in the ICE condition, but no differences between the conditions were observed. The hydration state before and
after the experiment is summarised in Table 1. In the CON condition, body mass significantly increased after the experiment (_p_ < 0.001); however, no significant differences were
detected between CON and ICE. Urine specific gravity significantly reduced after the experiment in both conditions (_p_ < 0.05), but no significant differences were found between CON and
ICE. SUBJECTIVE MEASUREMENTS Changes in TS and TC are summarised in Table 1. TS of the entire body yielded a significantly greater reduction with ICE than with CON after the experiment (_p_
< 0.001). Moreover, ICE yielded a significantly greater reduction in TC of the entire body after the experiment compared to before the experiment (_p_ < 0.01). Further, TC of the
entire body was significantly reduced in the ICE condition compared with in the CON condition (_p_ < 0.05). DISCUSSION The speculation that ice slurry ingestion can impair central fatigue
during exercise in the heat by reducing Tbrain has attracted attention. Some studies have suggested that ice slurry ingestion may reduce Tbrain25,26,27. Because directly measuring Tbrain in
humans is difficult, only an indirect index can be used, particularly during exercise. Therefore, we measured brain temperature at rest using the MRS method to improve the validity of data
obtained from the indirect index. Our results showed that ice slurry ingestion significantly reduced Tbrain, as well as Tre, TS, and TC. These results are important as they provide
foundational data for future applied studies. To the best of our knowledge, the present study is the first to successfully reveal a reduction in Tbrain in humans through ice slurry ingestion
using MRS. Two mechanisms could explain how ice slurry ingestion reduces Tbrain: inflow of cooled carotid blood and conductive cooling of the facial skin and brain10. Our previous report of
reduced forehead skin temperature with ice slurry ingestion in a warm environment supports the latter mechanism11. Given the limitation of MRI, we could not measure forehead skin
temperature using a thermistor probe in the present study; however, ice slurry ingestion may possibly reduce facial skin temperature, thereby reducing Tbrain as previously reported.
Interestingly, the reduction in Tbrain observed in this study (−0.4 °C) was the same as that of head cooling achieved through fanning28. Therefore, ice slurry ingestion can cool the brain
more easily than fanning, contributing to the development of effective body cooling strategies. Further investigation of this phenomenon is needed. Fuller _et al_.29 reported that despite
different pre-exercise temperatures, environmental conditions, and running time, rats reached a point of fatigue at the same Tbrain, suggesting a critical level in Tbrain, as well as core
temperature. If the same concept is applied in humans, reaching this critical level can be delayed by pre-cooling the brain with ice slurry ingestion, thereby extending the exercise time.
Previous studies reported that a −0.4 °C reduction of core temperature can improve exercise performance1,27; hence, a −0.4 °C reduction of Tbrain may also improve exercise performance.
Moreover, the reduction in Tbrain may explain the ergogenic effect of ice slurry ingestion during exercise. Previous studies reported that ice slurry ingestion during exercise improved
performance without changes in core temperature4,5. The authors cited an improvement in subjective sensation, such as TC, as the contributing factor. Sensory improvement was possibly
influenced by a reduction in Tbrain as core and skin temperatures were not significantly altered. Although TC became worse with ice slurry ingestion in the present study, we believe that
this effect resulted from ingestion in a temperate environment, and heat production was likely not caused by shivering because the mean Tsk was unchanged and according to the participants’
perspective. In previous studies investigating the effects of beverage ingestion on MRI scans30,31, the liquid was expelled from a syringe into a capillary tube using a separate syringe
pump. However, given that the ice slurry cannot be administered through a tube, the participants in this study picked up the ice slurry bottle by themselves and consumed the beverage ad
libitum. Two limitations should be noted for this aspect. First, we cannot exclude the possibility that the movement of the participant’s head disturbed the acquisition of spectra. Indeed,
Tbrain during beverage ingestion was excluded because of spectral issues. However, as the spectra after beverage ingestion was stable, similar to that before beverage ingestion, we believed
that it was possible to investigate the effect of ice slurry ingestion on Tbrain. Second, some ice remained in the bottle despite pouring the ice slurry. Hence, we could not control the
volume of beverage that the participants ingested. Therefore, ΔTre with ICE was smaller in the present study than that (0.66 °C) in a previous study in a temperate environment1. If the
participants were able to ingest the required volume, ΔTre with ICE may be greater in the present study. Moreover, the present study found a significant correlation between ΔTre and ΔTbrain,
indicating that the greater the ΔTre, the greater the ΔTbrain; thus, ΔTbrain with ICE might also be greater if participants ingest the required volume. Our results are robust because we
show that even a lower volume of ice slurry can reduce Tbrain. An indirect method using tympanic temperature was reported as an alternative to measuring Tbrain during exercise. However, many
studies have demonstrated that this method is challenging, especially during physical exertion in the heat, and can lead to measurement errors caused by dirt, inaccurate placement, and
insufficient skill of the measurer32,33,34,35,36. Thus, it does not provide an accurate reflection of core temperature37. Conversely, measuring Tbrain before and after exercise using MRS is
possible; therefore, investigating the effect of ice slurry ingestion on Tbrain when the body temperature increases from exercise is also possible. In the present study, we measured Tbrain
at the frontal cortex, which is related to cognitive function as the index of central fatigue. However, Tbrain in other brain regions may be different. For example, some studies have
reported temperature gradients of up to 1 °C from cooler superficial to warmer deep brain tissues in humans13,38,39. Therefore, it is worthwhile to measure Tbrain in other deep regions that
are related to thermoregulation, such as the hypothalamus. Ice slurry ingestion significantly reduced Tbrain, as well as Tre and TS in humans. Our findings can contribute to the development
of effective body cooling strategies. However, further research is needed to clarify the underlying mechanism of the ergogenic effect of ice slurry ingestion. REFERENCES * Siegel, R. _et
al_. Ice slurry ingestion increases core temperature capacity and running time in the heat. _Med. Sci. Sports. Exerc._ 42(4), 717–725 (2010). Article PubMed Google Scholar * Onitsuka, S.,
Ueno, T., Zheng, X. & Hasegawa, H. The effect of ice slurry ingestion during half-time breaks on intermittent exercise capacity and thermoregulation in the warm environment. _Gazz. Med.
Ital-Arch. Sci. Med._ 174, 113–121 (2015a). Google Scholar * Stevens, C. J., Dascombe, B., Boyko, A., Sculley, D. & Callister, R. Ice slurry ingestion during cycling improves Olympic
distance triathlon performance in the heat. _J. Sports. Sci._ 31(12), 1271–1279 (2013). Article PubMed Google Scholar * Burdon, C. A., Hoon, M. W., Johnson, N. A., Chapman, P. G. &
O’Connor, H. T. The effect of ice slushy ingestion and mouthwash on thermoregulation and endurance performance in the heat. _Int. J. Sport. Nutr. Exerc. Metab._ 23(5), 458–469 (2013).
Article PubMed Google Scholar * Schulze, E. _et al_. Effect of thermal state and thermal comfort on cycling performance in the heat. _Int. J. Sports. Physiol. Perform._ 10(5), 655–663
(2016). Article Google Scholar * Stanley, J., Leveritt, M. & Peake, J. M. Thermoregulatory responses to ice-slush beverage ingestion and exercise in the heat. _Eur. J. Appl. Physiol._
110(6), 1163–1173 (2010). Article PubMed Google Scholar * Burdon, C. A., Easthope, C. S., Johnson, N. A., Chapman, P. G. & O’Connor, H. The influence of ice slushy on voluntary
contraction force following exercise-induced hyperthermia. _Appl. Physiol. Nutr. Metab._ 39(7), 781–786 (2014). Article CAS PubMed Google Scholar * Siegel, R., Maté, J., Watson, G.,
Nosaka, K. & Laursen, P. B. The influence of ice slurry ingestion on maximal voluntary contraction following exercise-induced hyperthermia. _Eur. J. Appl. Physiol._ 111(10), 2517–2524
(2011). Article PubMed Google Scholar * Vanden Hoek, T. L. _et al_. Induced hypothermia by central venous infusion: saline ice slurry versus chilled saline. _Crit. Care. Med._ 32,
S425–431 (2004). Article PubMed Google Scholar * Siegel, R., Maté, J., Watson, G., Nosaka, K. & Laursen, P. B. Pre-cooling with ice slurry ingestion leads to similar run times to
exhaustion in the heat as cold water immersion. _J. Sports. Sci._ 30, 155–165 (2012). Article PubMed Google Scholar * Onitsuka, S., Zheng, X. & Hasegawa, H. Ice slurry ingestion
reduces both core and facial skin temperatures in a warm environment. _J. Therm. Biol._ 51, 105–109 (2015b). Article PubMed Google Scholar * Cady, E. B., D’Souza, P. C., Penrice, J. &
Lorek, A. The estimation of local brain temperature by _in vivo_ 1H magnetic resonance spectroscopy. _Magon. Reson. Med._ 33, 862–867 (1995). Article CAS Google Scholar * Corbett, R.,
Laptook, A. & Weatherall, P. Noninvasive measurements of human brain temperature using volume-localized proton magnetic resonance spectroscopy. _J. Cereb. Blood. Flow. Metab._ 17,
363–369 (1997). Article CAS PubMed Google Scholar * Jayasundar, R. & Singh, V. P. _In vivo_ temperature measurements in brain tumors using proton MR spectroscopy. _Neurol. India._
50, 436–439 (2002). CAS PubMed Google Scholar * Corbett, R. J., Laptook, A. R., Tollefsbol, G. & Kim, B. Validation of a noninvasive method to measure brain temperature _in vivo_
using 1H NMR spectroscopy. _J. Neurochem._ 64, 1224–1230 (1995). Article CAS PubMed Google Scholar * Ishihara, Y. _et al_. A precise and fast temperature mapping using water proton
chemical shift. _Magn Reson Med._ 34, 814–823 (1995). Article CAS PubMed Google Scholar * Corbett, R. J., Purdy, P. D., Laptook, A. R., Chaney, C. & Garcia, D. Noninvasive
measurement of brain temperature after stroke. _Am. J. Neuroradiol._ 20, 1851–1857 (1999). CAS PubMed Google Scholar * Kuroda, K. _et al_. Feasibility of internally referenced brain
temperature imaging with a metabolite signal. _Magn. Reson. Med. Sci._ 2, 17–22 (2003). Article PubMed Google Scholar * McDannold, N., King, R. L. & Hynynen, K. MRI monitoring of
heating produced by ultrasound absorption in the skull: _in vivo_ study in pigs. _Magn Reson Med._ 51, 1061–1065 (2004). Article PubMed Google Scholar * Trubel, H. K., Maciejewski, P. K.,
Farber, J. H. & Hyder, F. Brain temperature measured by 1H-NMR in conjunction with a lanthanide complex. _J. Appl. Physiol._ 94, 1641–1649 (2003). Article PubMed Google Scholar *
MacDonald, A. W., Cohen, J. D., Stenger, V. A. & Carter, C. S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. _Science._ 288,
1835–1838 (2006). Article ADS Google Scholar * Roberts, M. F., Wenger, C. B., Stolwijk, J. A. & Nadel, E. R. Skin blood flow and sweating changes following exercise training and heat
acclimation. _J. Appl. Physiol._ 43, 133–137 (1977). Article CAS PubMed Google Scholar * Gagge, A. P., Stolwijk, J. A. & Hardy, J. D. Comfort and thermal sensations and associated
physiological responses at various ambient temperatures. _Environ. Res._ 1, 1–20 (1969). Article Google Scholar * Bedford, T. The warmth factor in comfort at work: a physiological study of
heating and ventilation. _Industrial Health Research Board_. NO 76, HMSO, London (1936). * Jones, P. R., Barton, C., Morrissey, D., Maffulli, N. & Hemmings, S. Pre-cooling for endurance
exercise performance in the heat: a systematic review. _BMC Med._ 10, 166–184 (2012). Article PubMed PubMed Central Google Scholar * Tan, P. M. & Lee, J. K. The role of fluid
temperature and form on endurance performance in the heat. _Scand J Med Sci Sports._ 1, 39–51 (2015). Article Google Scholar * Naito, T., Iribe, Y., Ogaki, T. Ice ingestion with a long
rest interval increases the endurance exercise capacity and reduces the core temperature in the heat. _J Physiol Anthropol_. 36–39 (2017). * Harris, B. A., Andrews, P. J., Marshall, I.,
Robinson, T. M. & Murray, G. D. Forced convective head cooling device reduces human cross-sectional brain temperature measured by magnetic resonance: a non-randomized healthy volunteer
pilot study. _Br. J. Anaesth._ 100(3), 365–372 (2008). Article CAS PubMed Google Scholar * Fuller, A., Carter, R. N. & Mitchell, D. Brain and abdominal temperatures at fatigue in
rats exercising in the heat. _J. Appl. Physiol._ 84(3), 877–83 (1998). Article CAS PubMed Google Scholar * Chambers, E. S., Bridge, M. W. & Jones, D. A. Carbohydrate sensing in the
human mouth: effects on exercise performance and brain activity. _J. Physiol_. 15, 587(Pt 8), 1779–1794 (2009). * Guest, S. _et al_. Human cortical representation of oral temperature.
_Physiol. Behav._ 92, 975–984 (2007). Article CAS PubMed Google Scholar * Briner, W. W. Jr. Tympanic membrane vs rectal temperature measurement in marathon runners [letter]. _J Am Med
Assoc._ 276, 194 (1996). Article Google Scholar * Hooker, E. A. & Houston, H. Screening for fever in adult emergency department: oral vs tympanic thermometry. _South Med J._ 89,
230–234 (1996). Article CAS PubMed Google Scholar * Cetas, T. C. Thermometers in _Fever: basic mechanisms and management_ (ed. Philip, A. M.). 11–34 (LippinCott-Laven, 1997). *
Amoateng-Adjepong, Y., Del, M. J. & Manthous, C. A. Accuracy of an infrared tympanic thermometer. _Chest._ 115, 1002–1005 (1999). Article CAS PubMed Google Scholar * Cattaneo, C. G.
_et al_. The accuracy and precision of body temperature monitoring methods during regional and general anesthesia. _Anesth Analg._ 90, 938–945 (2000). Article CAS PubMed Google Scholar *
Daniel, S. M. & Liran, M. C. T. Measurement-Methods and Current Insights. _Sports Med._ 32(14), 879–885 (2002). Article Google Scholar * Shiraki, K. _et al_. Independence of brain and
tympanic temperatures in an unanesthetized human. _J Appl Physiol._ 65(1), 482–486 (1988). Article CAS PubMed Google Scholar * Mariak, Z., Lewko, J., Luczaj, J., Połocki, B. &
White, M. D. The relationship between directly measured human cerebral and tympanic temperatures during changes in brain temperatures. _Eur J Appl Physiol Occup Physiol._ 69(6), 545–549
(1994). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Sumire Onitsuka was supported as Research Fellows of the Japan Society for the Promotion of Science. This
work was supported by the Japan Institute of Sports Sciences, JSPS KAKENHI Grant Number JP 16J03843, JSPS KAKENHI Grant Number 16H03241, and the Sasakawa Scientific Research Grant from The
Japan Science Society. AUTHOR INFORMATION Author notes * Sumire Onitsuka, Daisuke Nakamura and Takahiro Onishi contributed equally to this work. AUTHORS AND AFFILIATIONS * Graduate School of
Integrated Arts and Sciences, Hiroshima University, Higashihiroshima, 739-8521, Japan Sumire Onitsuka & Hiroshi Hasegawa * Japan Society for the Promotion of Science, Tokyo, 102-0083,
Japan Sumire Onitsuka * Japan Institute of Sports Sciences, Tokyo, 115-0056, Japan Daisuke Nakamura, Takahiro Onishi & Hideyuki Takahashi * College of Sport and Health Science,
Ritsumeikan University, Kusatsu, 525-8577, Japan Takuma Arimitsu Authors * Sumire Onitsuka View author publications You can also search for this author inPubMed Google Scholar * Daisuke
Nakamura View author publications You can also search for this author inPubMed Google Scholar * Takahiro Onishi View author publications You can also search for this author inPubMed Google
Scholar * Takuma Arimitsu View author publications You can also search for this author inPubMed Google Scholar * Hideyuki Takahashi View author publications You can also search for this
author inPubMed Google Scholar * Hiroshi Hasegawa View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors have read and approved the
manuscript for submission. The following contributions were made: subject recruitment (S.O., D.N.), performing experiments (S.O., D.N., T.O., T.A.), study design and concept (S.O., D.N.,
T.O., T.A., H.H.), contribution to the writing, revising manuscripts and providing critiques (S.O., D.N., T.O., T.A., H.T., H.H.). CORRESPONDING AUTHOR Correspondence to Hiroshi Hasegawa.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Onitsuka, S., Nakamura, D., Onishi, T. _et al._ Ice slurry ingestion reduces human
brain temperature measured using non-invasive magnetic resonance spectroscopy. _Sci Rep_ 8, 2757 (2018). https://doi.org/10.1038/s41598-018-21086-6 Download citation * Received: 12 July 2017
* Accepted: 29 January 2018 * Published: 09 February 2018 * DOI: https://doi.org/10.1038/s41598-018-21086-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
