Elicitors derived from hazel (corylus avellana l. ) cell suspension culture enhance growth and paclitaxel production of epicoccum nigrum
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The microbial fermentation is considered as the potential source for large-scale production of paclitaxel. Since co-cultivation/mixed fermentation strategy has been reported as a
yield enhancement strategy for paclitaxel production, investigation of fungal endophyte response to plant culture medium, plant cell extract (CE) and medium filtrate (MF) of plant cell
suspension culture in terms of growth and paclitaxel production is interesting. In this study, 35 endophytic fungi were isolated from _Taxus baccata and Corylus avellana_ grown in Iran. The
analysis of high-performance liquid chromatography and mass spectrometry showed that one isolate (YEF2) produced paclitaxel. The isolate YEF2 was identified as _Epicoccum nigrum_ by
sequencing of ITS1-5.8S-ITS2 rDNA region and actin gene. YEF2 was slow-growing in Murashige and Skoog medium, but the synergistic interaction of gibberellic acid (GA3) and CE of _C.
avellana_ enhanced the growth of YEF2. The highest total yield of paclitaxel (314.7 µg/l; 11.5-folds) of _E. nigrum_ strain YEF2 was obtained by using 28% (v/v) filter sterilized CE of _C.
avellana_ and 2 µg ml−1 GA3 that was significantly higher than the control. In this study, the effects of the plant cell extract on growth and paclitaxel production of paclitaxel producing
endophytic fungus were studied for the first time. SIMILAR CONTENT BEING VIEWED BY OTHERS BIOPROSPECTING OF ENDOPHYTIC FUNGI FROM MEDICINAL PLANT _ANISOMELES INDICA_ L. FOR THEIR DIVERSE
ROLE IN AGRICULTURAL AND INDUSTRIAL SECTORS Article Open access 05 January 2024 IDENTIFICATION, FERMENTATION OPTIMIZATION, AND BIOCONTROL EFFICACY OF ACTINOMYCETE YG-5 FOR THE PREVENTION OF
_ALTERNARIA_ LEAF SPOT DISEASE IN STAR ANISE Article Open access 10 August 2024 INFLUENCE OF DIFFERENT ELICITORS ON BIA PRODUCTION IN _MACLEAYA CORDATA_ Article Open access 12 January 2021
INTRODUCTION Paclitaxel, a main impressive chemotherapeutic agent against a wide range of cancers1 was originally extracted from the bark of _Taxus brevifolia_, yew native to the
North-Western Pacific area and its chemical structure was elucidated in 19712. Paclitaxel stabilizes microtubules to depolymerization, thus arrest the division of actively growing tumor
cells at G1 or M phases3. Unfortunately, yew trees are slow-growing and large amounts of bark are required for paclitaxel production4. Owing to overexploitation, many species are now
endangered and on the brink of extinction5. Therefore, search for alternative sources of the drug is prompted. In 1993, the first paclitaxel-producing fungus was isolated from the Pacific
yew6. This initial discovery was followed by the plethora of different endophytic fungi reported producing paclitaxel7,8. A microbial fermentation process would be the most favorable means
of paclitaxel supply. Microorganisms are fast growing and their genetic manipulation is relatively easy and can be scaled-up to an industrial level. Therefore, microbial fermentation is
considered as the potential source for large-scale production of paclitaxel9. Low productivity of paclitaxel in endophytic fungi is a drawback for commercial production. Despite the various
genera of endophytes capable of producing paclitaxel9, there have been no major breakthroughs regarding to commercial production of paclitaxel by fungal fermentation. The problems including
the inconsistent production of fungal paclitaxel by repeated sub-culturing on defined artificial media10 have raised doubts about the commercial possibility of endophytic fungi as
sustainable production platforms. Venugopalan _et al_.11 stated that optimization of culture parameter including the exogenous addition of elicitors to _in vitro_ cultivation of endophytic
fungi improves production of secondary metabolites. It is reported that adding the host plant extracts to the fungal culture was successful in some cases12. Host plants affect metabolic
processes of the endophytes13 and reciprocally transcription of rate-limiting genes in plant paclitaxel biosynthetic pathway is increased by fungal endophytes14. Indeed, during the long
period of co-evolution, a friendly relationship has been gradually organized between each endophytic fungus and its host plant, so that host plant provides plentiful nourishment for
endophytes and fascinates their inhabitation leading to the survival of these endophytes. The endophytes, in turn, synthesize several bioactive compounds for protecting the host plants
against biotic and abiotic stresses and boosting their growth15,16. In some cases, endophytic fungi have gained the capability of producing identical or similar bioactive compounds as those
produced by their host plants. It seems the co-cultivation or the mixed fermentation can mimic the natural habitat of endophytes. Co-cultivation/mixed fermentation strategy has been reported
as a yield enhancement strategy for paclitaxel production17. It is stated that the addition of _Catharanthus roseus_ extract and ethanol in the medium enhanced the camptothecin production
in the suspension culture of _Fusarium solani_18. It assumes the addition of plant cell extract to suspension culture of paclitaxel-producing endophytic fungi or the co-cultivation of these
endophytes with plant cells can provide the required stimulus to the fungal endophyte in the axenic culture for enhanced and sustainable production of paclitaxel. In addition to _Taxus_
spp., hazel (_Corylus avellana_) has also been described as a paclitaxel-producing species through bioprospection among angiosperms19,20,21. The major advantage of producing taxanes through
hazel cell suspension culture (CSC) is that hazel is widely available, grows more quickly _in vivo_, and is easier to cultivate _in vitro_ than yew22. In the light of importance _in vitro_
cultures of _C. avellana_ as a promising and cheaper source for paclitaxel production23, It seems that the co-cultivation of _C. avellana_ cells with paclitaxel-producing endophytic fungus
can be promising for enhancing paclitaxel production. Therefore, investigation of fungal endophyte response to plant culture medium, cell extract (CE) and medium filtrate (MF) of _C.
avellana_ CSC in terms of growth and paclitaxel production is crucial. The objectives of this study were (a) to isolate endophytic fungi from _Taxus baccata_ and _C. avellana_ grown in Iran,
(b) to screen and identify paclitaxel producing isolates and (c) to investigate the response of paclitaxel-producing endophytic fungus (growth and paclitaxel production) to CE and MF of _C.
avellana_ CSC as well as GA3. RESULTS ISOLATION, SCREENING AND IDENTIFICATION OF THE PACLITAXEL-PRODUCING ENDOPHYTIC FUNGUS A total of 35 isolates was separated from _T. baccata_ and _C.
avellana_. Paclitaxel was extracted from culture filtrates and mycelia of fungi and then analyzed by HPLC. The HPLC analysis of these isolates showed that the peak positions of two strain
(YEF2 and HEF12) were identical to that of standard paclitaxel (retention time = 4.59 ± 0.05 min) (Fig. S1), indicating these fungal isolates may produce paclitaxel. Analysis of paclitaxel
production of these two isolates in six passages showed that only production of strain YEF2 was stable. This strain was isolated from _T. baccata_ bud. Further confirmation for the identity
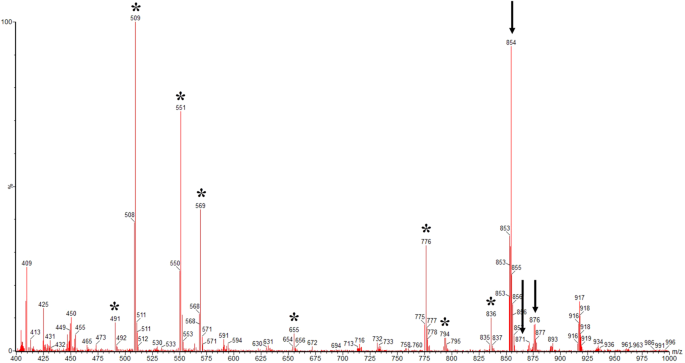
of the paclitaxel was gained by LC-MS/MS. Figure 1 shows representative mass spectra of paclitaxel from strain YEF2. The selected ions for the paclitaxel standard are an (M + Na+) ion with
mass-to-charge ratio (m/z) 876, an (M + H+) ion with m/z 854 and an (M + NH4+) ion with m/z 87124,25. The peaks of fungal paclitaxel exhibited m/z ratios corresponding to these molecular
ions that confirm this fungal strain can generate paclitaxel. The asterisks on the spectra (Fig. 1) indicate fragments ions which were most helpful for identifying the paclitaxel20,25. By
analysis of the sequences of ITS1-5.8S-ITS2 Region and actin gene, this paclitaxel producing strain was identified as _Epicoccum nigrum_ (YEF2). Endophytic fungal strain YEF2 was deposited
in Iranian Fungal Culture Collection (WDCM939) under the accession number IRAN 2950C. The partial sequences of the ITS rDNA and actin gene obtained from strain YEF2 were deposited in GenBank
(NCBI) under the accession numbers MF371418 and MF381043, respectively. It was very attractive to know if elicitors derived from cell suspension of the non-host plant, _C. avellana_, affect
growth and paclitaxel production of strain YEF2. SYNERGISTIC INTERACTION OF GIBBERELLIC ACID AND CE OF _C. AVELLANA_ CSC ENHANCED THE GROWTH OF _E. NIGRUM_ _E. nigrum_ is slow-growing in MS
medium supplemented with GA3 (Fig. S2c). The addition of _C. avallana_ CE to MS medium at the time of culture did not increase the growth of _E. nigrum_ (Fig. S2b), whereas this strain was
relatively fast-growing in MS supplemented with GA3 and CE of _C. avallana_ (Fig. S2a). The best GA3 concentration for _C. avellana_ cell growth was shown to be 2 µg/ml (data not shown). So,
in all next experiments, 2 µg/ml GA3 was added to the medium at the time of culture. At the first, three concentrations (3%, 5% and 7% (v/v)) of _C. avallana_ CE were added to culture
medium and a slight increase in growth was obtained by adding 3% and 5% CE of _C. avellana_ (Fig. S3). Therefore, three concentrations (7%, 14%, and 28%) were selected for next experiment.
THE CE AND MF OF _C. AVELLANA_ CSC ENHANCED GROWTH AND TOTAL YIELD OF PACLITAXEL OF _E. NIGRUM_ STRAIN YEF2 Paclitaxel in culture medium containing the elicitors derived from _C. avellana_
CSC with no inoculums was analyzed (Table S2) and paclitaxel in all treatments was modified based on paclitaxel of the culture medium containing the elicitors with no inoculums. The results
of induction of growth and paclitaxel production in _E. nigrum_ using CE and MF of _C. avellana_ CSC showed that the fresh weight (FW), dry weight (DW), intracellular paclitaxel (µg/l),
extracellular paclitaxel and total paclitaxel of the fungus significantly affected by CE and MF of _C. avellana_ CSC. The main effects of treatments and their interactions (reciprocal and
trilateral effects) on mentioned traits were highly significant. Considering the treatments individually, CE was performed better as plant elicitor in terms of FW (218.1 g/l) (5.3- folds),
DW (9 g/l) (2.9- folds), intracellular (30.1 μg/l) (3.3-folds), extracellular (191.3 μg/l) (10.8-folds) and total yield of paclitaxel (221.4 μg/l) (8.2-folds) as compared to the control
(Fig. 2). The significant interaction effect of elicitor type (CE or MF) and sterilization method (Filter sterilized or autoclaved) indicated that elicitor type affected measured traits
differently depending on used sterilization technique. Because of the significant interaction effect of elicitor type and sterilization technique, the effects of elicitor type were analyzed
on each sterilization method. The means comparison indicated that FCE was more effective than ACE for the increase of FW (254. 1 g/l) (6.2-folds), DW (9.6 g/l) (3.1-folds), intracellular
(33.8 μg_/_l) (3.6-folds), extracellular (224.0 μg/l) (12.7-folds) and total yield of paclitaxel (257.8 μg/l) (9.6-folds) (Fig. 3). The interaction effect of elicitor type × concentration
level indicated that the effect of elicitors was level-dependent (Fig. 4) and the means comparison showed that the highest FW (384. 4 g/l) (9.9-folds), DW (12.6 g/l) (4.1-folds),
intracellular (40.3 μg/l) (4.3-folds), extracellular (274.4 _μ_g/l) (15.3-folds) and total yield of paclitaxel (314.7 μg/l) (11.5-folds) of _E. nigrum_ strain YEF2 were obtained with 28%
(v/v) FCE and 2 µg/ml GA3 that were significantly higher than the control (supplemented with 2 µg/ml GA3 and 28% (v/v) filter sterilized water) with a mean of 38.9 g/l, 3.1 g/l, 9.3 μg/l,
17.9 μg/l and 27.3 μg/l, respectively (Table 1). The results of ANOVA indicated that CE and MF of _C. avellana_ CSC increased intracellular (per liter of medium), extracellular and total
yield of paclitaxel, whereas no significant increase in intracellular yield of paclitaxel per gram dry weight of mycelia was observed. Total yield of paclitaxel (dependent variable (Y)) was
regressed against FW and DW. The regressions of total paclitaxel against FW and DW for FCE, ACE, AMF were positive and significant (Table 2). Additionally, the fitted models for ACE and FCE
exhibited high R-Squared. Meanwhile, AMF increased FW (79.7 g/l) (1.9-folds), and DW (4.9 g/l) (1.6-folds) of _E. nigrum_ more than FMF (Fig. 5b). However, FMF was more effective for
paclitaxel production than the AMF (Fig. 5a). Also, The regressions of total paclitaxel against FW and DW for FMF were not significant (Table 2). DISCUSSION _E. NIGRUM AS A_
PACLITAXEL-PRODUCING ENDOPHYTIC FUNGUS _E. nigrum_ is a fungal species from the phylum _Ascomycota_ and a saprophyte on crop residues26. This species can live as an endophyte in different
plants27,28. In some studies, _Epicoccum_ genus was reported as a paclitaxel producing endophytic fungus10,24,29. Also, _E. nigrum_ is especially known for its biocontrol activity against
pathogens30,31. _E. nigrum_ is yellow initially and then become orange and later brown in late growth stage (Fig. S4). A yellow pigment was extracted from the culture of _E. nigrum_ and
identified as flavipin (3,4,5- trihydroxy-6-methylphthalaldehyde) which had antifungal activity32. Also, the red pigments produced by _E. nigrum_ were analyzed and detected to consist of
four carotenoids27,33. These colorants produced by _E. nigrum_ could be used in food, pharmaceutical, textile, and cosmetic industries34. SYNERGISTIC INTERACTION OF GIBBERELLIC ACID AND CELL
EXTRACT OF _C. AVELLANA_ ENHANCED THE GROWTH OF _E. NIGRUM_ Probably the co-cultivation of _E. nigrum_ with _C. avellana_ cells can enhance the yield of paclitaxel. Therefore, investigating
the ability of _E. nigrum_ to grow in plant culture media is essential. _C. avellana_ cell clone used in this study have cultured in MS medium. MS medium is one of the most commonly used
media for plant tissue culture that developed by Murashige and Skoog35. The preliminary experiments showed that _E. nigrum_ strain YEF2 is very slow-growing in MS medium (Fig. S2d). GA3 is a
hormone found in plants and fungi and reported that it stimulates growth in cellulolytic fungi, i.e., _Chaetomium globosum, Memnoniella echinata_ and _Ourvularia lunata_36. Therefore, we
hypothesized that this hormone may stimulate the growth of _E. nigrum_. El-bahrawy36 stated that the fungi differed from each other to the response of GA3 effect. It is observed that _E.
nigrum_ is slow-growing in MS medium supplemented with GA3. (Fig. S2c). Since _E. nigrum_ strain YEF2 is an endophytic fungus of plants and host plant supply plenteous nutrients for the
survival of its endophytes in symbiosis relationship, in the next stage we tested the effects CE and MF of _C. avellana_ CSC on the growth of _E. nigrum_. The addition of CE of _C. avellana_
CSC to MS medium did not increase the growth of _E. nigrum_ (Fig. S2b), whereas this strain was relatively fast-growing in MS supplemented with GA3 and cell extract of _C. avallana_ CSC
(Fig. S2a). Indeed, the effects of CE of _C. avallana_ CSC and GA3 on _E. nigrum_ growth were synergistic. Based on our observations, GA3 stimulates the growth of _E. nigrum_ strain YEF2 in
the presence of hazel CE. Indeed, GA3 was the essential prerequisite for the growth of strain YEF2 in the presence of _C. avallana_ CE. CE AND MF OF _C. AVELLANA_ CSC ENHANCED GROWTH AND
TOTAL YIELD OF PACLITAXEL OF _E. NIGRUM_ STRAIN YEF2 Resident endophytes within plants are steadily interacting with their hosts. It is found that plants would have a substantial influence
on _in planta_ metabolic processes of the endophytes13. For example, the study of the gene cluster expression for lolitrem biogenesis in endophytic _Neotyphodium lolii_ resident in
_perennial ryegrass_ revealed that expression of these genes is high _in planta_, but low _in vitro_ fungal cultures37. So it seems that fungal paclitaxel production such as fungal growth
would be affected by CE and MF of _C. avellana_ CSC. Therefore, the effects of CE and MF of _C. avellana_ CSC on fungal paclitaxel production were investigated. The CE was performed better
as plant elicitor in terms of the growth and paclitaxel production. Indeed, The CE of _C. avellana_ supplies plenteous nutrients and enhances the growth of strain YEF2. The means comparison
indicated that FCE was more effective than ACE for the increase of growth and paclitaxel production (Fig. 3). Autoclaving denatures proteins and degrades amino acids and may decrease the
nutritional value of CE which resulted in decreased growth and paclitaxel production. The results of ANOVA indicated that CE and MF of _C. avellana_ CSC increased intracellular (per liter of
medium), extracellular and total yield of paclitaxel whereas no significant increase in intracellular yield of paclitaxel per gram dry weight of mycelia was observed. Indeed, the part of
produced paclitaxel accumulated in mycelia and much of it secreted in the culture medium. Simple linear regression was developed to determined relationship between total yield of paclitaxel
and FW and DW. The fitted models for ACE and FCE exhibited high R-Squared which showed these models explain high percent of the variability in total paclitaxel. Also, the high correlation
coefficients indicate relatively strong relationship between total paclitaxel and FW and DW (Table 2). It seems that CE (autoclaved and filter sterilized) and AMF of _C. avellana_ CSC
enhanced the growth of _E. nigrum_ strain YEF2 and the increase in total paclitaxel production is due to increase biomass rather than a direct effect of the CE and AMF of _C. avellana_ CSC.
Meanwhile, AMF increased the growth of _E. nigrum_ more than FMF (Fig. 5b). However, FMF was more effective for paclitaxel production than the AMF (Fig. 5a). Also, Regressions of total
paclitaxel against FW and DW for FMF were not significant (Table 2). It is assumed that there are many precursors of paclitaxel in _C. avellana_ CSC and used to produce paclitaxel that these
precursors were degraded by autoclaving and lower growth in MS medium supplemented with FMF rather than AMF can be due to increasing paclitaxel production. The inverse relationship between
cell growth and paclitaxel accumulation has been reported previously38,39. There is one study which has reported the plant cells produced inhibitory substances in the late growth curve of
cell suspension that decreased the fungal biomass17. The presence of these inhibitors in FMF may be a drawback for the increase of YEF2 growth but autoclaving partly degraded these
substances. Therefore, MS medium supplemented with AMF increased growth more than FMF. The presence of paclitaxel precursors in plant cell cultures and the decrease of the final contents of
these precursors in fungus through fungi fermentation were reported17. In this context, further studies may be useful on the initial and final contents of paclitaxel precursors and
investigation of converting them to paclitaxel in fungus during fermentation by labeled precursors. Improved growth of _Fusarium mairei_ by adding supernatants of yew cell suspension
cultures of days 10 and 15 was reported17. Also, it is showed that yew needle extract can elicit fungal paclitaxel production. However, no information has been reported regarding the
influence of plant cell extract in the culture medium on growth and paclitaxel production of endophytic fungi. We studied for the first time the effects of _C. avellana_ CE on growth and
paclitaxel production of paclitaxel producing _E. nigrum_ strain YEF2. CONCLUSION The major limitation of using endophytic fungi for industrial paclitaxel production are the low and unstable
productivity. In this study, the addition of _C. avellana_ cell extract to MS medium enhanced growth and paclitaxel production of _E.nigrum_ strain YEF2. It is thought that addition of
hazel cell extract to MS medium in _E. nigrum_ culture simulated relatively the chemical environment of its host and resulted in increased growth and paclitaxel production. Since paclitaxel
production by endophytic fungi is significantly reduced by repeated sub-culturing on defined artificial media10, using plant cell extract in artificial media may be useful. It is essential
to examine whether stable enhanced production of paclitaxel can be obtained using plant cell extract in the fungal culture and the co-cultivation of endophytic fungus with plant cells. Also,
it is stated that endophytic fungi in artificial media have no access to the specialized microenvironment of the host plant which may lead to silencing of their secondary metabolite genes,
and standard culture conditions may not be sufficient to trigger expression of the cryptic biosynthetic gene clusters40. Based on achieved results, using plant cell extract in the fungal
culture or the co-cultivation of fungi with plant cells can be useful for enhancement of paclitaxel production. Large-scale production and extraction of pharmaceutical compounds from the
plants are high-priced and tedious. However, the endophytic fungi isolated from the medicinal plants can be easily cultured and large-scale production of the drugs is possible through the
fermentation process. It seems that using of plant cell extract in fungal artificial media is promising for the stable enhanced production of paclitaxel in fungi. MATERIAL AND METHODS FUNGI
AND PLANT CELL CULTURE REAGENTS The medium components, plant growth regulators and paclitaxel standard used in this experiment were supplied by Sigma (USA) and Merck (Germany) Chemical
Companies. ISOLATION OF ENDOPHYTIC FUNGI FROM _T. BACCATA_ AND _C. AVELLANA_ Healthy samples including the bark pieces, stem, bud and leaves were collected from _T. baccata_ and _C.
avellana_ grown at the botanical garden of College of Agriculture and Natural Resources (36°40'01″N, 51°10'18″ E at an altitude of 1321 m), University of Tehran, located in Karaj,
Alborz Province of Iran, in July and September 2014. The samples were treated with 75% ethanol (v/v) for 1 min and 2.5% sodium hypochlorite (w/v) for 2 min and rinsed two times with
sterilized water. In order to test the effectiveness of surface sterilization41, 10 ml of the last rinsing water was centrifuged for 10 min at 10,000 g. The supernatant was removed and
plated onto PDAC (PDA; supplemented with 250 mg/l Chloramphenicol). The surface sterilization was validated because no mycelial growth occurred. The surface disinfected small pieces (4 mm2)
of inner bark, bud and leaf segments were excised and placed on the surface of PDAC in unique Petri dishes (100 × 15 mm), incubated at 25 °C to allow the growth of endophytic fungi. Pure
fungal cultures of the endophytic isolates were prepared by the hyphal tip culture42. All fungal endophytes isolated from _T. baccata_ and _C. avellana_ were numbered as YEF# and HEF#
series, respectively and stored on PDA at 4 °C. CULTURE, EXTRACTION AND DETECTION OF PACLITAXEL Two agar plugs (5 mm diameter) containing mycelia of the fungal isolates were cultured
individually in 100 ml Erlenmeyer flasks containing 30 ml potato dextrose broth (PDB) medium. Cultures were incubated at 110 rpm at 25 °C for 12 days. The fungal mycelia were separated from
the broth by filtration. This filtered culture was subsequently extracted by adding two volumes of dichloromethane43. The extracted solvent was evaporated using rotary evaporator (Eyela,
Tokyo, Japan) to dryness at 35 °C. The dry residue was re-dissolved in 0.5 ml of absolute methanol. Intracellular paclitaxel was extracted from the mycelia with a procedure described below.
Freeze-dried mycelia (100 mg) were soaked in 4 ml methanol, sonicated for 40 min. After centrifugation, the supernatant was removed and extracted with dichloromethane:water (1:1, v/v). The
organic fraction was collected, dried under vacuum and resuspended in 0.5 ml methanol44. All samples were filtered through 0.22 µm cellulose acetate syringe filters before further analysis
with high-performance liquid chromatography (HPLC) and high-performance liquid chromatography-mass spectrometry (LC-MS/MS). Paclitaxel in samples was analyzed by the HPLC system (Waters,
USA) with a C18 analysis column (MachereyeNagel EC 4.6 × 250 mm, 5 µm Nucleodur). The sample (20 µl) was injected each time and detected at 230 nm using a UV detector. The mobile phase was
methanol:water (80:20 v/v) at a flow rate of 1.0 ml/min. The quantification of paclitaxel was based on an external standard of genuine paclitaxel (Sigma). Electrospray mass spectroscopy was
done on fungal paclitaxel sample using the electrospray technique by an Alliance 2695 waters with a C18 analysis column (Eclipse Agilent 4.6 × 150 mm, 5 µm). The sample in 100% methanol was
injected with a spray flow of 0.5 ml/min and a spray voltage of 4.5 kV by the loop injection method. The mobile phase was composed of acetonitrile acidified with 0.1% (v/v) formic acid (A)
and water acidified with 0.1% (v/v) formic acid (B) with binary solvent-delivery gradient elution (0–5 min, 40–90% A, 60–10% B). MOLECULAR STUDIES: GENOMIC DNA EXTRACTION, PCR AND SEQUENCING
The endophytic fungus was cultured in PD broth at 25 °C with constant shaking for 7 days. The fungal mycelia were freeze-dried and the genomic DNA was extracted as described by Safaie _et
al_.45. Briefly, 50 mg of fungal mycelia were vigorously crushed in liquid nitrogen to make a fine powder. The cells were lysed in 400 µl of DNA salt solution (Tris-HCl 100 mM, EDTA pH =
7.5–8 5 mM and NaCl 1.4 mM), mixed thoroughly and incubated at 65 °C for 15 min. The samples were cooled on crushed ice for 10 min and centrifuged at 13,000 g for 10 min at 4 °C. About 300
µl of the aqueous phase was transferred into a new labeled sterile tube. 210 µl cold isopropanol was added and mixed by inverting the tubes several times. The tubes were centrifuged for 15
min at 10,000 g and the supernatant was discarded, air dried and dissolved in 50 µl of sterile Millipore water. The fungal internal transcribed spacer (ITS) fragments (ITS1-5.8S-ITS2) were
amplified by PCR using the universal primers ITS1 and ITS446. The actin gene (ACT) was partly amplified with primer pair ACT-512F and ACT-783R47 (Table S1). The PCR reaction mixtures (25 µl)
consisted of 1 µl genomic DNA (~100 ng), 1 µl forward and reverse primers (10 pM), and 12.5 µl Premix Taq (TaKaRa Biotechnology Ltd., Japan), and 10.5 µl PCR quality water. The PCR reaction
programs were an initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation (94 °C for 30 s), annealing (56 °C (ITS) and (59 °C (ACT) for 30 s), extension (72 °C for 1
min) and a final extension at 72 °C for 5 min. The PCR products were analyzed by agarose gel electrophoresis and purified using a DNA gel extraction kit (Axygen Biotechnology Ltd., China).
The purified PCR product was directly sequenced using the same primers by Bioneer (Shanghai, China). The sequences of ITS1-5.8S-ITS2 region and actin gene of the endophytic fungus were
compared with the data in National Center for Biotechnology Information, USA (NCBI) using BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to estimate the phylogenetic relationship.
CLUSTAL X software (version 2.0, Conway Institute, USA) was used to generate the alignment of endophytic fungus48. Phylogenetic analysis was carried out by the neighbor-joining method using
MEGA software (version 4.0, Biodesign Institute, USA). The bootstrap was 1,000 replications to assess the reliable level to the nods of the tree49. PREPARATION OF CELL EXTRACT (CE) AND
MEDIUM FILTRATE (MF) The callus of hazel (_C. avellana_) was obtained from seed cotyledons on MS medium supplemented with 0.2 mg/l 6-benzylaminopurine and 2 mg/l 2,4-dichlorophenoxyacetic
acid and solidified with 8 g/l agar agar50. The _C. avellana_ CSC was established with transferring 5 g callus into 250 ml flasks containing 100 ml medium and were maintained at 25 °C in
darkness on gyratory shakers at 110 rpm. Suspensions were also subcultured every 15 days until the cells reached homogeneity. Then 1.5 ± 0.1 g of hazel cells (fresh mass) was transferred to
100 ml flasks containing 30 ml of the cell culture medium. The cell culture medium and the culture conditions for growing cells of _C. avellana_ remained the same as described above. Then
the fresh cells were harvested on the 21st day (the stationary growth phase)50 by passing CSC through a filter paper (Whatman No. 1). The cells washed several times with sterile double
distilled water and dried at 60 °C. Then crushed thoroughly in liquid nitrogen. Crushed cells were soaked in water (100 mg/ml), sonicated for 20 min, mixed thoroughly and incubated at 65 °C
for 30 min with continuous shaking. The hydrolysate centrifuged at 10,000 g for 15 min. After centrifugation, the supernatant (cell extract) was collected. The cell extract of hazel was
divided into two parts: one part was autoclaved at 121 °C for 20 min and designated as autoclaved cell extract (ACE) and another part was filtered through 0.22 µm cellulose acetate syringe
filters and designated as filter sterilized cell extract (FCE). The spent medium was centrifuged at 12,000 g for 20 min to remove completely suspended cells. This cell-free medium was
designated as medium filtrate. The medium filtrate of hazel CSC was divided into two parts: one part was autoclaved at 121 °C for 20 min and designated as autoclaved medium filtrate (AMF)
and another part was filtered through 0.22 µm cellulose acetate syringe filters and designated as filter sterilized medium filtrate (FMF). ESTABLISHMENT OF SUSPENSION CULTURE OF PACLITAXEL
PRODUCING ENDOPHYTIC FUNGUS The experiment was carried out with three replications. Each replication consisted of 100 ml flask containing 30 ml MS medium supplemented with 0.2 mg/l BAP, 2
mg/l GA3 and 2 mg/l 2,4-D. The inoculum was prepared from 7-day-old cultures of strain YEF2 grown on PDA medium at 25 °C. Two mycelial agar plugs (5 mm diameter) per replication were cut
from the margin of the growing colony using a sterilized cork borer. Cultures were maintained at 25 °C in darkness on gyratory shakers at 110 rpm. Four plant elicitor preparations viz. ACE,
FCE, AMF and FMF were added at different concentrations (7, 14, 28% (v/v)) to the medium at the time of culture. The control received an equal volume of the MS medium (for MF)/water (for
CE). Since CSC of _C. avellana_ produces paclitaxel50, elicitors derived from it may contain paclitaxel. As the control for fungal paclitaxel production, four elicitors derived from hazel
CSC were added at different concentrations (7, 14, 28% (v/v)) to the culture medium with no inoculation and maintained in mentioned condition. The cultures were harvested on the 14th day and
analyzed for growth and paclitaxel production. This experiment was designed as factorial based on a Completely Random Design (CRD) to determine how the CE and MF of _C. avellana_ CSC
affected the growth and paclitaxel production of paclitaxel-producing endophytic fungus strain YEF2. The factorial arrangement of the treatments was designed and consisted of three factors
containing the type of elicitor with four levels, sterilization method with two levels and elicitor concentration with three levels, given 24 treatments. STATISTICAL ANALYSIS The hypothesis
of normality and equal variance were met and conventional parametric statistics used for the analysis. Analysis of variance and means comparison using least significant difference (LSD) were
performed by SAS (SAS 9.3, 2011) and SPSS (SPSS 15.0, 2006). Excel software (Excel, 2011) was used for making graphs. Simple linear regression of total paclitaxel against FW and DW for
different treatments was developed by Statgraphics (54. Statgraphics Centurion XVII, 2015). AVAILABILITY OF DATA AND MATERIAL The dataset supporting the conclusions of this article is
included in the article. REFERENCES * Kohler, D. R. & Goldspiel, B. R. Paclitaxel (Taxol). 14, 3–34. https://doi.org/10.1002/j.1875-9114.1994.tb02785 (1994). * Wani, M. C., Taylor, H.
L., Wall, M. E., Coggon, P. & Mc Phail, A. T. Plant antitumor agents. VI. The isolation and structure of taxol. Anovel antileukemic and antitumor agent from T_axus brevifolia_. _J. Am.
Chem. Soc._ 93, 2325–2327, https://doi.org/10.1021/ja00738a045 (1971). Article PubMed CAS Google Scholar * Schiff, P. B., Fant, J. & Horwitz, S. B. Promotion of microtubule assembly
_in vitro_ by taxol. _Nature_ 277(5698), 665–667, https://doi.org/10.1038/277665a0 (1979). Article ADS PubMed CAS Google Scholar * Cragg, G. M., Schepartz, S. A., Suffness, M. &
Grever, M. R. The taxol supply crisis. New NCI policies for handling the large-scale production of novel natural product anticancer and anti-HIV agents. _J. Nat. Prod_. 56(10), 1657–1668.
https://doi.org/10.1021/np50100a001 _Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy_ (1993). * Shinwari, Z. K. & Qaisar, M. Efforts on conservation and sustainable
use of medicinal plants of Pakistan. _Pak. J. Bot._ 43, 5–10 (2011). Google Scholar * Stierle, A., Strobel, G. A. & Stierle, D. Taxol and taxane production by _Taxomyces andreanae_, an
endophytic fungus of Pacific yew. _Science_ 260, 214–216, https://doi.org/10.1126/science.8097061 (1993). Article ADS PubMed CAS Google Scholar * Guo, B. H. _et al_. An endophytic
taxol-producing fungus BT2 isolated from _Taxus chinensis_ var. mairei. _Afr J Biotechnol_ 5(10), 875–877 (2006). CAS Google Scholar * Zhao, K. _et al_. Aspergillus niger var. taxi, a new
species variant of taxol-producing fungus isolated from _Taxus cuspidata_ in China. _J. Appl. Microbiol._ 107(4), 1202–1207, https://doi.org/10.1111/j.1365-2672.2009.04305.x (2009). Article
PubMed CAS Google Scholar * Flores-Bustamante, Z. R., Rivera-Orduna, F. N., Martinez-Cardenas, A. & Flores-Cotera, L. B. Microbial paclitaxel: advances and perspectives. _J.
Antibiot._ 63, 460–467, https://doi.org/10.1038/ja.2010.83 (2010). Article CAS Google Scholar * Somjaipeng, S., Medina, A. & Magan, N. Environmental stress and elicitors enhance taxol
production by endophytic strains of _Paraconiothyrium variabile_ and _Epicoccum nigrum_. _Enzyme Microb. Technol._ 90, 69–75, https://doi.org/10.1016/j.enzmictec.2016.05.002 (2016). Article
PubMed CAS Google Scholar * Venugopalan, A., Potunuru, U. R., Dixit, M. & Srivastava, S. Effect of fermentation parameters, elicitors and precursors on camptothecin production from
the endophyte _Fusarium solani_. _Bioresour. Technol._ 206, 104–111, https://doi.org/10.1016/j.biortech.2016.01.079 (2016). Article PubMed CAS Google Scholar * Soliman, S. S. M. &
Raizada, M. N. Interactions between co-habitating fungi elicit synthesis of taxol from an endophytic fungus in host _Taxu_splants. _Front. Microbiol._ 4, 1–14,
https://doi.org/10.3389/fmicb.2013.00003 (2013). Article CAS Google Scholar * Kusari, S., Hertweck, C. & Spiteller, M. Chemical ecology of endophytic fungi: origins of secondary
metabolites. _Chem. Biol._ 19(7), 792–798, https://doi.org/10.1016/j.chembiol.2012.06.004 (2012). Article PubMed CAS Google Scholar * Soliman, S. S. M., Trobacher, C., Tsao, R.,
Greenwood, J. & Raizada, M. A. Fungal Endophyte Induces Transcription of Genes Encoding a Redundant Fungicide Pathway in Its Host Plant. _BMC Plant. Biol._ 13(1), 93,
https://doi.org/10.1186/1471-2229-13-93 (2013). Article PubMed PubMed Central CAS Google Scholar * Rodriguez, R. J., White, J. F., Arnold, A. E. & Redman, R. S. Fungal endophytes:
diversity and functional roles. _New Phytol._ 182, 314–330, https://doi.org/10.1111/j.1469-8137.2009.02773.x (2009). Article PubMed CAS Google Scholar * Firakova, S., Sturdikova, M.
& Muckova, M. Bioactive secondary metabolites produced by microorganisms associated with plants. _Biologia_ 62, 251–257, https://doi.org/10.2478/s11756-007-0044-1 (2007). Article CAS
Google Scholar * Li, Y. C. & Tao, W. Y. Interactions of taxol-producing endophytic fungus with its host (_Taxus_ spp.) during taxol accumulation. _Cell Biol. Int._ 33(1), 106–112,
https://doi.org/10.1016/j.cellbi.2008.10.007 (2009). Article MathSciNet PubMed CAS Google Scholar * Venugopalan, A. & Srivastava, S. Enhanced camptothecin production by ethanol
addition in the suspension culture of the endophyte, _Fusarium solani_. _Bioresour. Technol._ 188, 251–257, https://doi.org/10.1016/j.biortech.2014.12.106 (2015). Article PubMed CAS
Google Scholar * Hoffman, A. _et al_. Bioprospecting for Taxol in angiosperm plant extracts-Using high performance liquid chromatography thermospray mass spectrometry to detect the
anticancer agent and its related metabolites in Filbert trees. _Spectrosc._ 13(6), 22–32 (1998). CAS Google Scholar * Hoffman, A. & Shahidi, F. Paclitaxel and other taxanes in hazel.
_J. Funct. Foods_ 1, 33–37, https://doi.org/10.1016/j.jff.2008.09.004 (2009). Article CAS Google Scholar * Service, R. F. Hazel trees offer new source of cancer drug. _Science_ 288,
27–28, https://doi.org/10.1126/science.288.5463.27a (2000). Article MathSciNet PubMed CAS Google Scholar * Bestoso, F. _et al_. _In vitro_ cell cultures obtained from different explants
of _Corylus avellana_ produce Taxol and taxanes. _Biotechnol._ 6, 45–56, https://doi.org/10.1186/1472-6750-6-45 (2006). Article CAS Google Scholar * Gallego, A. _et al_. Taxol from
_Corylus avellana_: paving the way for a new source of this anti-cancer drug. _Plant Cell Tissue Organ Cult._ 129, 1–16, https://doi.org/10.1007/s11240-016-1164-5 (2017). Article CAS
Google Scholar * Somjaipeng, S., Medina, A., Kwaśna, H., Ortiz, J. O. & Magan, N. Isolation, identification, and ecology of growth and taxol production by an endophytic strain of
_Paraconiothyrium variabile_ from English yew trees (_Taxus baccata)_. _Fungal boil._ 119(11), 1022–1031, https://doi.org/10.1016/j.funbio.2015.07.007 (2015). Article CAS Google Scholar *
Kerns, E. H., Volk, K. J., Hill, S. E. & Lee, M. S. Profiling taxanes in _Taxus_ extracts using LC/MS and LC/MS/MS techniques. _J. Nat. Prod._ 57, 1391–1403,
https://doi.org/10.1021/np50112a008 (1994). Article PubMed CAS Google Scholar * Samsom, R. A., Houbraken, J., Thrane, U., Frisvad, J. C. & Anderson, B. Food and Indoor Fungi.
CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherland (2010). * Da Silva Araujo, F. D. _et al_. Epicolactone–Natural Product Isolated from the Sugarcane Endophytic Fungus _Epicoccum
nigrum_. _Eur. J. Org. Chem._ 2012(27), 5225–5230, https://doi.org/10.1002/ejoc.201200757 (2012). Article CAS Google Scholar * De Lima Favaro, L. C., Melo, F. L., Aguilar-Vildoso, C. I.
& Araújo, W. L. Polyphasic analysis of intraspecific diversity in _Epicoccum nigrum_ warrants reclassification into separate species. _PLOS One_ 6, 14828,
https://doi.org/10.1371/journal.pone.0014828 (2011). Article ADS CAS Google Scholar * Caruso, M. _et al_. Isolation of endophytic fungi and actinomycetes taxane producers. _Ann
Microbiol_ 50, 3–13 (2000). ADS CAS Google Scholar * De Lima Favaro, L. C., de Souza Sebastianes, F. L. & Araújo, W. L. _Epicoccum nigrum_ P16, a sugarcane endophyte, produces
antifungal compounds and induces root growth. _PLOS One_ 7(6), 36826, https://doi.org/10.1371/journal.pone.0036826 (2012). Article ADS CAS Google Scholar * Larena, I. _et al_. Biological
control of postharvest brown rot (_Monilinia_ spp.) of peaches by field applications of _Epicoccum nigrum_. _Biol. Control_ 32(2), 305–310, https://doi.org/10.1016/j.biocontrol.2004.10.010
(2005). Article Google Scholar * Bamford, P. C., Norris, G. L. F. & Ward, G. Flavipin production by Epicoccum spp. _Trans. Br. Mycol. Soc._ 44(3), 354–356,
https://doi.org/10.1016/S0007-1536(61)80028-4 (1961). Article CAS Google Scholar * Foppen, F. H. & Gribanovski-Sassu, O. Lipids produced by _Epicoccum nigrum_ in submerged culture.
_Biochem. J._ 106(1), 97–100, https://doi.org/10.1042/bj1060097 (1968). Article PubMed PubMed Central CAS Google Scholar * Cretu, R., Bahrim, G., Stefan, D. & Olteanu, M. Evaluation
of physical and chemical characteristics of yellow colorant produced by _Epicoccum nigrum_ MIUG 2.15 in crude extracts and emulsions. _Rom. Biotechnol. Lett_. 13(5), 59–68 (2008). *
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. _Physiol. Plant_ 15, 473–497, https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
(1962). * El-Bahrawy, S. A. Effect of gibberellic acid on some microbial growth and spore germination of some fungi. _Zentralbl. Mikrobiol._ 137(1982), 238–240,
https://doi.org/10.1016/S0232-4393(82)80034-6 (1982). Article Google Scholar * Yang, Z., Rogers, L. M., Song, Y., Guo, W. & Kolattukudy, P. E. Homoserine and asparagine are host
signals that trigger in planta expression of a pathogenesis gene in. _Nectria haematococca. Proc. Nat. Acad Sci. USA_ 102, 4197–4202, https://doi.org/10.1073/pnas.0500312102 (2005). Article
ADS PubMed CAS Google Scholar * Wang, C., Wu, J. & Mei, X. Enhancement of taxol production and excretion in _Taxus chinensis_ cell culture by fungal elicitation and medium renewal.
_Appl. Microbiol. Biotechnol._ 55(4), 404–410, https://doi.org/10.1007/s002530000567 (2001). Article PubMed CAS Google Scholar * Wickremesinhe, E. R. & Arteea, R. N. _Taxus_ callus
cultures: initiation, growth optimization, characterization and taxol production. _Plant Cell Tissue Organ Cult._ 35(2), 181–193, https://doi.org/10.1007/BF00032968 (1993). Article CAS
Google Scholar * Venugopalan, A. & Srivastava, S. Endophytes as _in vitro_ production platforms of high value plant secondary metabolites. _Biotechnol. Adv._ 33(6), 873–887,
https://doi.org/10.1016/j.biotechadv.2015.07.004 (2015). Article PubMed Google Scholar * Rakotoniriana, E. F. _et al_. Endophytic fungi from leaves of _Centella asiatica_: occurrence and
potential interactions within leaves. _Antonie Van Leeuwenhoek_ 93, 27–36, https://doi.org/10.1007/s10482-007-9176-0 (2008). Article PubMed CAS Google Scholar * Strobel, G. _et al_.
Taxol from _Pestalotiopsis microspora_, an endophytic fungus of _Taxus wallachiana_. _Microbiology_ 142, 435–440, https://doi.org/10.1099/13500872-142-2-435 (1996). Article PubMed CAS
Google Scholar * Strobel, G., Daisy, B., Castillo, U. & Harper, J. Natural products from endophytic microorganisms. _J. Nat. Prod._ 67, 257–268, https://doi.org/10.1021/np030397v
(2004). Article PubMed CAS Google Scholar * Luo, J., Liu, L. & Wu, C. D. Enhancement of paclitaxel production by abscisic acid in cell suspension cultures of _Taxus chinensis_.
_Biotechnol. Lett._ 23(16), 345–1348, https://doi.org/10.1023/A:1010597802741 (2001). Article Google Scholar * Safaie, N., Alizadeh, A., Saidi, A., Rahimian, H. & Adam, G. Molecular
characterization and genetic diversity among Iranian populations of _Fusarium graminearum_, the causal agent of wheat head blight. _Iran. J. Plant Pathol._ 41, 171–189 (2005). Google Scholar
* White, T. J., Bruns, T., Lee, S. & Taylor, J. PCR Protocols: a guide to methods and applications (eds Innis M. A. _et al_.) Ch. 38, 315–322,
https://doi.org/10.1016/B978-0-12-372180-8.50042-1 (Academic Press, 1990). * Carbone, I. & Kohn, L. M. A method for designing primer sets for speciation studies in filamentous
ascomycetes. _Mycologia_ 91, 553–556, https://doi.org/10.2307/3761358 (1999). Article CAS Google Scholar * Larkin, M. A. _et al_. Clustal W and Clustal X version 2.0. _Bioinformatics_ 23,
2947–2948, https://doi.org/10.1093/bioinformatics/btm404 (2007). Article PubMed CAS Google Scholar * Tamura, K., Dudley, J., Nei, M. & Kumar, S. MEGA4: Molecular Evolutionary
GeneticsAnalysis (MEGA) software version 4.0. _Mol. Biol. Evol._ 24, 1596–1599, https://doi.org/10.1093/molbev/msm092 (2007). Article PubMed CAS Google Scholar * Salehi, M., Moieni, A.
& Safaie, N. A Novel Medium for Enhancing Callus Growth of Hazel (_Corylus avellana_ L.). _Sci. Rep._ 7(1), 15598, https://doi.org/10.1038/s41598-017-15703-z (2017). Article ADS PubMed
PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS This research did not receive any specific grant from funding agencies in the public, commercial, ornot-for-profit
sectors. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Plant Breeding and Biotechnology Department, Faculty of Agriculture, Tarbiat Modares University, Tehran, P.O. Box 14115-336, Iran Mina
Salehi & Ahmad Moieni * Plant Pathology Department, Faculty of Agriculture, Tarbiat Modares University, Tehran, P.O. Box 14115-336, Iran Naser Safaie Authors * Mina Salehi View author
publications You can also search for this author inPubMed Google Scholar * Ahmad Moieni View author publications You can also search for this author inPubMed Google Scholar * Naser Safaie
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A. Moeini and N. Safaie supervised the project, N. Safaie and M. Salehi designed the
project. M. Salehi carried out the experiments, data analysis and drafted the manuscript. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Naser
Safaie. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Salehi, M., Moieni, A. & Safaie, N. Elicitors
Derived from Hazel (_Corylus avellana_ L.) Cell Suspension Culture Enhance Growth and Paclitaxel Production of _Epicoccum nigrum_. _Sci Rep_ 8, 12053 (2018).
https://doi.org/10.1038/s41598-018-29762-3 Download citation * Received: 29 December 2017 * Accepted: 18 July 2018 * Published: 13 August 2018 * DOI:
https://doi.org/10.1038/s41598-018-29762-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
