Orange jasmine as a trap crop to control diaphorina citri
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Novel, suitable and sustainable alternative control tactics that have the potential to reduce migration of _Diaphorina citri_ into commercial citrus orchards are essential to
improve management of huanglongbing (HLB). In this study, the effect of orange jasmine (_Murraya paniculata_) as a border trap crop on psyllid settlement and dispersal was assessed in citrus
orchards. Furthermore, volatile emission profiles and relative attractiveness of both orange jasmine and sweet orange (_Citrus_ × _aurantium_ L., syn. _Citrus sinensis_ (L.) Osbeck) nursery
flushes to _D. citri_ were investigated. In newly established citrus orchards, the trap crop reduced the capture of psyllids in yellow sticky traps and the number of psyllids that settled
on citrus trees compared to fallow mowed grass fields by 40% and 83%, respectively. Psyllids were attracted and killed by thiamethoxam-treated orange jasmine suggesting that the trap crop
could act as a ‘sink’ for _D. citri_. Additionally, the presence of the trap crop reduced HLB incidence by 43%. Olfactometer experiments showed that orange jasmine plays an attractive role
on psyllid behavior and that this attractiveness may be associated with differences in the volatile profiles emitted by orange jasmine in comparison with sweet orange. Results indicated that
insecticide-treated _M. paniculata_ may act as a trap crop to attract and kill _D. citri_ before they settled on the edges of citrus orchards, which significantly contributes to the
reduction of HLB primary spread. SIMILAR CONTENT BEING VIEWED BY OTHERS A NOVEL SUSTAINABLE BIOCIDE AGAINST THE FRUIT FLY _DROSOPHILA SUZUKII_ MADE FROM ORANGE PEELS Article Open access 14
November 2024 DETECTION AND MONITORING OF _DROSOPHILA SUZUKII_ IN RASPBERRY AND CHERRY ORCHARDS WITH VOLATILE ORGANIC COMPOUNDS IN THE USA AND EUROPE Article Open access 25 March 2021
BEHAVIORAL EFFECTS INDUCED BY ORGANIC INSECTICIDES CAN BE EXPLOITED FOR A SUSTAINABLE CONTROL OF THE ORANGE SPINY WHITEFLY _ALEUROCANTHUS SPINIFERUS_ Article Open access 25 September 2020
INTRODUCTION Asian citrus psyllid, _Diaphorina citri_ Kuwayama (Hemiptera: Liviidae) is considered the main threat to orange production due to its ability to transmit the putative causal
agents (‘_Candidatus_ Liberibacter spp.’) of huanglongbing (HLB), the most destructive citrus disease worldwide1. There is yet no effective cure for HLB, which affects all commercial citrus
varieties and produces billion dollar losses to citriculture2. Current management of HLB is based on the prevention of citrus tree infection through planting of healthy nursery trees,
inspections and eradication of symptomatic trees and control of its vector _D. citri_ using insecticides3. HLB spread is mainly associated with constant _D. citri_ short and long-range
flight movements by psyllid adults4,5,6,7. Primary HLB infection occurs mainly on orchard borders8,9 where the psyllid prefers to settle10. Despite the intensification of chemical control of
_D. citri_ on grove borders in recent years, concomitant reductions of HLB infections is limited because of the intense and constant influx of migrating psyllids9,11. Considering the HLB
‘edge effect’ and the difficulties to avoid primary infections, it is important to consider the development of novel and suitable alternative tactics to be incorporated into HLB management
programs. The trap cropping tactic has been studied for the integrated pest management of many insect pests, including aphids, leafhoppers, planthoppers, and whiteflies, which are important
hemipteran vectors of plant diseases12. Trap crops are plants used to attract insects or other organisms in order to protect important economic crops from direct damage or indirect damage
related to vector-transmitted diseases13. The trap plant could act as a barrier, protecting the crop of interest from the pest or vector, by concentrating them on attractive leaves or organs
where their control could be more efficient and specifically applied. Shelton and Badenes-Perez12 proposed a broader definition of trap crops, by including plants that are, per se or via
manipulation, responsible for attracting, diverting, intercepting, and/or retaining pests or vectors, and consequently, decreasing the damage inferred to the main crop. Moreover, in order to
increase the efficiency of controlling pests, trap crops can be associated with insecticides. Successful trap cropping strategies to manage pests have been described previously. For
example, using alfalfa (_Medicago sativa_ L.) as a trap crop reduced populations of the Western tarnished plant bug _Lygus hesperus_ Knight (Hemiptera: Miridae) in cotton (_Gossypium
hirsutum_ L.)14,15. More recently, borders of transgenic Rainbow papaya (_Carica papaya_ L.) plants resistant to the _Papaya ringspot virus_ were used as trap crop to reduce the spread of
viruliferous aphid vectors to non-transgenic papaya plants in Hawaii16. Preliminary field studies in São Paulo (SP) State, Brazil, showed that the use of suitable host plants for _D. citri_
as barriers reduced the number of marked psyllids recaptured on yellow stick traps deployed on citrus trees7. The lowest recapture rates were recorded when orange jasmine [_Murraya
paniculata_ (L.) Jack, syn. _Murraya exotica_ L.] was used as a barrier, envisaging its potential use as a trap crop to manage HLB. Orange jasmine is a preferred host for _D. citri_ in
comparison with other rutaceous host plants17,18 and this effect has been related to its volatiles19. Although orange jasmine can be infected with ‘_Candidatus_ Liberibacter asiaticus’
(CLas), bacterial titer is much lower in this host than in _Citrus_ species and cultivars and decreases over time, probably due to the lower multiplication rates in the former, being CLas
infections usually transient in orange jasmine20,21. Moreover, recent results showed that _D. citri_ could not acquire CLas upon feeding and developing on CLas-qPCR positive orange jasmine
seedlings22. These results indicate that orange jasmine is a poor host for CLas (as well as a ‘dead-end’ host plant23) and suggest that it may be used as a potential trap crop, attracting
psyllids which could be controlled in these plants by insecticides, thus limiting spread of HLB. In this study, the effect of orange jasmine as a border trap crop on _D. citri_ settlement
and dispersal was assessed in sweet orange orchards. Additionally, the volatile emission profiles and relative attractiveness of both orange jasmine and sweet orange flushes to _D. citri_
were investigated. The results of this study may help citrus growers to control immigrating psyllids that arrive to the edges of citrus orchards from external inoculum sources and thus limit
HLB incidence inside citrus orchards. RESULTS EFFECTS OF ORANGE JASMINE AS A TRAP CROP ON _DIAPHORINA CITRI_ NATURAL INFESTATION AND HLB INCIDENCE The presence of orange jasmine as a trap
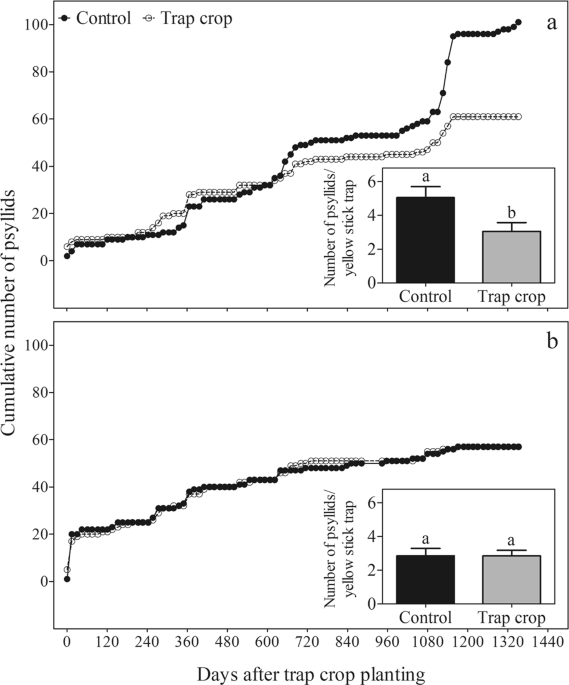
crop on the edge of a new (6-month-old) citrus orchard (Area A, Fig. 1a) reduced incidence of _D. citri_ in the orchard, when compared to the control, over a 45-month survey (d.f. = 1; _P_ =
0.0030). In this area significantly fewer psyllids were captured on yellow sticky traps placed in citrus trees bordered on the east side by the trap crop compared to identical traps placed
in control trees bordered on the east side by fallow mowed grass (F = 5.74; d.f. = 1, 38; _P_ = 0.0216) (Fig. 1a). In contrast, when the orange jasmine trap crop was planted on the edge of
well established (7-year-old) citrus plots (Area B, Fig. 1b), cumulative trends in numbers of psyllids captured on yellow sticky traps placed in trees within the plots did not differ
significantly, regardless of the presence or absence of an orange jasmine trap crop on the orchard edge (d.f. = 1; _P_ = 0.8196), and the total numbers of psyllids recorded on the yellow
sticky traps in both the trap crop and control treatments over 45 months did not differ significantly (F = 0.00; d.f. = 1, 38; _P_ = 1.0000) (Fig. 1b). The frequency of _D. citri_ detection
also varied between new and established citrus orchards. In new citrus plots (area A), the frequency of psyllid detection in the control treatment (40.6%) was significantly higher than in
the trap-crop treatment (25.7%) (χ2 = 4.377; d.f. = 1; _P_ = 0.0364). In contrast, in the 7-year-old citrus plots (area B), the frequency of _D. citri_ detection in each treatment was
similar (control: 25.3%; trap crop: 27.3%; χ2 = 0.026; d.f. = 1; _P_ = 0.8717). Regarding the assessments of ‘_Ca_. L. asiaticus’ or ‘_Ca_. L. americanus’-positive trees in the new citrus
orchard, the HLB incidence in the control plot was ~2.8-fold (control: 1.4%; trap crop: 0.5%) and ~1.8-fold (control: 2.8%; trap crop: 1.6%) higher than on orange jasmine trap crop
treatment, for assessments performed on May 2014 and on January 2015, respectively. EFFECTS OF ORANGE JASMINE AS A TRAP CROP ON _DIAPHORINA CITRI_ SETTLEMENT AND DISPERSAL To further
investigate orange jasmine trap crop effect on _D. citri_ settlement and dispersal, adult psyllids were marked with different colors of fluorescent powder and released near the experimental
sweet orange orchard with and without trap crop plots. Trap crop plots showed a significant reduction in the number of psyllids that settled on citrus trees compared to control plots at 1 (F
= 12.28; d.f. = 1, 430; _P_ = 0.0005), 3 (F = 9.13, d.f. = 1, 430; _P_ = 0.0027) and 7 (F = 15.42; d.f. = 1, 430; _P_ = 0.0001) days after release (Fig. 2). Overall, the trap crop treatment
provided a significant reduction in the number of _D. citri_ adults that settled on citrus trees compared to the control (χ2 = 4.13; d.f. = 1; _P_ = 0.0421), independently of time (χ2 =
2.86; d.f. = 1; _P_ = 0.0908) and treatment and time interaction (χ2 = 0.17; d.f. = 1; _P_ = 0.6768). Assessments on release platforms showed that approximately 90% of the released psyllids
had taken off from the platforms at 1 day after release (DAR) and no psyllids were found there by 3 DAR. In general, 83% of all marked psyllids that were found in the experimental area were
in the first citrus row regardless of the assessment time (Fig. 3). Overall, 150, 205 and 56 marked psyllids (out of 16800) were found in the experimental area at 1, 3 and 7 DAR,
respectively. At all inspection times, most psyllids (94.3, 78.7 and 86.3% at 1, 3 and 7 DAR, respectively) were found on control plots. Psyllids released in front of orange jasmine plots
were mostly restricted to the first citrus row. In contrast, individuals released in front of control plots were found mainly in the first four citrus rows, and one psyllid reached the last
citrus row (35 m from the release platforms). Visual assessments on orange jasmine trees at 1 and 3 DAR indicated the presence of 115 and 158 marked _D. citri_, respectively (Fig. 4). Few
psyllids (5 individuals) were found on orange jasmine trees at 7 DAR. In addition, among all psyllids that were found on orange jasmine trees, 19% were individuals released in the control
treatments. INSECTICIDE EFFECT ON _DIAPHORINA CITRI_ MORTALITY Psyllid mortality was significantly higher for psyllids confined on orange jasmine trees treated with thiamethoxam compared to
the untreated control (log rank: 322.00; d.f. = 1; _P_ < 0.0001), considering the whole assessment period. The same results were observed when comparing the mortality rates at 1 (F =
24.79; d.f. = 1, 57; _P_ < 0.0001), 3 (135.67; d.f. = 1, 57; _P_ < 0.0001), and 7 (F = 209.14; d.f. = 1, 57; _P_ < 0.0001) days after confinement (DAC) (Fig. 5). OLFACTOMETRIC
ASSAYS AND VOLATILE EMISSION ANALYSIS In 4-arm olfactometer assays, results indicated that _D. citri_ females spent more time on orange jasmine than on the clean air fields (V = 1270.00;
d.f. = 1, 60; _P_ = 0.0185) (Fig. 6a). However, no significant effect was observed on the _D. citri_ female foraging activity to the ‘Pera’ sweet orange vs. clean air (V = 1036.00; d.f. = 1,
64; _P_ = 0.8139) (Fig. 6b). This different _D. citri_ behavior to sweet orange and orange jasmine suggests different volatile profiles between both genotypes. To gain insight into the
attractiveness of orange jasmine volatiles, comparative untargeted volatile analysis of sweet orange and orange jasmine flushes was performed. Principal component analysis revealed two
separated clustering groups, one for each studied genotype (Fig. 7a). PC1 explained at least 75% of the variance at two independent replicate dates. Area integration of peaks corresponding
to relevant loadings at both sampling dates (detailed in Supplementary Table 1) revealed important quantitative and qualitative differences in volatiles emitted from both genotypes (Fig.
7b). For example, 37 compounds (of which 26 are sesquiterpenes) were detected only in the orange jasmine emission profile. On the other hand, the emission of monoterpenes characteristic of
sweet orange leaves, such as α- and β-phellandrene, d-limonene and linalool, was highly reduced in orange jasmine. DISCUSSION In the current study, we evaluated first the use of orange
jasmine as a trap crop in two types of orange orchards, recently planted and several years old, that differed in plant height and biomass (new orchard: 1 m-tall; well established orchard: 3
m-tall; Supplementary Fig. 1). The presence of orange jasmine as a trap crop in the border of new citrus orchards caused overall reductions of 40% in the accumulated number of psyllids
captured (Fig. 1a) and 83% in the number of psyllids settled on citrus trees (Fig. 2), when compared to the fallow field plots (control). Moreover, in the control plot the frequency of _D.
citri_ detection was 1.6-fold higher than in the orange jasmine plot (Fig. 1a). Similar insect reduction rates associated to the trap crop use were obtained after planting green leaf
desmodium [_Desmodium intortum_ (Mill.) Urb.] and _Brachiaria_ cv. Mulato II as ‘push’ and ‘pull’ crops, respectively, on maize (_Zea mays_ L.), in order to reduce the fall armyworm
[_Spodoptera frugiperda_ (J.E. Smith)] (Lepidoptera: Noctuidae) infestation and damage24. Likewise, Stern _et al_.14 demonstrated a reduction in the _L. hesperus_ movement into cotton in
California (USA), when alfalfa was intercropped with cotton. Regarding _D. citri_ spatial distribution, most psyllids were found in the first rows of citrus (Fig. 3) regardless of the
treatment and assessment period, reinforcing psyllid preference for trees located in the border of the orchards10,25,26. Psyllids released in front of the trap crop plots did not disperse
further than the first row of citrus, whereas individuals released in front of control plots were able to cross the first row and were present in almost all rows. On the other hand, a high
number of individuals were observed on orange jasmine trap crop during the first assessments (Fig. 4), including insects that were released on control plots. These results may be explained
by a strong attraction effect associated to the orange jasmine trees, which could have reduced psyllid movement into the orchard, and suggest that the psyllid has the ability to detect and
move to the preferred host rather than performing a passive random dispersal. This hypothesis is in accordance with previous studies that report the importance of visual and olfactory cues
in the host plant finding ability of _D. citri_27,28,29. The low effect of orange jasmine trap crop on _D. citri_ infestation when tested on well-established citrus orchards also supports
the hypothesis of visual and olfactory cues for _D. citri_ behavior. Probably, large differences in plant size between orange jasmine and citrus trees on mature plots may lead to higher
presence of visual/olfactory cues from citrus trees than from orange jasmine plants, thus explaining the absence of trap crop effect over the _D. citri_ population in this case. According to
Shelton and Badenes-Perez12, a successful trap crop relies on the combination of trap crop (e.g. size, phenology, and attractiveness) and pest characteristics. To evaluate the effectiveness
of orange jasmine olfactory cues as _D. citri_ attractants, 4-arm olfactometric assays were performed (Fig. 6). An increase of ~30% in _D. citri_ preference to orange jasmine volatiles in
relation to that of ‘Pera’ sweet orange was observed. High attractiveness of orange jasmine flushes to _D. citri_ has been also observed in Y-olfactometer19. Therefore, our olfactometric
data reinforces the _D. citri_ preference to volatiles from orange jasmine instead of sweet orange flushes. In this sense, the orange jasmine odors plume could contribute for psyllid
mobility towards orange jasmine, which increases the _D. citri_ settling preference to this host, as observed in field experiments. Regarding the volatile analysis, we found that orange
jasmine volatile profile is clearly distinguishable to that of sweet orange (Fig. 7). Then, analyses were conducted to identify which compounds were relevant to make this distinction either
quantitatively or qualitatively (Supplementary Table 1). Green leaf volatiles, such as hexenyl acetates30, were more emitted by orange jasmine than by sweet orange leaves, as previously
reported31. Monoterpene emission was also different between genotypes, being all them less abundantly emitted by orange jasmine. Lower emission of d-limonene and β-ocimene was also found in
orange jasmine in relation to lemon (_Citrus_ × _limon_ (L.) Osbeck), rough lemon (_Citrus_ × _taitensis_ Risso syn. _Citrus jambhiri_ Lushington), sweet orange, grapefruit (_Citrus_ ×
_aurantium_ L. syn. _Citrus paradisi_ Macfad.) and _Citrus_ × _macrophylla_ Wester in other studies19,31,32. Orange jasmine emits less β-pinene and linalool than ‘Red Rio’ grapefruit,
‘Meyer’ lemon (_Citrus_ × _limon_) and ‘Valencia’ sweet orange19,31. Most of the relevant compounds emitted by orange jasmine corresponded to sesquiterpenes, usually absent or poorly emitted
by _Citrus_ leaves19,31,32,33. Nearly all of the identified sesquiterpenes have been reported before as emitted by orange jasmine leaves19,31,32. These results are in accordance with the
highest attractiveness of psyllids to orange jasmine volatiles in the olfactometer device (chemical cues) and field experiments (chemical and visual cues). Delving in this area until
determination of which compound/s are responsible for higher attraction of orange jasmine leaves to _D. citri_ would allow the development of more efficient traps to control/monitor insect
populations. Besides reducing _D. citri_ population and its spread, the presence of orange jasmine trap crop at the edge resulted in 43% reduction in HLB incidence at the orange orchard.
Similarly, a cereal border crop reduced in 51.5% the incidence of Bean yellow mosaic virus (transmitted by aphids) compared to fallow fields in narrow-leaved lupin (_Lupinus angustifolius_
L.)34. Despite orange jasmine has been reported as a CLas host, bacterial titers are much lower in this host than on citrus trees20,21,35,36,37, and consequently, it may be considered as an
irrelevant inoculum source epidemiologically. Additionally, it is known that _D. citri_ adults are less efficient than nymphs to acquire CLas38,39,40, and the transmission process demands a
latent period of at least 7–10 days before psyllids become bacteriliferous41. Moreover, at 7 DAR the number of psyllids on orange jasmine plants decreased 97% (Fig. 4), probably due to the
treatment with thiamethoxam. This observation was confirmed with data from the experiment in which psyllids confined on thiamethoxam-treated orange jasmine plants presented mortality close
to 100% at 7 DAC (Fig. 5). The low number of released psyllids recaptured on citrus trees (0.3%) in comparison to that of insects recaptured when no trap crop was used7,25 supports that
thiamethoxam treated-orange jasmine acted as a ‘sink’ for _D. citri_, attracting, killing and consequently, reducing psyllid movement into the citrus orchard. Therefore, it is reasonable to
consider that the acquisition of CLas from a psyllid that landed on treated orange jasmine and subsequent inoculation in heathy citrus trees from a commercial orchard would be close to zero.
In summary, this study demonstrated for the first time that _M. paniculata_ treated with insecticides may act as a trap crop to attract and kill _D. citri_ before settling on the border of
citrus orchards. However, the use of orange jasmine as a trap crop should be implemented before, at the same time or soon after citrus tree planting. Moreover, our work opens the possibility
of performing studies assessing the trap crop integration with other tactics (e.g. kaolin), as a ‘push’ and ‘pull’ strategy, which could decrease further infestation rates inside citrus
orchards. Finally, in order to avoid the use of insecticides, a genetically modified trap crop, able to interfere with _D. citri_ survival, could be used in the citrus edges to attract and
kill _D. citri_. An analogous approach has been used in Hawaii, where borders of transgenic papaya plants resistant to _Papaya ringspot virus_ reduced the spread of aphid-vectors and then
viral incidence on non-transgenic papaya crops16. METHODS EFFECTS OF ORANGE JASMINE AS A TRAP CROP ON _DIAPHORINA CITRI_ NATURAL INFESTATION AND HLB INCIDENCE The study was carried out from
October 2011 to July 2015 in two areas from a commercial orchard located in Matão, SP State, Brazil subjected to HLB control. The area A (21.60806°S, 48.42611°W) was located in a plot of
‘Hamlin’ sweet orange (_Citrus_ × _aurantium_ L., syn. _Citrus sinensis_ (L.) Osbeck) trees (6 months old; ~1.0 m-tall; Supplementary Fig. 1a,b) grafted on ‘Swingle’ citrumelo [_Citrus_ ×
_aurantium_, syn. _Citrus paradisi_ Macfad. × _Citrus trifoliata_ L., syn. _Poncirus trifoliata_ (L.) Raf.] with spacing of 7.5 × 2.5 m. The area B (21.58556°S, 48.54556°W) was located in a
plot of ‘Valencia’ sweet orange trees grafted on Rangpur lime ‘_Citrus limonia_’ Osbeck (7 years old; ~3.0 m-tall; Supplementary Fig. 1c,d) with spacing of 6.5 × 2.8 m. Both areas were
historically subjected to continuous influx of _D. citri_ from neighbor areas with high HLB incidence. Each area was divided into two plots of 100 × 120 m. In one plot of each area, the
orange jasmine trap crop was planted on east (area A) and north (area B) edges, 20 m from the first citrus tree, in two double-rows (1 m separated) with spacing of 0.4 × 0.4 m per plant. In
total, 325 orange jasmine trees (~0.6 m-tall) were planted per row forming a canopy with 2 m in width and 120 m in length. The remaining plots of each area were maintained as fallow mowed
grass and used as controls. Orange jasmine trees were treated with drench applications of thiamethoxam (Actara® 250 WG, Syngenta Proteção de Cultivos Ltda., Paulínia, SP, Brazil) 10 days
before planting (0.25 g tree−1) and every 70 days thereafter (0.31 g of active ingredient per meter of tree height). In addition, foliar applications of insecticides with different modes of
action (pyrethroid, organophosphate and neonicotinoid) were applied at interval of 14 days to both, trap crop and citrus orchards. The trap crop was fertilized every 60 days with NPK
(10-10-10) at 100 g tree−1. Psyllid populations were monitored by placing yellow sticky traps (30 × 10 cm) (ISCA® Technologies, Ijuí, RS, Brazil) in 20 sweet orange trees located at 25 and
30 m from the trap crop. The same trap arrangement was used for the fallow field plots. Traps were assessed and replaced fortnightly. The number of _D. citri_ adults was recorded on each
trap. Two visual assessments (May 2014 and January 2015) of HLB-symptomatic trees were performed in all citrus trees from each new citrus plot (area A) and leaf samples from suspected
HLB-infected trees were tested by quantitative polymerase chain reaction (qPCR) for the presence of CLas or ‘_Ca_. L. americanus’42. EFFECTS OF ORANGE JASMINE AS A TRAP CROP ON _DIAPHORINA
CITRI_ SETTLEMENT AND DISPERSAL The experiment was carried out in an experimental area of ‘Pera’ sweet orange (2 years old and ~1.5 m-tall) grafted on Rangpur lime with spacing 5 × 2 m,
located in Araraquara, SP, Brazil (21.71500°S, 48.20083°W). The area was divided into three blocks and each one split into two plots (36 trees plot−1) of 30 × 13 m (Fig. 8). Treatments were
defined according to the presence or absence of orange jasmine as a trap crop in the citrus orchard border. In each plot, 65 nursery _M. paniculata_ trees (1.5 m-tall) were planted, 4 m from
the first citrus tree, in a double-row spacing of 0.4 × 0.4 m, forming a canopy with 0.4 m in width and 13 m in length (Fig. 8). Control plots were maintained as fallow mowed grass. The
orange jasmine trees were treated with drench application of thiamethoxam (0.47 g tree−1) 10 days before planting. In order to ensure the insecticide efficacy, 10 nursery _M. paniculata_
trees treated with thiamethoxam and 10 untreated were planted on the central west side of the experimental area at 2 m from the last citrus row. In these plants, groups of 10 adult psyllids
(10–15 days old) were confined on a young shoot of each orange jasmine tree using sleeve cages, and _D. citri_ mortality was assessed at 1, 3 and 7 days after the beginning of the
experiment. Psyllid confinements on untreated orange jasmine trees were used as a control. Adult psyllids, obtained from a colony free of ‘_Ca_. Liberibacter spp.’, maintained for several
generations on orange jasmine seedlings at Fundecitrus (Araraquara, SP State, Brazil), were marked with different colors of fluorescent powder (Day-Glo Color Corp., Cleveland, OH, USA)43 in
order to differentiate psyllids released on orange jasmine and fallow plots. Before release, marked insects were acclimated for 48 h on orange jasmine seedlings. Field release was conducted
as described by Tomaseto _et al_.7 in 1.5 m-tall platforms located on the east side of the area at 10 m from the first citrus trees of each plot. The experiment was replicated three times,
and psyllid releases were always performed in the afternoon (~15:00), which is the period of the highest _D. citri_ flight activity27,44,45, with 800–1000 marked psyllids per plot. The
number of settled psyllids was assessed by visual inspection on 24 citrus trees (central trees of each row) from each plot (Fig. 8), at 1, 3 and 7 days after release (DAR). Temperature and
rainfall were monitored using a weather station (Vantage Pro2 6152; Davis Instruments, Hayward, CA, USA) 20 m far from the experimental area. For the first replicate, the mean temperature
ranged from 22.0 to 25.1 °C with total precipitation of 19.2 mm, while for the second and third replicates, temperature and rainfall values ranged from 23.3 to 27.8 °C with 64.5 mm and from
21.7 to 24.06 °C with 6.1 mm, respectively (Supplementary Fig. 2). OLFACTOMETRIC ASSAYS The assays were carried out in a climate-controlled room at temperature 25 ± 2 °C, relative humidity
65 ± 10%, and 3000 lux luminosity. The preference of _D. citri_ toward volatiles was investigated using a 4-arm olfactometer (30.0 × 30.0 × 2.5 cm in length, width, and height,
respectively), adapted with a yellow acrylic floor arena, essentially as described in Zanardi _et al_.46. Individual constant (0.1 L min−1) charcoal-filtered humidified airflow, provided by
an oil-free air compressor (Schulz MSV6, Schulz, Joinville, SC, Brazil) converged through 0.635 cm-diameter individual PTFE tubes (Sigma-Aldrich, Bellefonte, PA, USA) to the center of the
acrylic arena. A single mated 7–15-day old female was released on the center of the arena. Psyllids that did not perform a choice after 5 min were recorded as “no response” in the analysis.
In case of response, 10 min were allowed to observe the time spent in each one of the four odor fields. For each psyllid (replicate), the time spent in each odor source was recorded. Data
were collected from 10:00 a.m. to 4:00 p.m. from five different assay days and a total of 61 (orange jasmine × clean air) and 65 (‘Pera’ sweet orange × clean air) replicates were used. Two
of the four possible arms received plant volatiles whereas the two remaining arms received clean air33. Odor sources were switched each assay, and the arena was rotated after each responding
insect to prevent bias. Orange jasmine and ‘Pera’ sweet orange nursery trees (~1 year old) to be used as odor sources were pruned 20 days before assays to stimulate the emergence of new
shoots in a greenhouse. VOLATILE EMISSION ANALYSIS New flushes from 1m-tall _M. paniculata_ and _Citrus_ × _aurantium_ sweet orange trees were detached and kept 30 min with the petioles
submerged in water to acclimate. Subsequently they were enclosed in 20 mL screw-cap Pyrex tubes carrying a Teflon septum on the top and containing 1 mL of milli-Q water (for avoiding leaf
hydric stress) and maintained 24 h at a controlled temperature of 22 °C. Volatile capture and GC-MS analysis were performed using a 6890 N gas chromatograph (Agilent Technologies Inc., Las
Rozas, Spain) coupled to a Thermo DSQ mass spectrometer equipped with a DB-5 ms (Agilent J & W Columns) column (60 m × 0.25 mm i.d. × 1.00 μm film) as described before33. In short, SPME
fiber (100 μm poly (dimethyl) siloxane/divinylbenzene (Supelco Inc., Bellefonte, PA) was exposed for 30 min at 22 °C and immediately afterwards transferred to GC injector (220 °C) where
thermal desorption was prolonged to 4 min. The GC interface and MS source temperatures were 260 °C and 230 °C, respectively. Oven programming conditions were 40 °C for 2 min, 5 °C min−1 ramp
until 250 °C, and a final hold at 250 °C for 5 min. Helium was the carrier gas at 1.5 mL min−1 in the splitless mode. Data was recorded in a 5975B mass spectrometer (Agilent Technologies
Inc., Las Rozas, Spain) in the 35‒250 m/z range at 7 scans, with electronic impact ionization at 70 eV. Samples from orange jasmine and sweet orange were analyzed by triplicate and at two
different sampling dates. At each sampling date, a quality control sample (composed by mixtures of both genotypes) was analyzed by triplicate. Datasets were processed independently by the
MetAlign software (www.metalign.nl) for full mass spectral alignment, baseline correction, noise estimation, and ion-wise mass spectral alignment. The MetAlign output scan peak values were
corrected by leaf fresh weight and volatile accumulation time and then subjected to PCA analysis with Past3.17 software (folk.uio.no/ohammer/past). Significant scans were manually analyzed
on TIC chromatograms to quantify the area and tentatively identify compounds by matching the acquired mass spectra with those stored in reference libraries (NIST, MAINLIB, REPLIB). The area
for each compound was corrected by leaf fresh weight and volatile accumulation time and used to generate a heat-map using ClustVis47. DATA ANALYSIS Data from the commercial and the
experimental citrus orchards were analyzed by Poisson generalized linear mixed models (GLMM) using the “_glmmADM_”48 (zero-inflated) and the “_lme4_”49 packages, respectively. Treatment was
considered as fixed effect, while each assessment dates (commercial citrus orchards) or repeated measures on assessed trees (experimental orchard) as random. Time was considered fixed effect
on experimental orchard data. The effect of treatment, time and interaction was assessed by likelihood-ratio tests (_P_ < 0.05) between a full and a reduced model. In order to compare
the effect of trap crop on cumulative mean number (commercial citrus orchards) or on counts of marked psyllids on citrus trees on each independent assessment time (experimental orchard),
data were analyzed by a quasi-Poisson generalized linear model (GLM)50. Goodness-of-fit was assessed through half-normal plots with simulation envelopes using the “_hnp_” package51. In case
of significant differences, means were separated by computing the 95% confidence intervals for linear predictors using the “_lsmeans_” package52. A 2 × 2 chi-squared contingency table was
used to determine the treatment effects on frequency of _D. citri_ detection (percentage of assessments with psyllid detection in relation to total number of assessments) in each commercial
orchard plot. In order to analyze the efficacy of systemic insecticide applied on orange jasmine trees, survival rates in the whole assessment time were compared by a log-rank test (_P_ <
0.05) and survival data at each time after release were compared by a quasi-binomial GLM. Infestation maps were generated using the Surfer® software (Golden Software Inc., Golden, CO, USA)
by the inverse of square of the distance interpolation method, considering the sum of insects found in all replicates. For the olfactometric assays, each pair of a plant (orange jasmine or
‘Pera’ sweet orange) and control (clean air) in behavioral measurements (time spent in each odor field) was compared by using two-tailed, Wilcoxon matched-pairs signed rank test. All
analyses were performed using the statistical software “R”, version 3.3.153. DATA AVAILABILITY The datasets generated during and/or analyzed during the current study are available from the
corresponding author on reasonable request. REFERENCES * Bové, J. M. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. _J Plant Pathol._ 88, 7–37 (2006). Google
Scholar * Alvarez, S., Rohrig, E., Solís, D. & Thomas, M. H. Citrus greening disease (Huanglongbing) in Florida: economic impact, management and the potential for biological control.
_Agric. Res._ 5, 109–118 (2016). Article CAS Google Scholar * Belasque, J. Jr. _et al_. Lessons from huanglongbing management in São Paulo state, Brazil. _J. Plant Pathol._ 92, 285–302
(2010). Google Scholar * Boina, D. R., Meyer, W. L., Onagbola, E. O. & Stelinski, L. L. Quantifying dispersal of _Diaphorina citri_ (Hemiptera: Psyllidae) by immunomarking and potential
impact of unmanaged groves on commercial citrus management. _Environ. Entomol._ 38, 1250–8 (2009). Article Google Scholar * Lewis-Rosenblum, H., Martini, X., Tiwari, S. & Stelinski,
L. L. Seasonal movement patterns and long-range dispersal of Asian citrus psyllid in Florida citrus. _J. Econ. Entomol._ 108, 3–10 (2015). Article CAS Google Scholar * Hall, D. G. &
Hentz, M. G. Seasonal flight activity by the Asian citrus psyllid in east central Florida. _Entomol. Exp. Appl._ 139, 75–85 (2011). Article Google Scholar * Tomaseto, A. F., Krugner, R.
& Lopes, J. R. S. Effect of plant barriers and citrus leaf age on dispersal of _Diaphorina citri_ (Hemiptera: Liviidae). _J. Appl. Entomol._ 140, 91–102 (2016). Article Google Scholar
* Gottwald, T. R. Current epidemiological understanding of citrus huanglongbing. _Annu. Rev. Phytopathol._ 48, 119–139 (2010). Article CAS Google Scholar * Bassanezi, R. B. _et al_.
Efficacy of area-wide inoculum reduction and vector control on temporal progress of huanglongbing in young sweet orange plantings. _Plant Dis._ 97, 789–796 (2013). Article Google Scholar *
Sétamou, M. & Bartels, D. W. Living on the edges: spatial niche occupation of Asian citrus psyllid, _Diaphorina citri_ Kuwayama (Hemiptera: Liviidae), in citrus groves. _PLoS One_ 10,
1–21 (2015). Article Google Scholar * Gottwald, T., Irey, M., Gast, T. & Parnell, S. Spatio-temporal analysis of an HLB epidemic in Florida and implications for spread. In _Proceedings
of the 17 th Conference of International Organization of Citrus Virologists, IOCV_, University of California, Riverside, CA, 84–97 (2010). * Shelton, A. M. & Badenes-Perez, F. R.
Concepts and applications of trap cropping in pest management. _Annu. Rev. Entomol_ 51, 285–308 (2006). Article CAS Google Scholar * Hokkanen, H. M. T. Trap cropping in pest management.
_Annu. Rev. Entomol._ 36, 119–138 (1991). Article Google Scholar * Stern, V. M., Mueller, A., Sevacherian, V. & Way, M. Lygus bug control in cotton through alfalfa interplanting.
_Calif. Agric_. 8–10 (1969). * Godfrey, L. D. & Leigh, T. F. Alfalfa harvest strategy effect on lygus bug (Hemiptera: Miridae) and insect predator population density: Implications for
use as trap crop in cotton. _Environ. Entomol._ 23, 1106–1118 (1994). Article Google Scholar * Gonsalves, D. & Ferreira, S. Transgenic papaya: a case for managing risks of Papaya
ringspot virus in Hawaii. _Plant Heal. Prog_. 1–6, https://doi.org/10.1094/PHP-2003-1113-03-RV (2003) * Aubert, B. _Trioza erytheae_ del Guercio and _Diaphorina citri_ Kuwayama (Homoptera:
Psylloidea), the two vectors of citrus greening disease: biological aspects and possible control strategies. _Fruits_ 42, 149–162 (1987). Google Scholar * Leong, S. C. T., Fatimah, A.,
Beattie, A., Heng, R. K. J. & King, W. S. Influence of host plant species and flush growth stage on the Asian citrus psyllid, _Diaphorina citri_ Kuwayama. _Am. J. Agric. Biol. Sci._ 6,
536–543 (2011). Article Google Scholar * Patt, J. M. & Sétamou, M. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants.
_Environ. Entomol._ 39, 618–24 (2010). Article CAS Google Scholar * Damsteegt, V. D. _et al_. _Murraya paniculata_ and related species as potential hosts and inoculum reservoirs of
‘_Candidatus_ Liberibacter asiaticus’, causal agent of huanglongbing. _Plant Dis._ 94, 528–533 (2010). Article CAS Google Scholar * Lopes, S. A. _et al_. Liberibacters associated with
orange jasmine in Brazil: Incidence in urban areas and relatedness to citrus liberibacters. _Plant Pathol._ 59, 1044–1053 (2010). Article CAS Google Scholar * Cifuentes-Arenas, J. C.
Huanglongbing e _Diaphorina citri_: Estudos das relações patógeno-vetor-hospedeiro. Ph.D. Thesis. Faculdade de Ciências Agrárias e Veterinárias/Universidade Estadual Paulista (UNESP),
Jaboticabal, SP, Brazil. 1–133 (2017). * Morilla, G. _et al_. Pepper (_Capsicum annuum_) is a dead-end host for _Tomato yellow leaf curl virus_. _Phytopathology_ 95, 1089–1097 (2005).
Article CAS Google Scholar * Midega, C. A. O., Pittchar, J. O., Pickett, J. A., Hailu, G. W. & Khan, Z. R. A climate-adapted push-pull system effectively controls fall armyworm,
_Spodoptera frugiperda_ (J. E. Smith), in maize in East Africa. _Crop Prot._ 105, 10–15 (2018). Article Google Scholar * Miranda, M. P. _et al_. Processed kaolin affects the probing and
settling behavior of _Diaphorina citri_ (Hemiptera: Liviidae). _Pest Manag. Sci._ 74, 1964–1972 (2018). Article CAS Google Scholar * Kobori, Y., Nakata, T., Ohto, Y. & Takasu, F.
Dispersal of adult Asian citrus psyllid, _Diaphorina citri_ Kuwayama (Homoptera: Psyllidae), the vector of citrus greening disease, in artificial release experiments. _Appl. Entomol. Zool._
46, 27–30 (2011). Article Google Scholar * Sétamou, M. _et al_. Diurnal patterns of flight activity and effects of light on host finding behavior of the Asian citrus psyllid. _J. Insect
Behav._ 25, 264–276 (2012). Article Google Scholar * Wenninger, E. J., Stelinski, L. L. & Hall, D. G. Roles of olfactory cues, visual cues, and mating status in orientation of
_Diaphorina citri_ Kuwayama (Hemiptera: Psyllidae) to four different host plants. _Environ. Entomol._ 38, 225–234 (2009). Article Google Scholar * Miranda, M. P., Dos Santos, F. L.,
Felippe, M. R., Moreno, A. & Fereres, A. Effect of UV-blocking plastic films on take-off and host plant finding ability of _Diaphorina citri_ (Hemiptera: Liviidae). _J. Econ. Entomol._
108, 245–251 (2015). Article CAS Google Scholar * Visser, J. H. Host odor perception in phytophagous insects. _Annu. Rev. Entomol._ 31, 121–144 (1986). Article Google Scholar * Robbins,
P. S., Alessandro, R. T., Stelinski, L. L. & Lapointe, S. L. Volatile profiles of young leaves of Rutaceae spp. varying in susceptibility to the Asian citrus psyllid (Hemiptera:
Psyllidae). _Florida Entomol._ 95, 774–776 (2012). Article CAS Google Scholar * Fancelli, M. _et al_. Attractiveness of host plant volatile extracts to the Asian citrus psyllid,
_Diaphorina citri_, is reduced by terpenoids from the non-host cashew. _J. Chem. Ecol._ 44, 397–405 (2018). Article CAS Google Scholar * Alquézar, B. _et al_. β-caryophyllene emitted from
a transgenic _Arabidopsis_ or chemical dispenser repels _Diaphorina citri_, vector of _Candidatus_ Liberibacters. _Sci. Rep._ 7, 5639 (2017). Article ADS Google Scholar * Jones, R. A. C.
Effects of cereal borders, admixture with cereals and plant density on the spread of bean yellow mosaic potyvirus into narrow‐leafed lupins (_Lupinus angustifolius_). _Ann. Appl. Biol._
122, 501–518 (1993). Article Google Scholar * Beloti, V. H., Alves, G. R., Coletta-Filho, H. D. & Yamamoto, P. T. The Asian citrus psyllid host _Murraya koenigii_ is immune to citrus
huanglongbing pathogen ‘_Candidatus_ Liberibacter asiaticus’. _Phytopathology_ 108, 1089–1094 (2018). Article CAS Google Scholar * Walter, A. J., Duan, Y. & Hall, D. G. Titers of
‘_Ca_. Liberibacter asiaticus’ in _Murraya paniculata_ and _Murraya_-reared _Diaphorina citri_ are much lower than in _Citrus_ and _Citrus_-reared psyllids. _HortScience_ 47, 1449–1452
(2012). Article Google Scholar * Walter, A. J., Hall, D. G. & Duan, Y. P. Low incidence of ‘_Candidatus_ Liberibacter asiaticus’ in _Murraya paniculata_ and associated _Diaphorina
citri_. _Plant Dis._ 96, 827–832 (2012). Article CAS Google Scholar * Ammar, E.-D. D., Ramos, J. E., Hall, D. G., Dawson, W. O. & Shatters, R. G. Acquisition, replication and
inoculation of _Candidatus_ Liberibacter asiaticus following various acquisition periods on huanglongbing-infected citrus by nymphs and adults of the Asian citrus psyllid. _PLoS One_ 11,
e0159594 (2016). Article Google Scholar * Inoue, H. _et al_. Enhanced proliferation and efficient transmission of _Candidatus_ Liberibacter asiaticus by adult _Diaphorina citri_ after
acquisition feeding in the nymphal stage. _Ann. Appl. Biol._ 155, 29–36 (2009). Article Google Scholar * Pelz-Stelinski, K. S., Brlansky, R. H., Ebert, T. A. & Rogers, M. E.
Transmission parameters for _Candidatus_ Liberibacter asiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). _J. Econ. Entomol._ 103, 1531–1541 (2010). Article CAS Google Scholar *
Canale, M. C. _et al_. Latency and persistence of ‘_Candidatus_ Liberibacter asiaticus’ in its psyllid vector, _Diaphorina citri_ (Hemiptera: Liviidae). _Phytopathology_ 107, 264–272 (2017).
Article Google Scholar * Li, W., Hartung, J. S. & Levy, L. Quantitative real-time PCR for detection and identification of _Candidatus Liberibacter_ species associated with citrus
huanglongbing. _J. Microbiol. Methods_ 66, 104–115 (2006). Article CAS Google Scholar * Nakata, T. Effectiveness of micronized fluorescent powder for marking citrus psyllid. _Diaphorina
citri. Appl. Entomol. Zool._ 43, 33–36 (2008). Article Google Scholar * Tomaseto, A. F. _et al_. Environmental conditions for _Diaphorina citri_ Kuwayama (Hemiptera: Liviidae) take-off.
_J. Appl. Entomol._ 142, 104–113 (2018). Article CAS Google Scholar * Paris, T. M., Croxton, S. D., Stansly, P. A. & Allan, S. A. Temporal response and attraction of _Diaphorina
citri_ to visual stimuli. _Entomol. Exp. Appl._ 155, 137–147 (2015). Article Google Scholar * Zanardi, O. Z. _et al_. Putative sex pheromone of the Asian citrus psyllid, _Diaphorina
citri_, breaks down into an attractant. _Sci. Rep._ 8, 455 (2018). Article ADS Google Scholar * Metsalu, T. & Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate
data using Principal Component Analysis and heatmap. _Nucleic Acids Res._ 43, W566–W570 (2015). Article CAS Google Scholar * Fournier, D. A. _et al_. AD Model Builder: using automatic
differentiation for statistical inference of highly parameterized complex nonlinear models. _Optim. Methods Softw._ 27, 233–249 (2012). Article MathSciNet Google Scholar * Bates, D.,
Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. _J. Stat. Softw._ 67, 1–48 (2015). Article Google Scholar * Nelder, J. A. & Wedderburn, R. W.
M. Generalized linear models. _J. R. Stat. Soc._ 135, 370–384 (1972). Google Scholar * Demétrio, C. G. B., Hinde, J. & Moral, R. A. In _Ecological_ Modelling _Applied to Entomology_
(eds Ferreira, C. P. & Godoy, W. A. C.) 219–259 (Springer, 2014). * Lenth, R. V. Least-Squares Means: the R package lsmeans. _J. Stat. Softw_. 69, (2016). * R Core Team R: A language and
environment for statistical computing. 2015. _R Foundation for Statistical Computing, Vienna, Austria_ (2015). Available at, http://www.r-project.org/. (Accessed: 20th July 2017). Download
references ACKNOWLEDGEMENTS This work was supported by Fund for Citrus Protection (Fundecitrus) and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Proc. 2015/07011-3). We
thank Moacir Celio Vizone, Felipe Marinho Martini and João Pedro Ancoma Lopes for technical support with experiments. Furthermore, we thank Cambuhy Agricola Ltda. and University of
Araraquara (Uniara) for providing the areas in which the field experiments were performed. Second author received scholarship from National Council for Scientific and Technological
Development (CNPq)/Brazil (Proc. 300153/2011-2). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Entomology, Fund for Citrus Protection (FUNDECITRUS), 14807-040, Araraquara, São
Paulo, Brazil Arthur F. Tomaseto, Odimar Z. Zanardi, Haroldo X. L. Volpe, Berta Alquézar, Leandro Peña & Marcelo P. Miranda * Centre of Nature Sciences, Federal University of São Carlos
(UFSCAR), Buri, São Paulo, Brazil Rodrigo N. Marques * Departamento de Protección Vegetal, Instituto de Ciencias Agrarias (ICA/CSIC), C/Serrano, 115 dpdo, 28006, Madrid, Spain Alberto
Fereres * Instituto de Biología Molecular y Celular de Plantas (IBMCP), Consejo Superior de Investigaciones Científicas (CSIC), Universidad Politécnica de Valencia (UPV), 46022, Valencia,
Spain Berta Alquézar & Leandro Peña Authors * Arthur F. Tomaseto View author publications You can also search for this author inPubMed Google Scholar * Rodrigo N. Marques View author
publications You can also search for this author inPubMed Google Scholar * Alberto Fereres View author publications You can also search for this author inPubMed Google Scholar * Odimar Z.
Zanardi View author publications You can also search for this author inPubMed Google Scholar * Haroldo X. L. Volpe View author publications You can also search for this author inPubMed
Google Scholar * Berta Alquézar View author publications You can also search for this author inPubMed Google Scholar * Leandro Peña View author publications You can also search for this
author inPubMed Google Scholar * Marcelo P. Miranda View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.F.T., M.P.M., A.F. and L.P. wrote the
manuscript; A.F.T., M.P.M. and L.P. conceived and designed the experiments; A.F.T. and R.N.M. performed the field experiments; O.Z.Z. and H.X.L.V. performed the olfactometric experiments;
B.A. performed VOC analyses; A.F.T. analyzed the data; M.P.M. and L.P. supervised the study. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Marcelo P. Miranda.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FILES RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tomaseto, A.F., Marques, R.N., Fereres, A. _et al._ Orange jasmine as a trap crop
to control _Diaphorina citri_. _Sci Rep_ 9, 2070 (2019). https://doi.org/10.1038/s41598-019-38597-5 Download citation * Received: 17 July 2018 * Accepted: 28 December 2018 * Published: 14
February 2019 * DOI: https://doi.org/10.1038/s41598-019-38597-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
