Anti-vegf injection frequency correlates with visual acuity outcomes in pro re nata neovascular amd treatment
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Clinical trials report substantial gains in visual acuity (VA) for eyes treated with intravitreal anti-VEGF for neovascular AMD (nAMD). In clinical reality, VA outcomes are more
variable. Here we investigate pro-re nata treatment frequencies and VA in a real-life cohort of 1382 eyes (1048 patients). Patients with nAMD and one year complete follow-up treated with
pro-re nata anti-VEGF between 2009 and 2016 were included. Injection frequency and VA was analyzed clustered by year of first treatment. Baseline parameters were compared between years.
Median injection frequency in the first year was 5 with an IQR (interquartile range) of 5 for patients treated in 2009 and 8 with an IQR of 3 for patients treated from 2012 onwards. Median
VA outcomes at one year were −5 to ±0 letters for patients treated between 2009 and 2013 and ±0 to +2 letters for patients treated from 2013 onwards. This cohort comprises all severities and
subtypes of nAMD. 39% of patients had baseline VA outside the range for the MARINA or ANCHOR clinical trials. Higher treatment frequency was associated with improved VA in our real-life
nAMD cohort. With adequate injection frequency, almost 90% of eyes had stable or improved VA over one year. Median VA gains, however, were lower compared to clinical trials. This may be due
to a wider range of baseline characteristics in real-life cohorts. SIMILAR CONTENT BEING VIEWED BY OTHERS TWO-YEAR OUTCOMES OF INTRAVITREAL AFLIBERCEPT IN A SWISS ROUTINE TREAT AND EXTEND
REGIMEN FOR PATIENTS WITH NEOVASCULAR AGE-RELATED MACULAR DEGENERATION Article Open access 20 November 2020 FROM PRO-RE-NATA TO FIXED-INTERVAL REGIMEN: EVOLVING REAL-WORLD TREATMENT
PARADIGMS IN ANTI-VEGF THERAPY FOR NEOVASCULAR AMD Article 29 January 2025 2-YEAR RESULTS FROM AN OBSERVATIONAL STUDY OF PROACTIVE TREATMENT REGIMENS WITH INTRAVITREAL AFLIBERCEPT 2 MG IN
PATIENTS WITH NAMD IN CLINICAL PRACTICE: XTEND STUDY UK COHORT Article Open access 24 December 2024 INTRODUCTION Anti vascular endothelial growth factor (VEGF) treatment has revolutionized
the treatment of neovascular age-related macular degeneration (nAMD). Since their introduction into clinical care in 2005, anti-VEGF agents have significantly helped to stabilize and improve
vision in hundreds of thousand AMD patients worldwide. However, almost all AMD patients require long-term therapy with frequent intravitreal injections. Several approaches to treat and
monitor AMD patients have been developed. They include (i) rigid monthly injections, (ii) pro-re-nata treatments (PRN) with patients only being injected if they show activity on optical
coherence tomography (OCT), fluorescein angiography or fundoscopy and (iii) treat-and-extend regimens (TAE) with patients being injected at increasing or decreasing intervals depending on
their disease activity. Our center has been following a PRN regimen since 2009 that closely resembles the IVAN study regimen1 (see Methods for details). Using this modified IVAN regimen, we
have previously published compound results from five years anti-VEGF treatment2. The purpose of the current study is to evaluate whether injection frequencies have changed between 2009 and
2017 and whether such changes impact visual acuity (VA) outcomes. In addition, this study describes the differences between a real-life nAMD cohort and clinical trial patients regarding
baseline demographics and VA outcomes. METHODS Patients being diagnosed with nAMD (represented by International Classification of Diseases (ICD)-code H35.3) receiving their first
intravitreal injection between January 2009 and December 2016 were included in this retrospective analysis. The diagnosis nAMD was based on the following OCT findings: drusen in association
with subretinal and/or intraretinal fluid +/− pigment epithelial detachment (PED). Active leakage of the CNV membrane was confirmed by fluorescein angiography at first presentation and
during follow-up as needed. All patients were treated using a pro-re-nata regimen with sets of three monthly anti-VEGF injections as standard treatment, but adjusted to disease activity
based on the examiners judgement (i.e. treatment intervals could be prolonged to 6 weeks if only minimal activity was noted; minimum treatment intervals, however, were always 4 weeks). All
decisions to inject as well as on injection frequencies were made based on both OCT and VA findings supplemented by fluorescein angiography where needed. Patients with inactive nAMD were
followed for six months with monthly OCT visits. In cases of sustained inactivity beyond 6 months patients were referred back to their primary care Ophthalmologist for further follow-ups.
Patients and physicians were free to choose between bevacizumab, aflibercept or ranibizumab and patients could be switched between anti-VEGF substances during treatment. All methods were
carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Ethics Board of the University of Freiburg Medical Centre (No. 26/15).
This is a retrospective study. All data was anonymized in the first step of data processing. Therefore the need to obtain informed consent was waived by the Ethics Committee. The method of
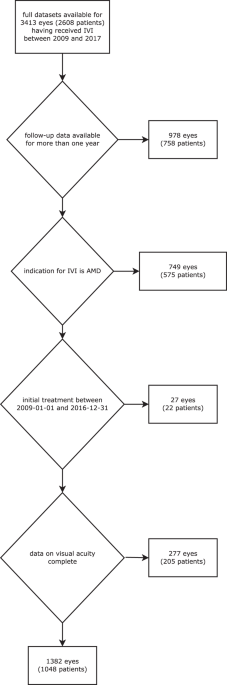
clinical data acquisition has been described previously2. Patients with a follow-up period of ≤1 year or without full electronically available visual acuity (VA) measurements were excluded
(Fig. 1). Patients were analysed on a per-eye-basis, i.e. if patients received bilateral injections both eyes were analyzed independently and all analyses reported in this study are per eye.
VA was acquired on decimal charts and converted to ETDRS-equivalents for further analysis and comparison. Different from controlled clinical trials this is a real life cohort. This means
that a number of different operators collected VA measurements and that operators changed over the years. Autorefraction was performed before each VA exam and VA was recorded with
autorefraction values as well as with the patient’s own glasses. The better VA was used for analyses. For VA readings “hand motion” and “counting fingers”, we determined an equivalent as
described previously3. It should be noted that measured Snellen acuities (or decimal acuities as in our study) may be lower than measured EDTRS acuities in the same eye4,5. We have corrected
our baseline VA values for comparison with trials using ETDRS charts based on Falkenstein _et al_.4 Lines read only partially during decimal VA testing were counted if at least three of
five characters were read correctly. Data processing and statistical analysis was done using GNU R and additional packages6,7,8,9. PRECIS This study reports anti-VEGF treatment frequencies
and visual acuity from a real-life neovascular AMD cohort (1382 eyes). Median injection frequency was 5 to 8.; higher injection frequency was associated with superior visual acuity. RESULTS
In order to identify possible changes in injection frequencies over time, we separated our data set by time of first injection. Figure 2A demonstrates an increase of median injection numbers
over time. In 2009, the median number of injections per eye was 5 with an IQR (interquartile range) of 5. The IQR indicates a high interindividual variability. Between 2010 and 2012, median
injection numbers in the first treatment year increased to 8 (IQR 3) and remained on that plateau from 2012 onwards. This increase in mean injection frequency is statistically significant
(p < 0.01 for years 2012 and later, Dunnett-test). Interestingly, the increase in injection frequency over the years was mirrored by a similar increase in VA outcomes: patients with
treatment initiated in 2009 displayed a median VA loss between −4 and −5 letters at the end of year one while patients treated from 2013 onwards showed a median VA outcome between ±0 and +2
letters (Fig. 2B). It should also be noted that no further increase in injection frequencies was observed from the year 2013 onwards indicating a stable plateau of PRN injections at 8
injections in the first treatment year. In all years analyzed, the majority of patients remained within ±3 lines VA change (Fig. 3). The proportion of eyes with significant VA loss (>3
lines), however, decreased from 29% in 2009 to an average of 12.5% between 2013 and 2016 while the proportion of eyes with significant VA gain increased from 10% in 2009 to an average of 17%
between 2013 and 2016. Achieving certain VA thresholds that are required for performing important everyday tasks like driving a car may be more relevant to patients than absolute VA
changes. We therefore analyzed baseline VA and outcomes clustered into three categories: (i) good VA of ≥0.5: able to read and drive; (ii) intermediate VA of 0.3–0.49; and (iii) poor VA ≤
0.3. Figure 4 displays the proportion of patients in each of the above categories at baseline and at the end of year one. Across all treatment years, most eyes remained in their respective
baseline VA category. Comparing the two ends of our analysed time window, however, yields interesting differences. For eyes with treatment initiated in 2009, the only significant group of
patients switching categories were eyes moving from VA ≥ 0.5 down to VA < 0.32. For patients with treatment initiated in 2016, the overall proportion of eyes switching VA categories
increased and we observed eyes moving up as well as down across VA categories. We next investigated whether baseline demographic criteria differed between patients over the years (Fig. 5).
In 2009, median patient age was 73.5 years (IQR 7.0) and increased slightly in the following years with a peak of 79.52 years (IQR 9.7) in 2014. These differences were statistically
significant for all years from 2012 onwards (compared to 2009; Dunnett p < 0.05) (Fig. 5A). Different from patient age, median VA at baseline did not differ significantly between the
treatment years (p = 0.44, Kruskal-Wallis-Test; Fig. 5B). DISCUSSION Anti-VEGF treatment significantly improves VA outcome of patients with nAMD10,11. The VA gains achieved in clinical
trials, however, are often not paralleled in real-life. For example, a recent study of a large US real-life cohort revealed stable mean VA with +/− 0 letters at one year12. This is
considerably better than the natural history of untreated nAMD but far from the VA gains found in randomized clinical trials (RCTs)13. The main difference between real-life data and RCTs is
that RCTs represent a highly selected cohort of patients. Besides disease-related inclusion criteria like limited lesion size, fairly good but not excellent VA at baseline and absence of
central macular scarring, patients in RCTs must meet other inclusion criteria. For example, RCT patients are selected based on their ability to understand the implications of study
participation and their ability to attend frequent study visits. As a consequence, a large proportion of patients requiring treatment in real-life settings are not represented in RCTs. In a
real life setting, in contrast, a certain degree of result variability may stem from the heterogeneity of the underlying disease. In our study, median VA outcome at one year was between −5
and +2 letters from baseline, depending on the year in which anti-VEGF treatment was initiated. Importantly, our treatment paradigm remained the same between 2009 and 2017. We observed,
however, that the stringency with which treatment was applied improved over the years. This reflects an improvement in treatment paths and improved patient communications regarding the
importance of treatment adherence. Decision to treat was always based on OCT parameters of active nAMD in combination with VA development and backed up by fluorescein angiography as needed.
It is known that the required number of injections for nAMD with PRN treatment is around 7 injections per year14,15. From 2013 onwards, our data demonstrates a plateau with a mean injection
frequency of 7.5 per year. This plateau of adequate anti-VEGF frequency is mirrored by median VA outcomes between 0 and +2 letters from 2013 onwards. Before 2013, our mean injection
frequencies were lower (indicating undertreatment) and median VA outcomes ranged between −5 and ±0 letters. Undertreatment is one of the main risk factors for poor VA outcome16. Our data
confirms these findings. In addition, our data from 2013 onwards provides evidence that with frequent PRN injections, median VA can be maintained in a real-life population that contains all
subforms and severities of nAMD. Our data also shows that PRN injections in our cohort did not increase to median values above 8 injections in the first year. It is important to find the
right balance between treatment cost, time and benefit for the patient in AMD treatment. Our data indicates that 8 injections in the first year may represent this balance point. Importantly,
a significant proportion of eyes in our real-life cohort had baseline VAs outside the range that would have permittted enrolment into the clinical trials that had led to the approval of
anti-VEGF therapies. Only 61% of eyes would have qualified for partizipation in the MARINA or ANCHOR clinical trials. A significant proportion of our eyes showed baseline VA values above the
threshold for RCT trial participation. These eyes with higher baseline VA have limited room for VA improvement. In our dataset, 39% of eyes had baseline VA either above or below the
standard thresholds for RCTs. These numbers illustrate the importance of real-life studies in anti-VEGF treatment. While for select patients (about 15% in our cohort) substantial VA gains
>3 lines can be achieved, a more realistic aim for the majority of patients in a real-life setting is VA stabilization. For patient guidance and treatment adherence, it is important to
communicate realistic treatment aims and provide patients with a realistic prognosis regarding VA outcomes. Our data and others show that injection frequency is closely associated with VA
outcomes in nAMD17 – a fact that should be emphasized during patient communication. DATA AVAILABILITY Compund anonymized data can be made available upon request. REFERENCES * IVAN Study
Investigators _et al_. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. _Ophthalmology_ 119, 1399–1411
(2012). Article Google Scholar * Wecker, T. _et al_. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. _Br J
Ophthalmol_ 101, 353–359 (2017). PubMed Google Scholar * Lange, C., Feltgen, N., Junker, B., Schulze-Bonsel, K. & Bach, M. Resolving the clinical acuity categories ‘hand motion’ and
‘counting fingers’ using the Freiburg Visual Acuity Test (FrACT). _Graefes Arch. Clin. Exp. Ophthalmol._ 247, 137–142 (2009). Article CAS Google Scholar * Falkenstein, I. A. _et al_.
Comparison of visual acuity in macular degeneration patients measured with snellen and early treatment diabetic retinopathy study charts. _Ophthalmology_ 115, 319–323 (2008). Article Google
Scholar * Kaiser, P. K. Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). _Trans Am Ophthalmol Soc_ 107,
311–324 (2009). PubMed PubMed Central Google Scholar * Harrell & Dupont. _Hmisc: Harrell Miscellaneous_. (2015). * Lang. _RJSONIO: Serialize R objects to JSON, JavaScript Object
Notation_. (2014). * Wickham, H. Reshaping data with the reshape package. _J Stat Softw_ (2007). * Wickham. Scales: scale functions for graphics (2014). * Brown, D. M. _et al_. Ranibizumab
versus verteporfin for neovascular age-related macular degeneration. _N. Engl. J. Med._ 355, 1432–1444 (2006). Article CAS Google Scholar * Rosenfeld, P. J. _et al_. Ranibizumab for
neovascular age-related macular degeneration. _N. Engl. J. Med._ 355, 1419–1431 (2006). Article CAS Google Scholar * Lotery, A., Griner, R., Ferreira, A., Milnes, F. & Dugel, P.
Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. _Eye (Lond)_ 31, 1697–1706 (2017). Article CAS Google Scholar
* Elshout, M., Webers, C. A., van der Reis, M. I., de Jong-Hesse, Y. & Schouten, J. S. Tracing the natural course of visual acuity and quality of life in neovascular age-related
macular degeneration: a systematic review and quality of life study. _BMC Ophthalmol_ 17, 120 (2017). Article Google Scholar * CATT Research Group _et al_. Ranibizumab and bevacizumab for
neovascular age-related macular degeneration. _N. Engl. J. Med._ 364, 1897–1908 (2011). Article Google Scholar * Comparison of Age-related Macular Degeneration Treatments Trials (CATT)
Research Group _et al_. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. _Ophthalmology_ 119, 1388–1398 (2012). Article Google
Scholar * Holz, F. G. _et al_. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. _Br J Ophthalmol_ 100,
1623–1628 (2016). Article Google Scholar * Ehlken, C., Helms, M., Böhringer, D., Agostini, H. T. & Stahl, A. Association of treatment adherence with real-life VA outcomes in AMD, DME,
and BRVO patients. _Clin Ophthalmol_ 12, 13–20 (2018). Article Google Scholar Download references ACKNOWLEDGEMENTS A.S. is supported by the DFG (STA 1102/5-1) and the German Ophthalmic
Society (DOG). This work was supported by a research grant from Novartis Germany. Novartis had no role in the design or conduct of this research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Eye Center, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany Thomas Wecker, Bastian Grundel, Sabine Reichl, Milena Stech, Clemens
Lange, Hansjürgen Agostini & Daniel Böhringer * Ophthalmic practice Dr. Wecker, Heilbronn, Germany Thomas Wecker * Department of Ophthalmology, University Medical Center, Greifswald,
Germany Andreas Stahl Authors * Thomas Wecker View author publications You can also search for this author inPubMed Google Scholar * Bastian Grundel View author publications You can also
search for this author inPubMed Google Scholar * Sabine Reichl View author publications You can also search for this author inPubMed Google Scholar * Milena Stech View author publications
You can also search for this author inPubMed Google Scholar * Clemens Lange View author publications You can also search for this author inPubMed Google Scholar * Hansjürgen Agostini View
author publications You can also search for this author inPubMed Google Scholar * Daniel Böhringer View author publications You can also search for this author inPubMed Google Scholar *
Andreas Stahl View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.W. study design, generating results, data analysis, writing manuscript.
B.G., B.G., S.R., M.S., C.L., H.A. and D.B. generating results, data analysis. A.S. study design, generating results, data analysis, writing manuscript. CORRESPONDING AUTHOR Correspondence
to Thomas Wecker. ETHICS DECLARATIONS COMPETING INTERESTS T.W. has received speaker fees from Novartis. H.A. has received research funds from Novartis as well as honoraria/speaker
fees/advisory fees from Roche, Allergan, Zeiss, Bayer Healthcare. A.S. has received research funds from Novartis Germany as well as honoraria/speaker fees/advisory fees from Allergan, Bayer,
Boehringer Ingelheim, Novartis, Orphan Europe. The other authors declare no competing interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wecker, T., Grundel, B., Reichl, S. _et al._ Anti-VEGF injection frequency
correlates with visual acuity outcomes in _pro re nata_ neovascular AMD treatment. _Sci Rep_ 9, 3301 (2019). https://doi.org/10.1038/s41598-019-38934-8 Download citation * Received: 03
August 2018 * Accepted: 15 January 2019 * Published: 01 March 2019 * DOI: https://doi.org/10.1038/s41598-019-38934-8 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
