Antimicrobial properties of the novel bacterial isolate paenibacilllus sp. Smb1 from a halo-alkaline lake in india
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Antibiotic-resistance is ever growing burden on our society for the past many years. Many synthetic chemistry approaches and rational drug-design have been unable to pace up and
tackle this problem. Natural resources, more specifically, the microbial diversity, on the other hand, make a traditional and still the best platform to search for new chemical scaffolds and
compounds. Here, we report the antimicrobial characteristics of novel bacterial isolate from a salt lake in India. We screened the bacterial isolates for their inhibitory activity against
indicator bacteria and found that four novel species were able to prevent the growth of test strains studied _in vitro_. Further, we characterized one novel species (SMB1T = SL4-2) using
polyphasic taxonomic approaches and also purified the active ingredient from this bacterium. We successfully characterized the antimicrobial compound using mass spectroscopy and amino acid
analysis. We also allocated two novel biosynthetic gene clusters for putative bacteriocins and one novel non-ribosomal peptide gene cluster in its whole genome. We concluded that the strain
SMB1T belonged to the genus _Paenibacilllus_ with the pairwise sequence similarity of 98.67% with _Paenibacillus tarimensis_ DSM 19409T and we proposed the name _Paenibacillus sambharensis_
sp. nov. The type strain is SMB1T (=MTCC 12884 = KCTC 33895T). SIMILAR CONTENT BEING VIEWED BY OTHERS SULFOXANTHICILLIN FROM THE DEEP-SEA DERIVED _PENICILLIUM_ SP. SCSIO SOF101: AN
ANTIMICROBIAL COMPOUND AGAINST GRAM-POSITIVE AND -NEGATIVE PATHOGENS Article 16 January 2023 ISOLATION, CHARACTERIZATION, ANTI-MRSA EVALUATION, AND IN-SILICO MULTI-TARGET ANTI-MICROBIAL
VALIDATIONS OF ACTINOMYCIN X2 AND ACTINOMYCIN D PRODUCED BY NOVEL _STREPTOMYCES SMYRNAEUS_ UKAQ_23 Article Open access 15 July 2021 FIRST REPORT ON ANTIBIOTIC RESISTANCE AND ANTIMICROBIAL
ACTIVITY OF BACTERIAL ISOLATES FROM 13,000-YEAR OLD CAVE ICE CORE Article Open access 12 January 2021 INTRODUCTION Increasing burden of antibiotic resistance around the globe is of grave
concern. The present antibiotics are almost ineffective to fight against the deadly infections caused by many bacteria1. It is estimated that till 2050, around 10 million people may die from
antimicrobial-resistant infections if current scenario persists. Presently, the methods and measures taken globally to tackle this problem are insufficient and slow2. Various strategies
such as high throughput screening of synthetic chemical compound libraries and determination of new targets with the help of genomic studies were not successful in finding potential
antimicrobial entities3. In recent years, natural products have gained significant attention to overcome the gap in drug discovery and development due to their structure versatility and
potential biological activity4,5,6,7,8. Majority of the antibiotics consumed at present are either the natural compounds or derivatives thereof, which were discovered from soil
_Actinomycetes_ in 1940–1960s i.e. golden era of antibiotics. The soil has been extensively mined since then for the new antimicrobial molecules and now seems exhausted. Therefore, looking
for alternative microbial sources or unique niches of microbes would be an asset to find novel antimicrobial compounds. Biodiversity of halophilic bacteria holds a huge potential to produce
new and unexplored antimicrobial entities. Tonima Kamat _et al_. reported the antimicrobial activities demonstrated by the halophilic bacteria isolated from salt pans9. Similarly, Toktham
_et al_. also reported the effectiveness of the halophiles as antimicrobials10,11. Recently, Atirah _et al_. discussed the purification of a bacteriocin from _Halomonas_ sp.12. Intracellular
proteins of _Virgibacillus marismortui_ and _Terribacillus halophilus_, i.e., glucanase and chinatase respectively have been reported for their antimicrobial activity13. Based on these
observations and since there are not many reports available from India showing the antimicrobial potential of halophiles, we studied the biodiversity of halophilic bacteria isolated from
Sambhar Lake in Rajasthan, India. In the present study, we screened the bacterial isolates from this lake for their antimicrobial activity. We have characterized one novel species strain
SMB1 in this manuscript. Moreover, we purified and characterized the antimicrobial compound from the fermentation broth. Tandem mass spectroscopy, amino acid analysis, and whole genome data
were used to identify this antimicrobial compound. Additionally, we predicted two novel bacteriocin gene clusters and one non-ribosomal peptide gene cluster in the whole genome of this novel
species. The novel species SMB1T was characterized using polyphasic taxonomic approaches. RESULTS ANTIMICROBIAL SCREENING OF BACTERIAL STRAINS More than hundred bacterial strains were
isolated from the Sambar Lake and screened for their antimicrobial activity against _E. coli_ (MTCC 1610)_, Staphylococcus aureus_ (ATCC 25923)_, Bacillus subtilis_ (ATCC 6633), and _Candida
albicans_ (MTCC 224). The cell-free supernatant and/or crude fermentation extract of fifteen isolates inhibited the growth of Gram-positive bacteria. These positive isolates were identified
based on their 16S rRNA gene sequences. They comprised of four novel species among them as shown in Table 1. Our group recently described three of these novel species viz; SMB4T 14, AK73T
15 and AK74T 16. In the current work, we characterized SMB1T as novel species and studied its antimicrobial activity. IDENTIFICATION AND CHARACTERIZATION OF THE ANTIMICROBIAL COMPOUND FROM
STRAIN SMB1T The strain SMB1T showed consistent antagonistic activity against Gram-positive bacteria. Partial purification of the antimicrobial compound was achieved through cation-exchange
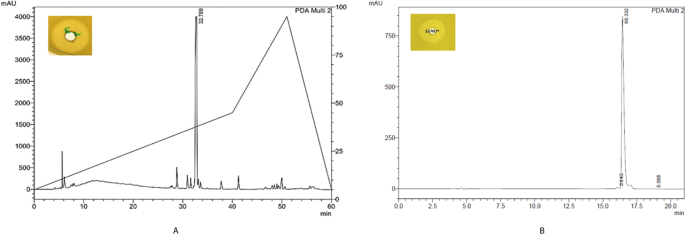
chromatography. The active fraction was eluted at 0.5 M NaCl concentration. Further, the purification of the active compound was performed on RP-HPLC. Figure 1A shows the HPLC chromatogram
of cation-active dialysate. The active compound eluted at RT = 32.7 min. The purity of the compound was more than 95% (Fig. 1B). The pure compound showed m/z value at 1422.76 [M + H]+ in
LC-ESI-MS and one doubly charged ion at 711.88 [M + 2H]+2 was also formed as illustrated in Fig. 2A. The molecular mass of the compound was deduced as 1421.75 Da. Tandem MS and amino acid
analysis were further carried out to identify the compound. In the amino acid analysis, we found the presence of aspartic acid, glutamic acid, histidine, isoleucine, leucine, phenylalanine,
lysine and one non-standard amino acid which later was identified as ornithine Fig. 2B. MS-MS data also supported the amino acid composition. Mass spectroscopy and amino acid profile
revealed that the active compound belongs to bacitracin family and its molecular mass was similar to that of bacitracin A (C66H103N17O16S, calc. mass 1421.749 Da). The MS/MS data was
consistent with what we observed for standard bacitracin A (Alfa Aeser, Thermofisher Scientific, India) (Fig. S1). We studied the MS/MS data extensively and annotated all the b and y ions
present in the raw spectrum (Fig. 3A). Bacitracin A is a cyclic peptide containing twelve amino acid residues17,18. The lysine at position 6 is involved in double linkage with ornithine at
position 7 using α- carbonyl group while with asparagine at position 12 using ε-amino group. In tandem mass spectroscopy, such residues are most vulnerable and thus we observed two series of
b and y ions upon linearization of the peptide after the breakage of either of the bonds. Each bond leads to a different primary sequence and we were able to assign both series of ions as
can be seen in Fig. 3B. For further confirmation, we performed the comparative analysis of the antimicrobial compound produced by the SMB1T strain with standard bacitracin A. As shown in
Fig. 4A,B, both compounds had significantly similar retention time in analytical RP-HPLC. Moreover, the antimicrobial activity spectrum against selected indicator strains was also similar
(Fig. S2). This data collectively suggested that compound isolated in this study is bacitracin A. WHOLE GENOME ANALYSIS AND PUTATIVE BIOSYNTHETIC GENE CLUSTERS The size of the genome of the
strain SMB1T is 5661449 bp with 4943914 total reads (N50 size 247161, L50 8 and N75 176162). This sequence was the draft genome of the strain SMB1T in which 63 contigs were obtained. We
predicted genes from the ABySS assembled contigs using Glimmer19. We found 5,282 genes in the assembly. The G + C content as predicted in the genome analysis was 53.0%, this falls in the G +
C content range i.e 45–54% generally found in the genus _Paenibacillus_20. The assembled fasta file was uploaded on Rapid Annotation using Subsystem Technology (RAST) tool. This is a fully
automated system for genome annotations. For further confirmation of the production of bacitracin by the strain SMB1T, we looked for the genes encoding for the synthesis of the bacitracin,
we were able to locate the partial cluster at contig 383 showing the presence of Bacitracin synthetase 3 (BA3) gene as shown in Fig. S3. This enzyme complex encodes for the amino acids that
are required for the synthesis of bacitracin. Hence, with this annotation results we confirmed that our strain SMB1T is having the genes for the synthesis of bacitracin which was purified in
the present study. Moreover, we subjected the whole genome sequence to other tools like as anti-SMASH (Antibiotics & Secondary Metabolites Analysis Shell) and BAGEL to find out the
genes associated with the antimicrobials and the secondary metabolites. Two novel biosynthetic gene clusters were obtained in the BAGEL analysis (Fig. 5A,B). The gene clusters encoding for
the lasso-peptide (cluster1) and thiopeptide (cluster2), the putative bacteriocins were identified using BAGEL 3. Another novel biosynthetic gene cluster was identified using anti-SMASH; it
belonged to non-ribosomal peptide (NRP) secondary metabolite, and was located on contig 41 as shown in Fig. 5C. The software predicted thirteen adenylation domains and six epimerase domains.
This analysis suggests that the predicted peptide contains thirteen amino acid residues; six out of them may be D-amino acids. The gene cluster exhibited less than 40% sequence identity
with its closest homologue from other bacterial species. This indicated that the compound encoded by this cluster may be a novel compound and needs further investigation. The other clusters
also displayed low levels of homology with other peptide gene clusters. The comparison of bacteriocin and NRPS gene cluster with their homologs are given in the Supplementary Material
(Supplementary Figs S4 and S5). The genome sequence of the strain SMB1T has been deposited in the GenBank database and Accession Number QKRB00000000 was obtained. CHARACTERIZATION OF NOVEL
SPECIES SMB1T The strain SMB1T was characterized using polyphasic taxonomic approach. The 16S rRNA (Accession Number LT161878) gene was showing 98.6% sequence similarity with _Paenibacillus
tarimensis_. The sequence similarity with other members of the genus _Paenibacillus_ is between 92.71% and 95.32%. The phylogenetic tree analysis demonstrated that the strain SMB1T belongs
to the genus _Paenibacillus_ and its closest homolog is _Paenibacillus tarimensis_ (Fig. 6). The strain SMB1T is non-motile, straight rod-shaped Gram-positive bacterium with dimension
0.43–0.69 µm wide × 2.25–4.18 µm long (Fig. S6). The colonies were irregular with raised elevation, diameter 2–3 mm, cream color on ZMA plates, whereas after 48 hours in same conditions the
colonies tend to appear reddish in color. The spore formation was noticed after 48 h. The strain SMB1T was able to grow between 30°C to 42°C and had an optimum temperature for growth at
37°C. The pH range for the growth was from pH 6.0 to 9.0, with the optimum growth at pH 7.0. Optimum growth occurred at salinities from 2% (NaCl, w/v) and the salinity range that the strain
can withstand is 0–3%(NaCl, w/v). The phenotypic characteristics of the strain SMB1T in comparison to its closely related species are described in Table 2. The results observed using
phenotypic fingerprinting (VITEK 2 GP) are represented in Table S1.The fatty acid profile (Table S2) revealed the presence of branched and saturated like C16:0 (18.17%), C17:0 (4.06%),
iso-C15:0 (4.17%), iso-C16:0 (5.83%), iso-C17:0 (5.09%), anteiso-C15:0 (48.86%) and anteiso-C17:0 (13.82%). Hydroxy fatty acids were absent. Overall fatty acid profile of strain SMB1T was
same as those of the strain DSM-19409T and however, the saturated fatty acids C17:0 and branched fatty acids iso-C17: 0 was absent in DSM-19409T. Fatty acids C14: 0, C16: 1 _ω11c_ and
iso-C14: 0 was absent in strain SMB1T but present in DSM-19409T. Hence, it clearly demonstrates the difference in their fatty acid profiles. The DNA base composition of strain SMB1T was 54
mol% G + C (_Tm_). According to the DNA-DNA hybridization, the relative binding ratio with _Paenibacillus tarimensis_, DSM 19409T was 46.45% (results are obtained from the average of the
triplicates)_. The relation was found to be significantly less in comparison to the threshold value for the species delineation i.e. 70%_21. These results demonstrate the distinction of the
strain SMB1T from its closest neighbor. The digital protologue of this strain has been registered on their website (http://imedea.uib-csic.es/dprotologue/) under the taxonumber TA00612.
DISCUSSION Sambhar Lake is the one of the largest Salt Lake in India. It has the extreme hypersaline environment. These extreme conditions harbor microorganisms with valuable and distinct
characteristics. The extremophiles survive the harsh and hyper environmental conditions and produce unique and uncommon bioactive molecules and secondary metabolites. These compounds are
industrially stable and have many biotechnological applications22. The main objective of our study was to explore the diversity of the Sambhar Lake for the screening of the antimicrobial
compounds. We isolated hundred bacterial strains from the lake samples and screened all of them for their antimicrobial activities using agar well diffusion assay. Fifteen isolates showed
inhibitory activity against the indicator strains. The 16S rRNA gene sequencing data revealed that these isolates included four novel bacterial species, having pairwise similarity percentage
less than or equal to 98.5%. We recently published three out of four strains as novel species. In the present work, we studied the strain SMB1T for the purification and identification of
the antimicrobial compound and also characterized it as a novel species. This strain had a pairwise sequence similarity of 98.67% with _Paenibacillus tarimensis_ DSM 19409T while the
sequence similarity with other members of this genus was between 92.71% and 95.32%. So, we performed polyphasic taxonomic characterization to describe the strain. Different species of genus
_Paenibacillus_ were isolated and characterized till date from various ecological niches such as soils, plants, animals, polar Antarctic habitats, alkaline environments, marine sources or
cold and desert environments23,24,25,26. The phylogenetic analysis based on the 16S rRNA gene sequences revealed that the strain SMB1T is closely related to the _Paenibacillus tarimensis_
DSM 19409T and they shared the same clade. The genus _Paenibacillus_ is reported to have antesio-C15:0 as major cellular fatty acids20, likewise our results showed the major fatty of
anteiso-C15:0 (48.86%) in case of SMB1T and anteiso-C15:0 (61.69%) for _Paenibacillus tarimensis_ DSM 19409T. Major differences were also observed in the cellular fatty acids, as unsaturated
fatty acids were absent in strain SMB1T, but they were present in the strain DSM 19409T (C16: 1 _ω11c)_. Similarly, fatty acids C17:0 and iso-C17: 0 were present in our strain while absent
in DSM 19409T. Moreover, the DNA-DNA hybridization results showed the relative binding percentage was below 70%. Hence, it clearly supports that the strain SMB1T is the novel species. Based
upon the phenotypic and genotypic analyses, we concluded the strain SMB1T belongs to the novel species of genus _Paenibacilllus_ and thus we proposed the name _Paenibacillus sambharensis_
sp. nov for this strain (sam.bhar.en’sis. N.L. masc. adj. _sambharensis_ pertaining to Sambhar Lake). The type strain is SMB1T (=MTCC 12884T = KCTC 33895T). The _Paenibacillus_ genus has
been studied widely for producing a diversity of secondary metabolites, including enzymes, exopolysaccharides, and antimicrobial peptides and other industrially important bioactive
molecules27. Polymyxins, which are active against Gram negatives and fusaricidins, the antifungal peptide are the best examples of antibiotic products of the _Paenibacillus_ genus27.
_Paenibacillus_ also produces bacteriocins, for example, _P. polymyxa_ NRRL B-30509 produces paenicidin28 and _Paenibacillus_ sp. strain A3 produces penisin29. In the present research work,
we have identified and characterized an antimicrobial peptide from our strain SMB1T. The whole genome analysis identified three novel biosynthetic gene clusters in this strain. We presumed
that one of these clusters might be responsible for the observed antimicrobial activity. Hence we purified and characterized the antimicrobial compound from the fermentation broth. Using MS,
MS/MS and amino analysis we confirmed that the compound is bacitracin A. Bioactivity was also found to be similar when assessed at the similar concentration, in comparison to standard
bacitracin A. Though we were not able to obtain the complete biosynthetic cluster for the antimicrobial peptide bacitracin, but we found that the contig 383 of the genome sequence contains
the genes encoding for the bacitracin synthesis. Recently, a draft genome sequence of a _Paenibacillus polymyxa_ strain also revealed bacitracin biosynthetic gene cluster30. This further
validates that the strain SMB1T produces antimicrobial peptide bacitracin A. Bacitracin is a polypeptide known to be produced by _Bacillus subtilis_ and _Bacillus licheniformis_31. Apart
from this, we also found novel biosynthetic clusters for the putative bacteriocin i.e. lassopeptide and thiopeptide. Thiopeptides and lasso peptides are known for their antimicrobial
activities32. In a recent report, _Paenibacillus dendritiformis_ C454 was reported to produce novel lasso peptide paeninodin33. Additionally, we also identified one biosynthetic gene cluster
for the non-ribosomally synthezized peptide. This suggests that the strain SMB1T holds the potential as antimicrobial producing species. To the best of our knowledge, this is the first
report describing the production and purification of the antimicrobial compound bacitracin A from the genus _Paenibacillus_. Moreover, the other novel species isolated from salt lake also
showed antimicrobial activity and are deposited in a public repository. These strains could be explored in future for the isolation of bioactive compounds. Additionally, the novel
biosynthetic gene cluster found in the whole genome of strain SMB1T could be heterologously expressed and checked for their antimicrobial activity. This approach has been used in several
antimicrobial clusters, for example malacidins34. These BGCs are cryptic gene clusters and alternatively, they may be expressed when placed under strong inducible promoters. As explained in
the report by, Zipperer A _et al_. 2016, the compound lugdunin was not initially produced by the strain _Staphylococcus lugdunensis_ in the fermentation broth, so they expressed the
biosynthetic gene cluster by adding the strong promoter to produce the strain in liquid broth8. This approach can also be done in case of these BGCs as they are showing similarity less than
40% to the already known BGCs. Overall, this research demonstrates that novel species harbored from extreme niche hold potential to produce antimicrobial compounds. MATERIALS AND METHODS
ISOLATION OF BACTERIAL STRAINS AND ANTIMICROBIAL SCREENING Sediment and water samples were collected in the sterile 50 ml polypropylene tubes (Tarsons, India) from different sites at Sambhar
Lake, Rajasthan (GPS coordinates 26°55.520′N 075°11.827′E). The pH at different sampling sites was 8–12 and the temperature was 28 °C- 35 °C. Serially diluted samples were plated on
different media such as Zobell marine agar (ZMA), Reasoner’s 2 A Agar (R2A agar) with 2% NaCl (w/v), modified Zobell marine agar containing NaCl (2–10% w/v) and pH range from 7–10 for the
isolation of various halophilic bacteria. Optimization for salinity, pH, and temperature was done to check the optimum growth parameters. The pH was adjusted to 8.0–10 with the Na2CO3
solution (20%, w/v) and incubated at 30 °C and 37 °C for 3–7 days. The plates were monitored regularly and each unique colony was purified and preserved in 20% glycerol stock at −80 °C. The
isolates were screened for their antimicrobial activity using agar well diffusion assay against _Staphylococcus aureus_ ATCC 25923, _Bacillus subtilis_ ATCC 6633 (Equivalent MTCC 441), _E.
coli_ MTCC 1610 and _Candida albicans_ MTCC 224. Two to three colonies were inoculated in Zobell marine broth and incubated at 37 °C for 24–48 h. The cultures were harvested by
centrifugation after 48 h, and the crude fermentation extracts using Diaion HP20 resins were prepared as described in the next section. The cell-free supernatants (100 µl) at 24 h and 48 h
along with the crude extracts were loaded on seeded agar plates containing the indicator strain. The plates were incubated for 12–24 h and zones of inhibition were observed. The sterile
medium without inoculation of culture was extracted in the similar way and served as negative control. Positive isolates having inhibitory activity were identified using 16S rRNA gene
sequencing. Genomic DNA was extracted using DNA isolation kit (Zymo Research, California, D6005) and 16S rRNA gene was amplified. The sequencing was performed with the Genetic Analyzer ABI
3130XL (Applied Biosystems, California, USA). The sequence obtained was analyzed using EzTaxon sequence based database (https://www.ezbiocloud.net). PURIFICATION OF THE ANTIMICROBIAL
COMPOUND FROM THE STRAIN SMB1T _Paenibacillus_ sp. SMB1T was grown in 700 ml ZMB in 2 L flask at 37°C and 180 rpm. After 36 h, the culture was harvested by centrifugation at 12,000 × g for
15 min. Subsequently, the cell-free supernatant was incubated with Diaion HP-20 (Supelco, Sigma-Aldrich, USA) resins (2% w/v) for 3 h. The resins were washed with 10% methanol and the bound
components were eluted with 100% methanol. The solvent was evaporated under vacuum (Rotary evaporator BUCHI R-300). The crude extract was re-dissolved in Milli-Q. The antimicrobial compound
was partially purified by cation-exchange chromatography (SP Sepharose, 10 mM ammonium acetate, pH 5.0). Bioactivity-guided fractionation was performed and the active fractions were pooled.
The cation-active fraction was dialyzed using the 0.5–1 kDa membrane. Final purification of the antimicrobial compounds was carried out by high-performance liquid chromatography (HPLC)
(SHIMADZU with PDA detector, XBridge Waters column, C18, 5 µm, 10 × 250 mm). The mobile phase consisted of solvent A, 5 mM ammonium acetate buffer (pH 5.5) and solvent B, 100% acetonitrile.
The gradient elution was performed as 5–45% solvent B in 40 min, 45–90% B in 12 min and reverse 90–5% B in 8 min. The flow rate was kept at 3.0 ml/min. 500 µl sample was injected and the
peaks were analyzed at 220 nm. All peaks were collected and assayed for bioactivity. The active peak was identified and purity was determined by analytical HPLC. Antimicrobial activity of
the purified compound was checked against _Staphylococcus aureus_ ATCC 25923. MASS SPECTROMETRY AND AMINO ACID ANALYSIS The purified compound was subjected to LC-ESI-MS (Agilent 6550 _I_
funnel QTOF) in positive ion mode. The mass spectrum was analyzed in the range of 400–4000 m/z. For MALDI-TOF analysis, the sample was mixed with α-cyano-4-hydroxycinnamic acid (CHCA) matrix
and mass spectrum was obtained on MALDI-TOF mass spectrometer (AB Sciex 5800). MS/MS analysis was performed on the same instrument with TOF-TOF analyzer. The amino acid analysis was carried
out with PICO-TAG amino analysis system (Waters) as per the manufacturer’s instructions. COMPARATIVE ANALYSIS OF ANTIMICROBIAL COMPOUND WITH STANDARD BACITRACIN A Bacitracin A was purchased
from Alfa Aeser, Thermofisher Scientific, India. MS and MS/MS analysis of bacitracin A were performed in a similar way as described for antimicrobial compound isolated in this study. The
MS/MS spectrum of isolated compound was compared with bacitracin A data and manually annotated in details. Also, the retention time of both compounds was compared in analytical HPLC under
the similar conditions. Bacitracin A and isolated compound were dissolved at the same concentration (0.5 mg/ml) and their antimicrobial activity was checked against _Micrococcus luteus_ and
_Staphylococcus aureus_. WHOLE GENOME SEQUENCING AND BIOINFORMATICS ANALYSIS Genomic DNA was isolated from the strain SMB1T and the whole genome sequencing was performed on Illumina HiSeq
sequencing platform using paired end library. Around 4–5 Mb data was obtained. _De Novo_ assembly was performed using Spades, MaSuRCa, ABySS, and Velvet. We used ABySS assembly for all
further downstream analysis since it had better statistics than all other assemblies generated35. 63 contigs were obtained with the ABySS assembly. The functional analysis was performed
using Rapid Annotation using Subsystems Technology (RAST) version 2.036. BAGEL337 and anti-SMASH38 were used to predict the biosynthetic gene clusters for secondary metabolites and
antimicrobial peptides. Default search parameters were used in antiSMASH and BAGEL3 mining. CHARACTERIZATION OF SMB1T STRAIN Sequence similarity search of SMB1T strain indicated that
_Paenibacillus tarimensis_ and _Paenibacillus lacus_ were the closest phylogenetic neighbors, with a pair-wise sequence similarity of 98.6%, 95.5% respectively. Thus, the strain SMB1T was
characterized in comparison to its closest type strain _Paenibacillus tarimensis_ DSM 19409T. The phylogenetic tree was constructed using the neighbor-joining method in the MEGA6 software.
MORPHOLOGICAL CHARACTERIZATION The strains SMB1T and DSM 19409T were grown in Zobell Marine Agar medium at 37 °C for 48 h. The shape, size, color, margin, and elevation of the colonies were
observed. The cell shape was observed using phase contrast microscopy at 1000X magnification (BX51; Olympus, Japan). The cell size was measured using the transmission electron microscopy.
The cells were grown in the Zobell Marine broth and the bacterial pellet was washed with PBS (pH 7.4). 10 µl of the sample was loaded on the copper carbon-coated grids and kept undisturbed
for 10 minutes (300mesh), (Polysciences, Inc. USA cat #24933–25). After drying, 2% PTA (phosphotungstic acid) at pH 6 was added for 3 minutes and the grid was dried and observed under TEM
(JEOL JEM-2100; Camera, ES500W Model 782). PHYSIOLOGICAL CHARACTERIZATION KB003:Hi25TM Carbohydrate Identification Kit and KB009 (HiMedia Laboratories, India) were used for other biochemical
tests. Strains SMB1T and DSM 19409T were grown in Zobell Marine Agar medium until the log phase; the turbidity was adjusted to 0.1 OD at 620 nm. The specified wells in the kit were
inoculated with 50 µl of the samples and the strips were incubated at 37 °C for 18–24 hours. The reagents were added in the selective wells as per the manufacturer’s instructions39. The
change in color was observed and results were recorded. EFFECT OF TEMPERATURE, PH AND NACL CONCENTRATIONS ON THE GROWTH Growth at varying temperature conditions (10, 15, 25, 30, 37, 38, 42,
50 and 55 °C) was measured with the Zobell marine agar plates streaked with the strains. To check the effect of pH the strains were inoculated in ZMA at pH 5.0, 5.7, 6.8, 8.0, 9.0 and 11.0
adjusted with different buffer systems such as; 0.1 M citric acid/0.1 M sodium citrate pH 4.0–5.0, 0.1 M KH2PO4/0.1 M NaOH pH 6.0–8.0, 0.1 M NaHCO3/0.1 M Na2CO3 (pH 9.0–10.0). Growth at
various NaCl concentrations (0, 0.5, 1, 2, 4, 6, 8, 9, 10, 12 and 14% (w/v)) was monitored as described previously40. PHENOTYPIC FINGERPRINTING USING VITEK -2 VITEK®2 system was used for the
phenotypic fingerprinting of the cultures SMB1T and DSM 19409T. The cultures were analyzed based on their metabolic activities for the utilization of various nitrogen, carbon, and other
nutrient sources. VITEK 2 is the automated system, it contains 64 welled VITEK®2 GN cards (France) having different substrates in each well. The bacterial cell culture (diluted in 0.45%
(w/v) of NaCl) with the OD 0.5 measured by DensiCheck meter (bioMe’rieux) was used in an automated sampling system the cards were incubated in the in-build incubator in VITEK 2 machine. The
results were recorded. ANTIBIOTICS SUSCEPTIBILITY ASSAY The susceptibility of strain SMB1T and the DSM 19409T to different antibiotics was checked using disc diffusion assay as per Clinical
and Laboratory Standards Institute guidelines41. The antibiotics discs (Hi-Media, India) used were Tetracycline (30 mcg), Neomycin (30), Penicillin G (2 units), Gentamycin (10 mcg),
Kanamycin (30 mcg), Amoxycillin (30 mcg), Cephanroxcil (30 mcg), Lincomycin (2 mcg), Cefalexin (30 mcg), Chloramphenicol (30 mcg), Cefazolin (30 mcg), Polymixin B (300 units), Cefprozil
(30), Vancomycin (30 mcg), Novobiocin (30 mcg) and Chlortetracycline (30 mcg). FATTY ACID METHYL ESTER (FAMES) ANALYSIS The strains SMB1T and DSM 19409T were grown till the logarithmic
phase. Cellular fatty acid methyl esters (FAMEs) were obtained according to manufacturer’s protocol. The samples containing the FAMEs were subjected to GC (6890) and the different fatty
acids were separated and analyzed with the Sherlock Microbial Identification System (MIDI-6890 with database TSBA6)42. DNA-DNA HYBRIDISATION The G + C content of genomic DNA of the strain
SMB-1T and the type strain was determined spectrophotometrically (lambda 35, Perkin Elmer, Waltham, MA, USA) by using thermal denaturation method43. For DNA-DNA Hybridisation genomic DNA was
isolated for both the strains. The fluorimetry method was used to measure the relative binding ratio of the samples. Step One Plus Real-Time PCR system (Applied Biosystems) was used with
the thermal cycler in 96-well plate as explained by44. 2X SSC buffer was used for dissolving the DNA. SYBR Green in the ratio 1:10000 was used for the detection of the binding. The program
used for the experiment was denaturation at 95 °C for 10 min, the re-association temperature was 74.4 °C for 10 sec (240 cycles) and holding stage 25 °C for 5 min. The florescence readings
were recorded and the relative binding percentage was calculated according to the method reported by45,46. ACCESSION NUMBERS 16S rRNA gene sequence was submitted to GenBank/EMBL/DDBJ under
Accession Number LT161878. The genome sequence of the strain SMB1T has been deposited in the GenBank/NCBI database and Accession Number QKRB00000000 was obtained. ETHICAL APPROVAL This
article does not contain any studies with human participants or animals performed by any of the authors. This manuscript is IMTECH communication number 039/2018. REFERENCES * Nordmann, P.,
Dortet, L. & Poirel, L. Carbapenem resistance in Enterobacteriaceae: here is the storm! _Trends in molecular medicine_ 18, 263–272 (2012). Article CAS Google Scholar * WHO. Worldwide
country situation analysis: response to antimicrobial resistance. (2015). * Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of
antibacterial discovery. _Nature reviews Drug discovery_ 6, 29 (2007). Article CAS Google Scholar * Chellat, M. F. & Riedl, R. Pseudouridimycin: The First Nucleoside Analogue That
Selectively Inhibits Bacterial RNA Polymerase. _Angewandte Chemie International Edition_ 56, 13184–13186 (2017). Article CAS Google Scholar * Fisch, K. M. & Schaeberle, T. F. Toolbox
for Antibiotics Discovery from Microorganisms. _Archiv der Pharmazie_ 349, 683–691 (2016). Article CAS Google Scholar * Ling, L. L. _et al_. A new antibiotic kills pathogens without
detectable resistance. _Nature_ 517, 455 (2015). Article ADS CAS Google Scholar * Pantel, L. _et al_. Odilorhabdins, antibacterial agents that cause miscoding by binding at a new
ribosomal site. _Molecular cell_ 70, 83–94. e87 (2018). Article CAS Google Scholar * Zipperer, A. _et al_. Human commensals producing a novel antibiotic impair pathogen colonization.
_Nature_ 535, 511 (2016). Article ADS CAS Google Scholar * Kamat, T. & Kerkar, S. Bacteria from Salt Pans: a potential resource of antibacterial metabolites. _Recent Research in
Science and Technology_ 3 (2011). * Hashemi, T., Baseri, S. M. & Bahador, N. Isolation of Halophilic Bacteria from Maharlu salt Lake-Iran and their evaluation for the production of
bioactive compounds (2014). * Sawale, A., Kadam, T., Karale, M. & Kadam, O. Antimicrobial activity of secondary metabolites from halophilic Bacillus pumilus sp. _Int. J. Curr. Microbiol.
App. Sci_ 3, 506–512 (2014). Google Scholar * Azemin, A., Klappa, P. & Omar, M. S. S. Bacteriocin isolated from Halomonas sp.: a bacterial DING protein. _The Malaysian Journal of
Analytical Sciences_ 4, 831–840 (2015). Google Scholar * Essghaier, B. _et al_. Antimicrobial behavior of intracellular proteins from two moderately halophilic bacteria: strain J31 of
Terribacillus halophilus and strain M3-23 of Virgibacillus marismortui. _Journal of Plant Pathology & Microbiology_ 5, 1 (2014). Article Google Scholar * Singh, H. _et al_.
Salibacterium nitratireducens sp. nov., a haloalkalitolerant bacterium isolated from a water sample from Sambhar salt lake, India. _International journal of systematic and evolutionary
microbiology_ (2018). * Singh, H. _et al_. Bacillus alkalilacus sp. nov., isolated from a sediment sample from a lake in India. _International journal of systematic and evolutionary
microbiology_ (2018). * Singh, H. _et al_. Bacillus lacus sp. nov., isolated from a water sample of a salt lake in India. _International journal of systematic and evolutionary microbiology_
(2018). * Lockhart, I. & Abraham, E. The amino acid sequence in bacitracin A. _Biochemical Journal_ 58, 633 (1954). Article CAS Google Scholar * Stone, K. J. & Strominger, J. L.
Mechanism of action of bacitracin: complexation with metal ion and C55-isoprenyl pyrophosphate. _Proceedings of the National Academy of Sciences_ 68, 3223–3227 (1971). Article ADS CAS
Google Scholar * Delcher, A. L., Bratke, K. A., Powers, E. C. & Salzberg, S. L. Identifying bacterial genes and endosymbiont DNA with Glimmer. _Bioinformatics_ 23, 673–679 (2007).
Article CAS Google Scholar * Ash, C., Priest, F. G. & Collins, M. D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test.
_Antonie van Leeuwenhoek_ 64, 253–260 (1993). Article CAS Google Scholar * Wayne, L. _et al_. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics.
_International Journal of Systematic and Evolutionary Microbiology_ 37, 463–464 (1987). Article Google Scholar * Hamedi, J., Mohammadipanah, F. & Ventosa, A. Systematic and
biotechnological aspects of halophilic and halotolerant actinomycetes. _Extremophiles_ 17, 1–13 (2013). Article CAS Google Scholar * Daane, L. _et al_. PAH-degradation by Paenibacillus
spp. and description of Paenibacillus naphthalenovorans sp. nov., a naphthalene-degrading bacterium from the rhizosphere of salt marsh plants. _International journal of systematic and
evolutionary microbiology_ 52, 131–139 (2002). Article CAS Google Scholar * Kishore, K. H., Begum, Z., Pathan, A. A. K. & Shivaji, S. Paenibacillus glacialis sp. nov., isolated from
the Kafni glacier of the Himalayas, India. _International journal of systematic and evolutionary microbiology_ 60, 1909–1913 (2010). Article CAS Google Scholar * Montes, M. J., Mercadé,
E., Bozal, N. & Guinea, J. Paenibacillus antarcticus sp. nov., a novel psychrotolerant organism from the Antarctic environment. _International journal of systematic and evolutionary
microbiology_ 54, 1521–1526 (2004). Article CAS Google Scholar * Scheldeman, P. _et al_. Paenibacillus lactis sp. nov., isolated from raw and heat-treated milk. _International Journal of
Systematic and Evolutionary Microbiology_ 54, 885–891 (2004). Article CAS Google Scholar * Grady, E. N., MacDonald, J., Liu, L., Richman, A. & Yuan, Z.-C. Current knowledge and
perspectives of Paenibacillus: a review. _Microbial cell factories_ 15, 203 (2016). Article Google Scholar * Lohans, C. T. _et al_. Structural characterization of the highly cyclized
lantibiotic paenicidin A via a partial desulfurization/reduction strategy. _Journal of the American Chemical Society_ 134, 19540–19543 (2012). Article CAS Google Scholar * Baindara, P.
_et al_. Characterization of the antimicrobial peptide penisin, a class Ia novel lantibiotic from Paenibacillus sp. strain A3. _Antimicrobial agents and chemotherapy_ 60, 580–591 (2016).
Article CAS Google Scholar * Li, Y., Li, Q., Li, Y., Gao, J. & Fan, X. Draft genome sequence of Paenibacillus polymyxa KF-1, an excellent producer of microbicides. _Genome
announcements_ 4, e00727–00716 (2016). PubMed PubMed Central Google Scholar * Azevedo, E., Rios, E., Fukushima, K. & Campos-Takaki, G. Bacitracin production by a new strain ofBacillus
subtilis. _Applied biochemistry and biotechnology_ 42, 1 (1993). Article CAS Google Scholar * Zhao, N., Pan, Y., Cheng, Z. & Liu, H. Lasso peptide, a highly stable structure and
designable multifunctional backbone. _Amino acids_ 48, 1347–1356 (2016). Article CAS Google Scholar * Zhu, S. _et al_. Insights into the unique phosphorylation of the lasso peptide
paeninodin. _Journal of Biological Chemistry_ 291, 13662–13678 (2016). Article CAS Google Scholar * Hover, B. M. _et al_. Culture-independent discovery of the malacidins as
calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. _Nature microbiology_ 3, 415 (2018). Article CAS Google Scholar * Gurevich, A., Saveliev,
V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. _Bioinformatics_ 29, 1072–1075 (2013). Article CAS Google Scholar * Aziz, R. K. _et al_. The RAST
Server: rapid annotations using subsystems technology. _BMC genomics_ 9, 75 (2008). Article Google Scholar * van Heel, A. J., de Jong, A., Montalban-Lopez, M., Kok, J. & Kuipers, O. P.
BAGEL3: automated identification of genes encoding bacteriocins and (non-) bactericidal posttranslationally modified peptides. _Nucleic acids research_ 41, W448–W453 (2013). Article Google
Scholar * Weber, T. _et al_. antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. _Nucleic acids research_ 43, W237–W243 (2015). Article CAS Google
Scholar * Kiran, S., Swarnkar, M. K., Mayilraj, S., Tewari, R. & Gulati, A. Paenibacillus ihbetae sp. nov., a cold-adapted antimicrobial producing bacterium isolated from high altitude
Suraj Tal Lake in the Indian trans-Himalayas. _Systematic and applied microbiology_ 40, 430–439 (2017). Article CAS Google Scholar * Kumar, A. _et al_. Shivajiella indica gen. nov., sp.
nov., a marine bacterium of the family “Cyclobacteriaceae” with nitrate reducing activity. _Systematic and applied microbiology_ 35, 320–325 (2012). Article Google Scholar * Wayne, P.
Performance standards for antimicrobial susceptibility testing: Twenty Fifth International Supplement M100-S25. _Clinical and Laboratory Standards Institute_ (2015). * Sasser, M.
Identification of bacteria by gas chromatography of cellular fatty acids. (1990). * Mandel, M. & Marmur, J. In _Methods in enzymology_ Vol. 12 195–206 (Elsevier, 1968). *
Loveland-Curtze, J., Miteva, V. I. & Brenchley, J. E. Evaluation of a new fluorimetric DNA–DNA hybridization method. _Canadian journal of microbiology_ 57, 250–255 (2011). Article CAS
Google Scholar * Ley, J. D., Cattoir, H. & Reynaerts, A. The quantitative measurement of DNA hybridization from renaturation rates. _The FEBS Journal_ 12, 133–142 (1970). Google Scholar
* Gillis, M., Ley, J. D. & Cleene, M. D. The determination of molecular weight of bacterial genome DNA from renaturation rates. _The FEBS Journal_ 12, 143–153 (1970). CAS Google
Scholar Download references ACKNOWLEDGEMENTS We are highly thankful to the Director CSIR-IMTECH for all the facilities provided to conduct this work. The research fellowship of H.S. is
provided by Indian Council of Medical Research (ICMR), Government of India is highly acknowledged. We thank Professor Aharon Oren for his expert suggestion concerning the correct species
epithet and Latin etymology. We also thank Mr. Deepak Bhatt for technical assistance in 16S rRNA gene sequencing and Dr. Venkata Ramana for his help in the data analysis for DDH experiment.
Dr. P Anil Kumar is thankful to the Department of Science and Technology (DST) -Science and Engineering Research Board (SERB) for funding (project SB/YS/LS-09/2014). AUTHOR INFORMATION
Author notes * Harjodh Singh and Manpreet Kaur contributed equally. AUTHORS AND AFFILIATIONS * Academy of Scientific and Innovative Research, (AcSIR), CSIR Campus, Chennai, India Harjodh
Singh, Manpreet Kaur, Sunita Mishra, Hemraj Nandanwar & Anil Kumar Pinnaka * Council of Scientific and Industrial Research (CSIR) - Central Scientific Instruments Organisation, Sector
30C, Chandigarh, 160030, India Harjodh Singh, Manpreet Kaur & Sunita Mishra * Clinical Microbiology & Bioactive Screening Laboratory, Council of Scientific & Industrial Research
-Institute of Microbial Technology, Sector -39A, Chandigarh, India Manoj Jangra & Hemraj Nandanwar * MTCC-Microbial Type Culture Collection & Gene Bank, CSIR-Institute of Microbial
Technology, Chandigarh, 160036, India Harjodh Singh, Manpreet Kaur & Anil Kumar Pinnaka Authors * Harjodh Singh View author publications You can also search for this author inPubMed
Google Scholar * Manpreet Kaur View author publications You can also search for this author inPubMed Google Scholar * Manoj Jangra View author publications You can also search for this
author inPubMed Google Scholar * Sunita Mishra View author publications You can also search for this author inPubMed Google Scholar * Hemraj Nandanwar View author publications You can also
search for this author inPubMed Google Scholar * Anil Kumar Pinnaka View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.S. and M.K. did the
sampling and isolation of the bacteria. H.S., M.K. and M.J. performed the experiments, data analysis, and manuscript write-up. A.K., H.N. and S.M. planned, supervised and analyzed the study.
H.S. and M.K. contributed equally to this work. CORRESPONDING AUTHOR Correspondence to Anil Kumar Pinnaka. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Singh, H., Kaur, M., Jangra, M. _et al._ Antimicrobial properties of the novel bacterial isolate _Paenibacilllus_ sp. SMB1 from a halo-alkaline lake in India.
_Sci Rep_ 9, 11561 (2019). https://doi.org/10.1038/s41598-019-47879-x Download citation * Received: 12 October 2018 * Accepted: 17 July 2019 * Published: 09 August 2019 * DOI:
https://doi.org/10.1038/s41598-019-47879-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
