fMRI indicates cortical activation through TRPV1 modulation during acute gouty attacks
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Gout is one of the most painful disease conditions. The central mechanism of pain processing in this condition remains elusive. Cerebral blood volume (CBV) responses are faithful correlates
of brain activity changes; the application of CBV-weighted functional magnetic resonance imaging (fMRI) may shed light on the issue of interest. Transient receptor potential vanilloid 1
(TRPV1) is a critical ion channel expressed both peripherally in nociceptors and centrally in the brain. Whether TRPV1 plays a critical role in gout pain was also explored. Results showed
that, in rats with gouty arthritis, noxious stimulation induced CBV increases in the primary somatosensory cortex and thalamus. These increases were correlated with up-regulated TRPV1
protein expression and pain behavior. Selective blockage of central TRPV1 channel activity by intrathecal administration of AMG9810 reversed the induced pain, and abolished the induced CBV
increase in thalamocortical regions. The findings support that TRPV1 activation in the central pain pathway is crucial to the augmentation of pain in gouty conditions. This new information
supports the development of TRPV1-based drugs for treating gout pain, while fMRI can be useful for repeated evaluation of brain activity changes induced by gout.
As one of the most painful disease conditions, gout is characterized by episodes of severe pain, tenderness, warmth, redness, swelling as well as fever1 in the affected tissue. However,
there is very little information regarding the central mechanism underlying the experience of severe gout pain2. The spinothalamocortical pathway is one of the key pain circuits responsible
for nociceptive processing in response to peripheral stimuli. The activation of this pathway upon pain stimulation can be detected in vivo by functional magnetic resonance imaging (fMRI) in
both healthy humans and animals. Nevertheless, whether gout induces activity changes in this pathway or whether the alterations are responsible for the severity of the pain in gout has not
been investigated.
Cerebral blood volume (CBV) weighted fMRI can be used in rodents. Its high signal-to-noise ratios are improved by the use of superparamagnetic iron oxide nanoparticles as the contrast
agent3,4,5,6. CBV fluctuations highly correlate with brain activity changes. Therefore, CBV weighted fMRI is an alternative to the commonly used blood oxygen level dependent (BOLD) fMRI
technique7; the quality of BOLD fMRI images tends to be poor because of low contrast to noise ratio and multiple hemodynamic factors4. The use of the CBV-weighted fMRI method in healthy rats
has been shown to detect activity changes in the cortex and thalamus in response to noxious stimulation. Therefore its application is justified in the exploration of our issue of interest:
how gout attacks affect the activity of the central nociceptive pathway.
To understand the gout-associated activity changes in the nociceptive signaling pathway, it is necessary to explore the underlying molecular mechanism. The significance of transient receptor
potential vanilloid 1 (TRPV1) was of particular interest because it is a critical ion channel that responds to various physical and chemical stimuli including heat, pain, and capsaicin8.
Activation of TRPV1 is linked to the painful conditions of inflammation9,10,11,12,13,14 and peripheral neuropathy15. Our hypothesis is that gout-induced change in nociceptive pathway
activity may be mediated by an ion channel such as TRPV1, and results in exacerbation of pain.
In this study using CBV-weighted fMRI, we first examined whether normal and gouty conditions elicit different brain activity patterns in response to noxious electrical stimulation. TRPV1
protein levels were examined by western blotting and immunohistology from the neural tissues of the peripheral and central nociceptive pathways. The involvement of TRPV1 in gout pain was
confirmed pharmacologically using a TRPV1-selective blocker called AMG9810. Altogether, these findings established the involvement of a TRPV1-mediated nociceptive mechanism in the
augmentation of pain responses in gout. Such new information may support the development of TRPV1 antagonists as new drugs for treating gout, while fMRI can be used diagnostically for
evaluating gout pain in the brain.
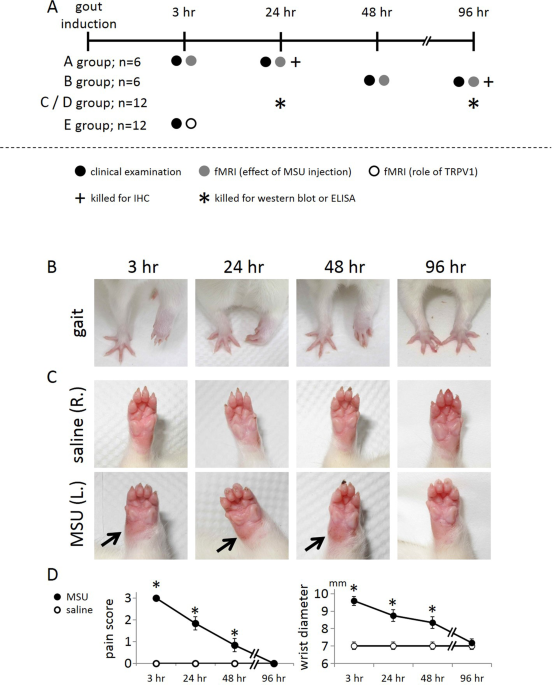
The left wrist was deposited with monosodium urate crystals (MSU) for the induction of gouty arthritis whereas the right wrist was treated with saline serving as the control. Signs of pain
were observed at 3, 24, 48, and 96 hr after MSU deposition, as indicated in the timeline of Fig. 1A. Figure 1B shows that MSU treatment of the wrist resulted in an obvious gait abnormality
when compared to the contralateral wrist. This gait change, indicative of the pain level, resolved with time. Figure 1C shows swelling of the MSU-treated wrist as compared to the
contralateral wrist. This swelling sign also weakened over time. Pain scores and wrist diameters were statistically analyzed as shown in Fig. 1D, and the results of this analysis indicated
that overt signs in this rat model of gouty arthritis were the most severe at 3 hr.
Overt signs in the wrist region of the rat with gouty arthritis with time. (A) Schematic showing the timeframe and experimental protocol. (B) Gait as an indicator of pain. (C) Swelling as a
sign of inflammation. (D) Quantification of the gait and swelling over time indicates that gait abnormality and swelling resolved with time. Arrows point to the swollen spot. (*Indicates
statistically significant difference with p
