Association between the relative abundance of gastric microbiota and the risk of gastric cancer: a case-control study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The human gut hosts a diverse community of bacteria referred to as the gut microbiome. We investigated the association between the relative abundance of gastric microbiota and
gastric cancer (GC) risk in a Korean population. The study participants included 268 GC patients and 288 controls. DNA was extracted from gastric biopsies, and 16S rRNA gene analysis was
performed. Unconditional logistic regression models were used to observe the associations. Of the participants, those who had the highest level (highest tertile) of relative _Helicobacter
pylori_ and _Propionibacterium acnes_ abundances showed a significantly higher risk for GC after adjusting for potential confounding variables (odds ratio (OR) = 1.86, 95% confidence
interval (CI) = 1.17–2.97, p for trend = 0.017 and OR = 4.77, 95% CI = 2.94–7.74, p for trend <0.001, respectively). Subjects who carried _Prevotella copri_ had a significantly higher
risk of GC than noncarriers (OR = 2.54, 95% CI = 1.42–4.55, p for trend = 0.002). There was a lower risk of GC in subjects carrying _Lactococcus lactis_ than in noncarriers (OR = 0.21, 95%
CI = 0.10–0.44, p for trend <0.001). _H. pylori, P. acnes_ and _P. copri_ are strong risk factors, whereas _L. lactis_ is a protective factor, for GC development in Koreans. Further
microbiome studies are warranted to verify the findings of the current study. SIMILAR CONTENT BEING VIEWED BY OTHERS MICROBIAL COMPOSITION OF GASTRIC LESIONS: DIFFERENCES BASED ON
_HELICOBACTER PYLORI_ VIRULENCE PROFILE Article Open access 21 November 2024 META-ANALYSIS OF MUCOSAL MICROBIOTA REVEALS UNIVERSAL MICROBIAL SIGNATURES AND DYSBIOSIS IN GASTRIC
CARCINOGENESIS Article Open access 09 June 2022 DYSBIOTIC CHANGE IN GASTRIC MICROBIOME AND ITS FUNCTIONAL IMPLICATION IN GASTRIC CARCINOGENESIS Article Open access 11 March 2022 INTRODUCTION
Gastric cancer (GC) ranks as the fifth leading cancer type, and it has been identified as one of the main causes of cancer-related deaths in the world1. The incidence of GC in eastern Asia,
including Korea, is the highest worldwide, which is over 4 times higher than the rates in Western Europe2. It has been reported that the age-adjusted incidence rate of GC was 33.8 per
100,000 in Korea3. According to a prediction of cancer incidence and mortality in Korea, GC accounts for a remarkable proportion of the overall cancer burden because it is the second most
common type of cancer among Koreans4. Recent studies about the human microbiome demonstrate a surge in interest in the context of disease, particularly in gastrointestinal cancers5. It is a
known fact that there are various types of bacteria in different body sites, which are colloquially referred to as normal flora. This microbiota has the potential to maintain human health by
interacting with the human body and can be considered pathological for the development of certain diseases5. Due to the complex and dynamic nature of the human gastrointestinal microbiota,
it is recently considered as a metabolically active organ and the complex nature of it evidently regulates gastrointestinal homeostasis by interacting with immune cells6. The normal flora in
the gastrointestinal tract supports several processes, including the host mucosal immune response, energy metabolism, pathogen elimination, and cancer development7. It is widely implicated
that human gut bacteria play a crucial role in the etiology of gastrointestinal cancers, particularly GC due to dysbiosis8. Dysbiosis is a condition in which there is an imbalance in the
gastrointestinal microbiota, which consequently leads to several pathological conditions, specifically GC. Furthermore, gastric microbial community profiling revealed that dysbiosis of gut
microbiota is associated with GC or precancerous lesions9. The relationship between particular microbial pathogens and carcinogenesis has been the subject of exploration in the context of
systems epidemiology. A considerable number of studies have focused on individual pathogens, such as _Helicobacter pylori (H. pylori)_, and their ability to initiate disease conditions, such
as gastritis or GC10. Of the numerous risk factors associated with GC occurrence, _H. pylori_ infection plays a pivotal role11. It is an established fact that _H. pylori_ infection is
widespread in East Asia, which is associated with GC development12. Thus, it has been extensively suggested that reduction in chronic _H. pylori_ infection is a useful strategy for
preventing GC. Notably, the identification of specific microbial species that are associated with various disease conditions, particularly cancers pertaining to the gastrointestinal tract,
has been achieved with the expansion of advance sequencing technologies. It has been suggested that colonization of non-_H. pylori_ bacteria in the stomach can also stimulate GC risk13.
Thus, understanding how dysbiosis influences host metabolic reactions and inflammatory responses is critical to defining the roles specific components of the microbiota play in
carcinogenesis. However, there is a paucity of data regarding the association between the gastric microbiota and GC. As it is well documented that the composition of the microbiota shapes
immune responses at local and systemic levels, as well as inflammatory signaling related to GC development, it is necessary to investigate the gastric microbiota-associated alterations that
may influence GC development. Moreover, it is necessary to explore the evidence related to this association based on epidemiological studies because various experimental approaches have
already been employed to elucidate the microbiota profiles of GC patients14. Therefore, in the present case-control study, we aimed to investigate the association between the relative
abundance of the gastric microbiota components and the risk of GC in a Korean population. RESULTS GENERAL CHARACTERISTICS OF THE STUDY POPULATION Table 1 presents the general characteristics
of the study participants with and without GC. The proportion of current smokers was higher in the patient group (29.1%) than in the control group (17.7%), and the patients were more likely
to have a family history of GC (p = 0.003). The patients engaged in exercise less regularly (p < 0.001), were less educated (p < 0.001), and exhibited lower employment rates (p =
0.037) and lower monthly incomes (p < 0.001) than the controls. The proportion of _H. pylori_ infection among the patients (99.6%) was higher than among the controls (93.4%). The patients
had a higher energy intake than the controls (p < 0.001). The Shannon index, which represents the alpha diversity, was significantly higher in the controls than in the patients (p <
0.001). COMPARISON OF RELATIVE ABUNDANCE AND DIVERSITY INDICES Table 2 presents the mean relative abundance comparison results between the patients with GC and controls. We found that
patients with GC had a higher relative abundance of _Helicobacteraceae, Propionibacteriaceae_, and _Prevotellaceae_ than the healthy subjects at the family level (p adjusted for false
discovery rate (FDR) = 0.025, <0.001, 0.013, respectively). At the genus level, the relative abundances of _Helicobacter, Propionibacterium_, and _Prevotella_ were higher in the patients
than in the controls (p adjusted for FDR = 0.025, <0.001, 0.013, respectively) whereas the relative abundance of _Lactococcus_ was higher in the controls than in the patients (p adjusted
for FDR < 0.001). Regarding the species level, we found that the patients had higher relative abundances of _H. pylori, Propionibacterium acnes_ (_P. acnes_), and _Prevotella copri_ (_P.
copri_) than the controls (p adjusted for FDR = 0.035, 0.002, and 0.049, respectively), while the relative abundance of _Lactococcus lactis_ (_L. lactis_) was higher in the healthy controls
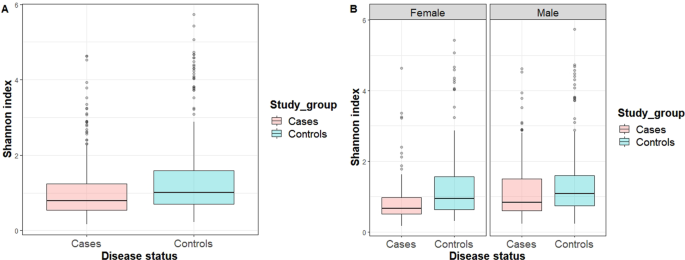
than in the patients (p adjusted for FDR = 0.003). Figure 1A presents a box plot for the Shannon index for the entire study population. We observed that the Shannon index was significantly
higher in the controls than in the patients (p < 0.001) in the entire study population. Figure 1B shows the box plots for the Shannon index by sex. We found that the Shannon index was
significantly higher in controls than in male or female patients (p = 0.013 and p < 0.001, respectively). Figure 2 represents a principal coordinates analyses (PCoA) plot of the
Bray-Curtis distance based on the operational taxonomic units (OTU) relative abundance table at the species level. The blue triangles represent the controls, while the red dots represent the
patient. The blue and red ellipses represent cases in which 95% of the data belong to the controls and patients at a 95% significance level. The 2-D plot of the first two principal
coordinates shows a marked divergence between the GC patients and the healthy controls. The total diversity captured by the first two principal coordinates was 26.4%. The microbiota
composition of the patients with GC was significantly different from that of the healthy controls (analysis of similarity (ANOSIM) R = 0.006, p = 0.030) according to the Bray-Curtis
dissimilarity measure. ASSOCIATION OF CANDIDATE SPECIES WITH GC RISK AND EVALUATION OF THEIR ABUNDANCE FOR GC DIAGNOSIS Table 3 shows the association between the relative abundance of
candidate bacterial species and GC risk. The subjects, who had a higher relative abundance of _H. pylori_ showed a significantly higher risk of GC in model II than the subjects who had a
lower relative abundance (odd ratio (OR) = 1.86, 95% confidence interval (CI) = 1.17–2.97, p-trend = 0.017). The females who had a higher relative abundance of _H. pylori_ showed a
significantly higher risk of GC than the females who had a lower relative abundance (OR = 3.36, 95% CI = 1.41–8.00, p-trend = 0.008). A positive association between the relative abundance of
_H. pylori_ and GC was observed in males, although the results were not significant. Those who had a higher relative abundance of _P. acnes_ showed a significantly higher risk for GC than
those in the lowest relative abundance group among the entire study population (OR = 4.48, 95% CI = 2.79–7.21, p-trend ≤ 0.001), in males (OR = 3.03, 95% CI = 1.62–5.66, p-trend ≤ 0.001) and
females (OR = 8.19, 95% CI = 3.41–19.65, p-trend ≤ 0.001). Regarding _P. copri_, those who carried this species showed a significantly higher risk of GC than the noncarriers among the
entire population (OR = 2.39, 95% CI = 1.36–4.19) and in males (OR = 2.78, 95% CI = 1.32–5.85). The subjects who carried _L. lactis_ showed a significantly lower risk of GC than the
noncarriers among the entire population (OR = 0.19, 95% CI = 0.10–0.39), in males (OR = 0.22, 95% CI = 0.08–0.57) and in females (OR = 0.18, 95% CI = 0.05–0.60) (Table 3). Based on principal
component analysis (PCA), two linear combinations of four species were obtained using Eigen value greater than 1.00. _H. pylori_ and _P. acnes_ were identified as dominant bacterial species
in components 1 and 2, respectively, based on the principal component loadings. Approximately 63.0% of the variation was explained by the first two principal components. According to the
linear discriminant analysis, the coefficients for the linear discriminants for PC1 (_H. pylori_ dominant) and PC2 (_P. acnes_ dominant) were −0.07 and 0.97, respectively. We investigated
whether the combination of these four bacterial species could demonstrate better predictive ability using a receiver operating characteristic (ROC) curve and area under curve (AUC) analyses.
An analysis using two linear combinations of four bacterial species showed 79.7% sensitivity and 67.1% specificity. The AUC was 77.7% indicating that there is a 77.7% chance that the model
will be able to distinguish between positive and negative classes (Supplementary Fig. S1). The best cutoff point was chosen as 0.492 in order to find a balance between sensitivity and
specificity. DISCUSSION In this case-control study involving 556 participants (268 patients and 288 controls), we observed that the relative abundances of the _H. pylori_, _P. acnes_ and _P.
copri_ species were significantly higher in the patients than in the controls, whereas the relative abundance of _L. lactis_ was significantly higher in the controls than in the patients.
Generally, the subjects who had a high relative abundance of the _H. pylori_, _P. acnes_ and _P. copri_ species showed a significantly higher risk of GC. In contrast, those who had a high
relative abundance of _L. lactis_ showed a significantly lower risk of GC. A significantly higher Shannon index was observed in the controls than in the patients. The ROC and AUC analysis
results suggest that the identified four candidate bacterial species will be clinically useful for the identification of GC in Koreans. _H. pylori_ infection is the strongest single risk
factor for GC, specifically in countries where _H. pylori_ infection is endemic15. Reduced gastric acidity caused by chronic _H. pylori_ infection lowers nutrient availability and local
innate immunity responses16. Corpus-dominant infection leads to gastric mucosal atrophy, with an increase in gastric pH due to the loss of acid-producing parietal cells17. Due to the high
relative abundance of _H. pylori_, our data represents a unique bacterial profile of the Korean population by virtue of the fact that the Korean people have been exposed to a similar diet
for a long period of time18. In contrast, a study suggested that _H. pylori_ colonization or stomach anatomic sites does not influence the gastric microbiota composition, although it
differed between paired nonmalignant and tumor tissues19. In our study population, we observed that _H. pylori_ had the highest mean relative abundance compared with other bacterial species,
specifically in GC patients. The results were similar at the family (_Helicobacteraceae_) and genus (_Helicobacter_) levels. A study that focused on the molecular characterization of the
human stomach microbiota in GC patients concluded that _H. pylori_ is the dominant member of the nonmalignant gastric tissue microbiota in many GC patients20. Interestingly, a study
conducted in Colombia revealed that the gastric microbiome composition is considerably different between people but observed a significant correlation with town of origin, although a
significant correlation between the _H. pylori_ phylogeographic population and microbial composition has not been identified21. Thus, the identification of the biological role of _H. pylori_
needs to be investigated in clinical practice. The critical role of _H. pylori_ in GC pathogenesis has been well documented. The carcinogenic potential of _H. pylori_ can be unraveled due
to the effect from two _H. pylori_ related virulence factors namely, vacuolating cytotoxin A (VacA) and cytotoxin associated gene A (CagA)22. _H. pylori_ evasion can be stimulated because of
the immunosuppressive activities of VacA which eventually leads to enhance gastric tumor survival15. The protein CagA can enter gastric epithelial cells for undergoing phophorylation15,
leading to structural changes of cells, including cell scattering, elongation22, and resistance to apoptosis23. Because of changes in physiological and immunological environments in stomach
due to the aforementioned _H. pylori_ related pathological issues, composition of the gastric microbiota can be altered resulting in a proinflammatory condition15. Studies showed a strong
association between proinflammatory cytokines polymorphisms and an increased risk of developing _H. pylori_-associated GC24,25,26. In addition to the carcinogenic role of _H. pylori_,
mucosal atrophy plays a critical role in GC pathogenesis23. Evidence from our study showed that a higher relative abundance of _H. pylori_ increases the risk of GC. Although it has been
widely accepted that there is no direct influence of _H. pylori_ to the adults’ gastric microbiota composition, few studies have noted that there is an effect owing to the fact that those
who carry _H. pylori_ are feasible to have higher abundance of _Spirochetes_, _Acidobacteria_, and non-_Helicobacter Proteobacteria_ and have comparatively lower abundances of
_Actinobacteria_, _Bacteroidetes_, and _Fermicutes_ phyla than uninfected adults27,28. Our analysis supports the theory that a higher relative abundance of _H. pylori_ increases the risk of
GC in males although the results are not statistically significant. This finding may be due to our limited sample size, although we had a relatively large overall sample. The Shannon index
box plot shows that the alpha diversity was significantly higher in the controls than in the GC patients in our study sample. A similar bacterial diversity has been noted in a study in which
there is a higher Shannon index in healthy subjects than in GC patients29. Such a change in bacterial composition may be the predominant causes of gastric atrophy resulting in GC even
though the number of _H. pylori_ bacteria decreases due to atrophy28. Additionally, based on our PCoA plot, the bacterial composition at the species level of our study was markedly divergent
between the GC patients and control subjects. This finding also resembles the finding observed by Li, T. H. _et al_.29, indicating that there is a clear divergence between GC patients and
healthy subjects in PCoA analyses. Regarding _P. acnes_, our study found that individuals with a high relative abundance of _P. acnes_ showed an increased risk of GC. A study based on
comparative microbial community profiling of human stomach biopsies, found that overabundance of _P. acnes_ is a cause of lymphocytic gastritis (LyG)30. Our study findings also show the mean
relative abundance of _P. acnes_ is higher in GC patients than in the controls. The same results were found for both the family (_Propionibacteriaceae_) and genus (_Propionibacterium_)
levels. It is notable that _P. acnes_, a classic skin bacterium that causes acne, has been recently identified as a gastric microbiota31. Furthermore, LyG caused by _P. acnes_ can enhance GC
development by producing proinflammatory cytokines such as IL 1530. It is important to mention that the gut brain skin hypothesis indicates there is a complex interrelationship between acne
and gut dysfunction mediated by the brain which has been recently validated by the microbiome studies32. This is supported by the fact that frequent associations of both anxiety and
depression and gastrointestinal distress occur with the occurrence of acne. Such condition can be a causative factor for releasing neuropeptides from the enteroendocrine cells due to the
production of neurotransmitters, such as serotonin, norepinephrine and acetylcholine by normal flora. These chemicals can trigger both intestinal and systemic inflammation which eventually
leads to GC development by increasing the gut permeability that allow for cross talk between the gut and skin32. Recent research in humans found that the localized and systemic diseases,
including periodontitis, bacterial vaginosis, rheumatoid arthritis, metabolic disorders, and low grade systemic inflammation can be caused by the overabundance of _Prevotella_ species at
mucosal sites33. _In vitro_ study suggested that _Prevotella_ has remarkable capability in driving T helper type 17 (Th17) immune responses which evidently propose the association between
increased _Prevotella_ abundance and augmented Th1733. Further, the ability of _Prevotella_ in producing redox proteins with an increased resistance to the host has also been proposed34.
Based on the current study findings, it was observed that the subjects who carried _P. copri_ had a significantly higher risk of GC than the subjects who did not carry _P. copri_.
Additionally, there was significantly higher mean _P. copri_ abundance in the GC patients than in the controls. It has been strongly suggested that _P. copri_ induces inflammatory conditions
in the human body, leading to the development of several types of diseases, including GC34. According to previous study on animal models, it has been shown that the overall bacterial
composition can be affected by long term _H. pylori_ infection35. A relative abundance of the _Prevotella_ genera was found in studies conducted with patients without _H. pylori_
infection36. Thus, _H. pylori_-induced changes in gastric microflora can be attributed to various factors and can lead to gastric atrophy, increased gastric pH, and the colonization of the
stomach by transient bacteria35. However, the role of _P. copri_ in GC development needs to be further investigated. _L. lactis_ was observed as a beneficial bacterium in the current study
because the subjects who carried _L. lactis_ had a lower GC risk. A study has revealed that there is a strong antiproliferative activity of the cytoplasmic fraction of _L. lactis_ upon human
colon cancer cells37. Furthermore, a study investigating the antiproliferative effects of the cytoplasmic fraction of _L. lactis_ on a human stomach cancer cell line revealed that there is
an inhibitory effect on cell proliferation with _L. lactis_ treatment in a time and dose dependent manner38. _L. lactis_ caused G0/G1 cell cycle arrest, which was associated with an increase
in p53 and p21 expression, a reduction in cyclin D1 expression, and retinoblastoma protein phosphorylation, thereby inducing apoptosis. Additionally, it has been noted that the _L. lactis_
bacterium has a probiotic effect in the human gut and can result in beneficial effects for human gastrointestinal health, including the prevention of gastrointestinal cancers38.
Interestingly, we observed that the mean relative abundance of _L. lactis_ was higher in healthy controls than in the GC patients in our study population. We observed that two linear
combinations (_H. pylori_ and _P. acnes_ dominant) of the four identified candidate bacterial species can be considered predictive of GC, therefore, representing a potential diagnostic
marker. Together, our data indicated that _H. pylori_ and _P. acnes_ were highly abundant in the GC patients and positively identified GC with 79.7% sensitivity. It is evident that the
abundance of _H. pylori_ becomes lower due to the succession of microbial species as GC locally advances14. In contrast, our data indicated that GC patients have significantly high
abundances of _H. pylori_. A possible explanation is that we collected the biopsy samples from patients with early gastric cancer in which bacterial succession had not progressed. A study
conducted in Taiwan concluded that regardless of the biological roles of _Clostridium_, _Fusobacterium_ and _Lactobacillus_ in oncogenesis, the overabundance of these microbes serves as a
diagnostic tool for GC14. Thus, it can be suggested that if there is a bacteria species that can promote the GC occurrence, the eradication of this bacteria is useful for decreasing GC
incidence. The human gut microbiome plays a critical role in gastrointestinal cancers39. A report on the next steps in studying the human microbiome and health in prospective studies pointed
out that the importance of continuing and expanding of the recent microbiome research40. Despite the utilization of optimized techniques for sample collection, processing and storage,
supplementary methodological approach is required specifically in the epidemiological context due to the inherent limitations of epidemiological studies. As the potential limitations in
case-control studies of the microbiome and cancer, changes of the composition of the microbiome due to dietary factors, selection and recall bias have been emerged. It has been recommended
that biorepositories for human samples establish a similar collection method to serve as a basis for nested case-control studies. In addition, conducting short interventions in human and
animal studies can identify the important effects of diet on the microbiome, particularly the fecal microbiome40. Furthermore, managing optimal storage conditions soon after a sample is
collected is necessary to reduce the bias arising from the quick changes in the genetic components of the microbes41,42. Our study has both strengths and limitations. A major strength of our
study is that the sample size was relatively large with 268 GC patients and 288 healthy controls, which provides sufficient statistical power to detect the relevant associations between the
gastric microbiota and GC risk. Additionally, several possible confounding variables were taken into consideration that are possible risk factors for GC development, including age, smoking,
family history of GC, regular exercise, education, occupation, income and total energy intake throughout the analysis. However, our study has potential limitations. In general, the presence
of bias associated with a hospital based case-control study, including selection bias and recall bias should be raised. As this is not a prospective study, the associations between
microbiome and GC can occur without a causal relation to GC because patients with early GC have altered microbiomes because of atrophy progression. Additionally, we only measured a single
sample for our microbial measurements, and it has been shown that microbiome measurements at multiple time points could result in more precise exposure estimates43. However, it is important
to emphasize that there are ethical issues in repeating biopsies in those with normal gastric histology and healthy subjects. In conclusion, _H. pylori, P. acnes_ and _P. copri_ are strong
risk factors, whereas _L. lactis_ is a protective factor, for GC development in Koreans. The identified four candidate bacterial species will be clinically useful for the identification of
GC in Koreans. Further microbiome studies, such as a prospective study that has the capability to infer causality, are warranted to confirm the findings of the current study. Furthermore,
the study can be expanded by evaluating the impact of diet on the bacterial composition of the gastric mucosa and by including other races and ethnicities, specifically other East Asian
populations, to improve the generalizability of the results. The identification of the gastric bacterial composition can also serve as a readily accessible, noninvasive biomarker for the
identification of GC risk44. Moreover, conducting GC related pathway and functional studies based on gastric microbiome data is warranted, as most cancer related pathways are yet to be
discovered45,46. MATERIALS AND METHODS STUDY SUBJECTS Participants were recruited at the National Cancer Center Hospital in Korea between March 2011 and December 2014. Individuals who had
been histologically confirmed at the Center for Gastric Cancer as having early GC within the preceding three months were included in the patient group. Early GC was defined as an invasive
carcinoma confined to the mucosa and/or submucosa, regardless of lymph node metastasis status. Patients who had been diagnosed with diabetes mellitus or had a history of cancer within the
past five years, advanced GC, or severe systemic or mental diseases, as well as women who were pregnant or breastfeeding, were excluded. The control group was selected from individuals
undergoing health-screening examinations at the Center for Cancer Prevention and Detection at the same hospital. Individuals in the control group with a history of cancer, diabetes mellitus,
gastric ulcers, and _H. pylori_ treatment were excluded. The final sample of 556 participants was composed of 268 patients and 288 controls (men, 353; women, 203). All study protocols were
conducted according to the Declaration of Helsinki principles. This study was approved by the Institutional Review Board of the National Cancer Center (IRB number: NCCNCS-11-438). Written
informed consent was obtained from all participants. DATA COLLECTION Five gastric mucosa biopsy samples were collected from each study participant following the Sydney system after endoscopy
and examination of the stomach. A biopsy sample in the greater curvature, at least 3 cm away from each tumor, was used for the metagenomics analysis. The _H. pylori_ infection status was
determined by a rapid urease test, a serological test and histological evaluation. Regarding the rapid urease test, one biopsy sample was taken from the greater curvature of the corpus. Four
biopsy samples were collected from the lesser curvature of the corpus and antrum for histological evaluation. The _H. pylori_ status was determined via Wright-Giemsa staining of the biopsy
specimens by a pathologist who specialized in GC. A current infection was defined as at least one positive test result in the rapid urease test or histological evaluation of four biopsy
sites47. Participants were asked to complete a self-administered questionnaire. Demographic, lifestyle, physical activity, and medical history data were collected from the participants.
Total energy intake was obtained from the semiquantitative food frequency questionnaire (SQFFQ), which has been previously reported as a reliable and valid questionnaire48. _H. pylori_
infection was assessed by a rapid urease test and histological evaluation. DNA EXTRACTION DNA was extracted from the biopsy samples using the MagAttract DNA Blood M48 kit (Qiagen, Hilden,
Germany) and BioRobot M48 automatic extraction equipment (Qiagen), according to the manufacturers’ instructions. METAGENOMIC 16S RRNA GENE SEQUENCING Input gDNA (12.5 ng) was amplified with
16S rRNA gene V3-V4 primers, and a subsequent limited cycle amplification step was performed to add multiplexing indices and Illumina sequencing adapters. The final products were normalized
and pooled using PicoGreen, and the library sizes were verified using the LabChip GX HT DNA High Sensitivity Kit (PerkinElmer, Massachusetts, USA). Then, we sequenced using the MiSeq™
platform (Illumina, San Diego, USA). Each sequenced sample was prepared according to the Illumina 16S rRNA gene Metagenomic Sequencing Library protocols. DNA quantification and quality were
measured by PicoGreen and Nanodrop analyses, respectively. The 16S rRNA genes were amplified using 16S rRNA gene V3-V4 primers for the 288 control samples and the 268 GC patient samples. The
primer sequences are as follows: 16S rRNA gene V3-V4 primer. 16S rRNA gene Amplicon PCR Forward Primer. 5′ TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG. 16S rRNA gene Amplicon PCR
Reverse Primer. 5′ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC. Preprocessed reads from each sample were used to calculate the number of OTUs. The number of OTUs was determined
by clustering the sequences from each sample using a 97% sequence identity cut-off using quantitative insights into microbial ecology (QIIME) software (v.1.8.0). Taxonomic abundance was
counted with a National Center for Biotechnology Information (NCBI) database using a confidence threshold of 0.8 derived from the preprocessed reads for each sample. The microbial
composition was normalized using the values calculated from the taxonomic abundance count divided by the number of preprocessed reads for each sample to obtain the relative abundance.
STATISTICAL ANALYSIS To compare the demographic and lifestyle characteristics between the controls and patients, the chi-square test and Student’s _t_-test were performed for categorical
variables and continuous variables, respectively. We compared the relative abundance of the bacterial taxa (class, family, genus, and species) between patients and controls. FDR was applied
for the multiple comparison correction. The relative abundance of the candidate species was categorized into tertiles based on the relative abundance in the control group. Exceptionally, if
more than one third of the subjects has a relative abundance of zero, that bacterial species was categorized into two groups (noncarriers and carriers) based on the median distribution of
the controls. Noncarriers were defined as the subjects who had a relative abundance of zero. The group with the lowest relative abundance was used as the reference group. The ORs and 95% CIs
were estimated using unconditional logistic regression models. The median values of relative abundance in each tertile category were used as continuous variables to test for trends. The OR
estimates were calculated for the crude model (model I) and model II. Model II was adjusted for age, smoking, first-degree family history of GC, regular exercise, education, occupation,
monthly income, and total energy intake. An association analysis was performed for the male and female groups. Boxplots were drawn for the Shannon index value comparisons between the
patients and controls using the ggplot2 package. A PCoA was performed on a Bray-Curtis dissimilarity based on the OTU relative abundance table for the species level by using the R package’s
“vegan”. Sample clustering in beta diversity analysis was tested using ANOSIM. The statistical significance of the observed R was assessed by 104 permutations9. To determine whether the
candidate bacterial species can be used as diagnostic tool for GC, a ROC curve analysis was performed based on linear discriminant function. Initially, PCA was conducted to obtain two linear
combinations of four variables that explain part of the variance of the model. Then, a linear discriminant function was constructed to distinguish between patients with GC and non-GC. For
constructing the linear discriminant function, 424 observations were selected as training data set whereas 132 observations were selected as test data set. The training data set was used to
calculate the linear discriminant function by using the “MASS” package in R. The model evaluation was performed using the ROC and AUC analysis results. Statistical analyses were conducted by
using SAS version 9.4 software (SAS Inc., Cary, NC, USA) and the R platform (version 3.5.1) (The R Foundation for Statistical Computing, Vienna, Austria). ETHICAL STATEMENT This study was
approved by the Institutional Review Board of the National Cancer Center and all the methods were performed in accordance with the approved guidelines and regulations (IRB number:
NCCNCS-11-438). Written informed consent was obtained from all participants. DATA AVAILABILITY The sequence data are available https://www.ncbi.nlm.nih.gov/nuccore/KEQH0000000000. All other
data used and analyzed during the current study are available from the corresponding author by reasonable request. CHANGE HISTORY * _ 29 OCTOBER 2021 A Correction to this paper has been
published: https://doi.org/10.1038/s41598-021-00573-3 _ REFERENCES * Bray, F. _et al_. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers
in 185 countries. _CA Cancer J Clin._ 68(6), 394–424 (2018). Article PubMed Google Scholar * Ferlay, J. _et al_. GLOBOCAN, Cancer incidence and mortality worldwide: IARC CancerBase No. 10
[Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from, http://globocan.iarc.fr, Accessed February 2019. * Jung, K.-W., Won, Y.-J., Kong, H.-J. &
Lee, E. S. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. _Cancer Res Treat._ 50(2), 303 (2018). Article PubMed PubMed Central Google Scholar * Jung,
K.-W., Won, Y.-J., Kong, H.-J. & Lee, E. S. Prediction of Cancer Incidence and Mortality in Korea, 2018. _Cancer Res Treat._ 50(2), 317 (2018). Article PubMed PubMed Central Google
Scholar * Ashton, J. J., Beattie, R. M., Ennis, S. & Cleary, D. W. Analysis and Interpretation of the Human Microbiome. _Inflamm Bowel Dis._ 22(7), 1713–22 (2016). Article PubMed
Google Scholar * Martha, A. _et al_. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. _Science_. 343 (2014). * Garrett, W. S. Cancer and the
microbiota. _Science._ 348, 80–86 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Thursby, E. & Juge, N. Introduction to the human gut microbiota. _Biochem J._
474(11), 1823–36 (2017). Article CAS PubMed Google Scholar * Ferreira, R. M. _et al_. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. _Gut._ 67,
226–236 (2018). Article CAS PubMed Google Scholar * Noto, J. M. & Peek, R. M. Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the
progression to stomach cancer. _PLoS Pathogens._ 13(10), e1006573 (2017). Article PubMed PubMed Central CAS Google Scholar * Jemal, A. _et al_. Global cancer statistics. _CA Cancer J
Clin._ 61, 69–90 (2011). Article PubMed Google Scholar * Fock, K. M. _et al_. Second asia–pacific consensus guidelines for helicobacter pylori infection. _J Gastroenterol Hepatol._ 24,
1587–1600 (2009). Article PubMed Google Scholar * Li, J. & Perez Perez, G. I. Is there a role for the non-Helicobacter pylori bacteria in the risk of developing gastric cancer? _Int J
Mol Sci._ 19(5), 1353 (2018). Article ADS PubMed Central CAS Google Scholar * Hsieh, Y. Y. _et al_. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of
patients with gastric cancer in Taiwan. _Sci Rep._ 8(1), 158 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Brawner, K. M., Morrow, C. D. & Smith, P. D. Gastric
microbiome and gastric cancer. _Cancer J._ 20(3), 211–6 (2014). Article CAS PubMed PubMed Central Google Scholar * Heimesaat, M. M. _et al_. Helicaobacter pylori indicedgastric
immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. _PLoS One_. 9 (2014). * Gao, J.-J. _et al_. Association
Between Gut Microbiota and Helicobacter pylori-Related Gastric Lesions in a High-Risk Population of Gastric Cancer. _Front Cell Infect Microbiol._ 8, 202 (2018). Article PubMed PubMed
Central CAS Google Scholar * Eun, C. S. _et al_. Differences in Gastric Mucosal Microbiota Profiling in Patients with Chronic Gastritis, Intestinal Metaplasia, and Gastric Cancer Using
Pyrosequencing Methods. _Helicobacter._ 19, 407–416 (2014). Article CAS PubMed Google Scholar * Kamangar, F. _et al_. Helicobacter pylori and oesophageal and gastric cancers in a
prospective study in China. _Br J Cancer._ 96(1), 172 (2007). Article CAS PubMed Google Scholar * Yu, G. _et al_. Molecular characterization of the human stomach microbiota in gastric
cancer patients. _Front Cell Infect Microbiol._ 7, 302 (2017). Article PubMed PubMed Central CAS Google Scholar * Yang, I. _et al_. Different gastric microbiota compositions in two
human populations with high and low gastric cancer risk in Colombia. _Sci Rep._ 6, 18594 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Rhead, J. L., Letley, D. P.
& Mohammadi, M. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. _Gastroenterol._ 133, 926–936 (2007). Article
CAS Google Scholar * Ohnishi, N., Yuasa, H. & Tanaka, S. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. _Proc Natl
Acad Sci._ 105, 1003–1008 (2008). Article ADS CAS PubMed PubMed Central Google Scholar * El-Omar, E. M. _et al_. Interleukin-1 polymorphisms associated with increased risk of gastric
cancer. _Nature._ 404(6776), 398 (2000). Article ADS CAS PubMed Google Scholar * El-Omar, E. M. The importance of interleukin 1beta in Helicobacter pylori associated disease. _Gut._
48(6), 743–7 (2001). Article CAS PubMed PubMed Central Google Scholar * Figueiredo, C. _et al_. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk
individuals for gastric carcinoma. _J Natl Cancer Inst._ 94(22), 1680–7 (2002). Article CAS PubMed Google Scholar * Lofgren, J. L., Whary, M. T. & Ge, Z. Lack of commensal flora in
Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. _Gastroenterol._ 140, 210–220 (2011). Article Google Scholar * Tan, M. P., Kaparakis, M.
& Galic, M. Chronic Helicobacter pylori infection does not significantly alter the microbiota of the murine stomach. _Appl Environ Microbiol._ 73, 1010–1013 (2007). Article ADS CAS
PubMed Google Scholar * Li, T. H. _et al_. Alterations in gastric microbiota after H. Pylori eradication and in different histological stages of gastric carcinogenesis. _Sci Rep._ 7, 44935
(2017). Article ADS CAS PubMed PubMed Central Google Scholar * Montalban-Arques, A. _et al_. Propionibacterium acnes overabundance and natural killer group 2 member D system
activation in corpus-dominant lymphocytic gastritis. _J Pathol._ 240(4), 425–36 (2016). Article CAS PubMed PubMed Central Google Scholar * Monstein, H. J., Tiyejung, A. & Kraft, C.
H. Profiling of bacterial flora in gastric biopsies from patients with Helicobacter pylori‐associated gastritis and histologically normal control individuals by temperature gradient gel
electrophoresis and 16S rDNA sequence analysis. _J Med Microbiol._ 49, 817–822 (2000). Article PubMed Google Scholar * Salem, I., Ramser, A., Isham, N., Ghannoum, M.A. The gut microbiome
as a major regulator of the gut-skin axis. _Front Microbiol_. 9 (2018). * Larsen, J. M. The immune response to Prevotella bacteria in chronic inflammatory disease. _Immunol._ 151(4), 363–74
(2017). Article CAS Google Scholar * Wu, J. _et al_. Tongue Coating Microbiota Community and Risk Effect on Gastric Cancer. _J Cancer._ 9(21), 4039 (2018). Article CAS PubMed PubMed
Central Google Scholar * Nardone, G. & Compare, D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? _United European Gastroenterol J._ 3(3),
255–60 (2015). Article CAS PubMed PubMed Central Google Scholar * Li, X.-X. _et al_. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal
anti-inflammatory drug use. _PLoS One._ 4(11), e7985 (2009). Article ADS PubMed PubMed Central CAS Google Scholar * Kim, J. Y., Woo, H. J., Kim, Y.-S. & Lee, H. J. Screening for
antiproliferative effects of cellular components from lactic acid bacteria against human cancer cell lines. _Biotechnol Lett._ 24(17), 1431–6 (2002). Article CAS Google Scholar * Kim, S.
Y., Kim, J. E., Lee, K. W. & Lee, H. J. Lactococcus lactis ssp. lactis inhibits the proliferation of SNU-1 human stomach cancer cells through induction of G0/G1 cell cycle arrest and
apoptosis via p53 and p21 expression. _Ann N Y Acad Sci._ 1171, 270–5 (2009). Article ADS PubMed Google Scholar * Meng, C., Bai, C., Brown, T.D., Hood, L., Tian, Q. Human gut microbiota
and gastrointestinal cancer. _Genomics Proteomics Bioinformatics_ (2018). * Sinha, R. _et al_. Next steps in studying the human microbiome and health in prospective studies, Bethesda, MD,
May 16–17, 2017. _BMC.Microbiome_ (2018). * Franzosa, E. A. _et al_. Relating the metatranscriptome and metagenome of the human gut. _Proc Natl Acad Sci_. 201319284 (2014). * Ahn, J. _et
al_. Human gut microbiome and risk for colorectal cancer. _J Natl Cancer Inst._ 105(24), 1907–11 (2013). Article CAS PubMed PubMed Central Google Scholar * Hayes, R. B. _et al_.
Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. _JAMA Oncol._ 4(3), 358–65 (2018). Article PubMed PubMed Central Google Scholar * Ahn, J., Chen,
C. Y. & Hayes, R. B. Oral microbiome and oral and gastrointestinal cancer risk. _Cancer Causes Control._ 23(3), 399–404 (2012). Article PubMed PubMed Central Google Scholar * Kwon,
M., Seo, S.-S., Kim, M. K., Lee, D. O. & Lim, M. C. Compositional and Functional Differences between Microbiota and Cervical Carcinogenesis as Identified by Shotgun Metagenomic
Sequencing. _Cancers._ 11(3), 309 (2019). Article CAS PubMed Central Google Scholar * Zhang, B., Xia, C., Lin, Q. & Huang, J. Identification of key pathways and transcription factors
related to Parkinson disease in genome wide. _Mol Biol Rep._ 39(12), 10881–7 (2012). Article CAS PubMed Google Scholar * Woo, H. D. _et al_. Genome-wide profiling of normal gastric
mucosa identifies Helicobacter pylori-and cancer-associated DNA methylome changes. _Int J Cancer._ 143(3), 597–609 (2018). Article CAS PubMed Google Scholar * Ahn, Y. _et al_. Validation
and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. _Eur J Clin Nutr._ 61(12), 1435–41 (2007). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by grants from the National Cancer Center, Republic of Korea (Nos 1410260, 1810090, and 1810980). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Cancer Control and Population Health, Graduate School of Cancer Science and Policy, Goyang-si, 10408, Gyeonggi-do, South Korea Madhawa Neranjan Gunathilake *
Department of Cancer Biomedical Science, Graduate School of Cancer Science and Policy, Goyang-si, 10408, Gyeonggi-do, South Korea Jeonghee Lee & Jeongseon Kim * Center for Gastric
Cancer, National Cancer Center Hospital, National Cancer Center, Goyang-si, 10408, Gyeonggi-do, South Korea Il Ju Choi & Young-Il Kim * Microbiome Division, Theragen Etex, 145
Gwanggyo-ro, Gyeongtong-gu, Suwon-si, Gyeonggi-do, 16229, South Korea Yongju Ahn & Chanhyeok Park Authors * Madhawa Neranjan Gunathilake View author publications You can also search for
this author inPubMed Google Scholar * Jeonghee Lee View author publications You can also search for this author inPubMed Google Scholar * Il Ju Choi View author publications You can also
search for this author inPubMed Google Scholar * Young-Il Kim View author publications You can also search for this author inPubMed Google Scholar * Yongju Ahn View author publications You
can also search for this author inPubMed Google Scholar * Chanhyeok Park View author publications You can also search for this author inPubMed Google Scholar * Jeongseon Kim View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.L., M.N.G., C.P. and Y.A. conducted the formal analysis; M.N.G. wrote the original manuscript draft;
I.J.C., Y.-I.K., Y.A., and J.K. reviewed and edited the manuscript; I.J.C., Y.-I.K., J.K., J.L., C.P. and Y.A. curated the data; I.J.C., Y.-I.K. and J.K., were involved in the study
investigation; I.J.C., Y.-I.K. and J.K. helped with the methodology; J.K. acquired the funding; J.K. was the project administrator; J.K. supervised the project. CORRESPONDING AUTHOR
Correspondence to Jeongseon Kim. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. The original online version of this Article was revised: The Data Availability section in the original
version of this Article was incomplete. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and
the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gunathilake, M.N., Lee, J., Choi, I.J. _et al._ Association between the relative
abundance of gastric microbiota and the risk of gastric cancer: a case-control study. _Sci Rep_ 9, 13589 (2019). https://doi.org/10.1038/s41598-019-50054-x Download citation * Received: 15
April 2019 * Accepted: 30 August 2019 * Published: 19 September 2019 * DOI: https://doi.org/10.1038/s41598-019-50054-x SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
