Proline improves switchgrass growth and development by reduced lignin biosynthesis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Transgenic switchgrass overexpressing _Lolium perenne_ L. delta1-pyrroline 5-carboxylate synthase (_LpP5CS_) in group I (TG4 and TG6 line) and group II (TG1 and TG2 line) had
significant P5CS and ProDH enzyme activities, with group I plants (TG4 and TG6) having higher P5CS and lower ProDH enzyme activity, while group II plants had higher ProDH and lower P5CS
enzyme activity. We found group II transgenic plants showed stunted growth, and the changed proline content in overexpressing transgenic plants may influence the growth and development in
switchgrass. RNA-seq analysis showed that KEGG enrichment included phenylpropanoid biosynthesis pathway among group I, group II and WT plants, and the expression levels of genes related to
lignin biosynthesis were significantly up-regulated in group II. We also found that lignin content in group II transgenic plants was higher than that in group I and WT plants, suggesting
that increased lignin content may suppress switchgrass growth and development. This study uncover that proline can appropriately reduce lignin biosynthesis to improve switchgrass growth and
development. Therefore, appropriate reduction in lignin content and increase in biomass are important for bioenergy crop to lower processing costs for biomass fermentation-derived fuels.
SIMILAR CONTENT BEING VIEWED BY OTHERS OVEREXPRESSION OF _PVWOX3A_ IN SWITCHGRASS PROMOTES STEM DEVELOPMENT AND INCREASES PLANT HEIGHT Article Open access 01 December 2021 THE REGULATION OF
CELL WALL LIGNIFICATION AND LIGNIN BIOSYNTHESIS DURING PIGMENTATION OF WINTER JUJUBE Article Open access 01 November 2021 REGULATION OF PHENYLPROPANOID BIOSYNTHESIS BY MDMYB88 AND MDMYB124
CONTRIBUTES TO PATHOGEN AND DROUGHT RESISTANCE IN APPLE Article Open access 01 July 2020 INTRODUCTION Proline is known as a compatible solute (osmolyte) and a scavenger of reactive oxygen
species (ROS) providing protection against oxidative damage1. In addition to its well established role in coping with environmental stress, proline also plays an increasingly significant
role in plant development. It was reported that proline might have certain regulatory functions during protein synthesis and may act as a signaling molecule during plant development1, and
proline plays an important role in plant growth and life cycle, such as regulating cyclin genes and affecting general protein synthesis2. Additionally, proline may also play critical roles
in cellular metabolism both as a component of proteins and as a free amino acid3. Many genes are involved in the proline synthesis and degradation pathways. In higher plants,
Δ1-pyrroline-5-carboxylate synthetase (P5CS) and proline dehydrogenase (ProDH) are rate-limiting enzymes during the synthesis and degradation of proline respectively. Glutamate is reduced to
pyrroline-5-carboxylate (P5C) by P5CS, which is then converted into proline by Δ1-pyrroline-5-carboxylate reductase (P5CR)1. On the other hand, proline degradation takes place in the
mitochondria, where it is catalyzed into glutamate by ProDH and P5C dehydrogenase (P5CDH)4. Proline metabolism occupies a central place in plant metabolism and is connected to other pathways
through both ornithine and glutamate. It is connected to the pentose phosphate pathway and the TCA cycle as a way of moving the reductants and buffering the redox status of the
chloroplast4,5. In the proline biosynthesis pathway, the consumption of the reductants (NADPH) buffers the redox status of the chloroplast, which is linked with the pentose phosphate
pathway. In the pentose phosphate pathway, glucose-6-phosphate (G-6-P) is reduced to ribulose-5-phosphate (Ru-5-P) by the rate-limiting enzymes glucose 6-phosphate dehydrogenase (G6PDH) and
6-phosphogluconate dehydrogenase (6PGDH), and concurrently consumes NADP+ to generate NADPH in this process6. In plants, transketolase (TK) plays a role in Calvin cycle while in
non-photosynthetic organisms it connects the phosphate pentose pathway and glycolysis for generating NADPH7. In addition, proline degradation contributes carbon for TCA cycle4. The pentose
phosphate pathway and Calvin cycle provide erythrose-4-phosphate (E4P) which together with phosphoenolpyruvate acts as precursor for phenylalanine biosynthesis through the shikimic acid
pathway5. Phenylalanine, as the precursor amino acid for lignin biosynthesis, is essential for secondary cell wall biosynthesis8. In most plants, lignin is mainly composed of hydroxyphenyl
(H), guaiacyl (G) and syringyl (S) monolignol subunits that are derived from _p_-coumaryl, coniferyl and sinapyl monolignols, respectively. Several enzymes are required for monolignol
biosynthesis, including phenylalanine ammonia-lyase (PAL), caffeic acid 3-Omethyltransferase (COMT), caffeoyl-CoA O-methyltransferase (CCoAOMT), hydroxycinnamoyl-CoA reductase (CCR),
cinnamyl alcohol dehydrogenase (CAD)9. A number of reports have showed that lignin content is reduced by down-regulating the expression of monolignol biosynthesis related genes in plants10.
Many studies had showed that lignin content had the negative relationship with the growth and development of plants. Transgenic aspen with suppressed _Pt4CL1_ expression exhibited up to a
45% reduction of lignin and a 15% increase in cellulose, but leaf, root, and stem growth were substantially enhanced11. Additionally, compared with WT plants, _AtLOV1_ transgenic switchgrass
plants had higher total lignin content, delayed flowering time and less aboveground biomass12. Besides, a number of studies suggested that the most severe deficiency of lignin showed
stunted growth in plants13. However, many studies also showed that lignin can be reduced without reducing yield or fitness14,15. Switchgrass (_Panicum virgatum_ L.) is a perennial C4 grass
native to North America, considered as a potential dedicated bioenergy crop due to its high biomass production and tolerance on marginal land9. We found that overexpression plants showed two
different phenotypes, the two groups transgenic plants had different P5CS and ProDH enzyme activities, as well as proline content. To shed light on potential changes in growth and
development, we analyzed the RNA-seq data. The results showed that phenylpropanoid biosynthesis pathway was significantly up-regulated in the group II plants compared with the group I and WT
plants. In particular, lignin content in group II transgenic plants was higher than that in group I and WT plants, suggesting that proline affects switchgrass growth and development by
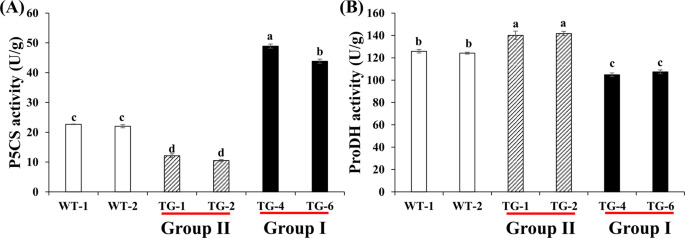
coordination with lignin biosynthesis. RESULTS P5CS AND PRODH ENZYME ACTIVITIES IN THE TWO GROUPS OF TRANSGENIC PLANTS P5CS and ProDH are the rate-limiting enzymes in the proline synthesis
and degradation pathway, respectively1. P5CS and ProDH activities showed significant differences among the group I (TG4 and TG6), group II (TG1 and TG2) and WT plants. P5CS activity was
lower in group II and higher in group I as compared to the wild type (Fig. 1A) where the P5CS activity in group I plants was 4.1 and 2.1 fold greater than group II and WT plants
respectively. On the contrary, ProDH activity was the highest in the group II plants (142 U/g), followed by the WT (124 U/g), and lowest in the group I plants (102 U/g) (Fig. 1B).
Additionally, group II overexpression lines showed relatively lower proline content, and the proline content of group I transgenic plants was higher compared with WT plants16. Combined with
the proline levels, these results suggested that proline synthesis is reduced and proline degradation is enhanced in the group II plants compared with the WT plants, and while in group I,
there is an increased proline synthesis and decreased proline degradation. COENZYME II NADP(H) CONTENTS IN THE TWO GROUPS OF TRANSGENIC PLANTS Group II transgenic plants contain lower
proline content, higher ProDH and lower P5CS enzyme activity compared with the WT plants. NADP+ and NADPH are generated during the synthesis and degradation of proline, respectively; proline
synthesis consumes NADPH to regenerate NADP+ in the chloroplast, which indicates the NADPH/NADP+ ratio is an important index for proline synthesis17. The coenzyme II NADP(H) content and
NADPH/NADP+ ratio was significantly different between the two groups of overexpression lines and WT plants (Fig. 2). The coenzyme II NADP+ content was the highest in group I plants, followed
by the WT, and lowest in group II plants. Conversely, coenzyme II NADPH was the highest in group II plants, 2.0- and 2.6-fold greater than that in WT and group I plants, respectively.
Additionally, the NADPH/NADP+ ratio was higher in group II plants and lower the group I plants compared with WT plants. These results suggested that the change in P5CS and ProDH enzyme
activities may result in the difference of coenzyme II NADP(H) contents in the group I, group II and WT plants, and simultaneously changing the proline level. MORPHOLOGICAL CHARACTERISTIC IN
THE TWO GROUPS OF TRANSGENIC PLANTS As shown in the Fig. 3A,B, group II plants exhibited more stunted growth, such as slender stem and fewer tillers. Compared to the WT plants, transgenic
plants in group II had a 52% and 23% reduction in tiller number and internode diameter, respectively; while transgenic plants in group I had a 48% and 19% increase in tiller number and
internode diameter, respectively (Fig. 3C,D). RNA-SEQ ANALYSIS In the RNA-seq data, the top 20 obviously enriched KEGG pathways are shown in Supplementary Fig. S1. In group II and group I
transgenic plants, these significantly enriched pathways include “Phenylpropanoid biosynthesis (KO: zma00940)”, “Stilbenoid, diarylheptanoid and gingerol biosynthesis (KO: zma00945)”,
“Cyanoamino acid metabolism (KO: zma00460)”, “Flavonoid biosynthesis (KO: zma00941)” and “Biosynthesis of secondary metabolites (KO: zma00564)”. Additionally, 13 pathways were significantly
enriched in the group II and WT plants. These include “Phenylpropanoid biosynthesis (KO: zma00940)”, “Biosynthesis of secondary metabolites (KO: zma01110)”, “Glutathione metabolism (KO:
zma00480)”, “Cysteine and methionine metabolism (KO: zma00270)”, “Stilbenoid, diarylheptanoid and gingerol biosynthesis (KO: zma00945)”, “Pentose phosphate pathway (KO: zma00030)”,
“Cyanoamino acid metabolism (KO: zma00460)”, “Flavonoid biosynthesis (KO: zma00941)”, “Selenocompound metabolism (KO: zma00450)”, “Nitrogen metabolism (KO: zma00910)”, “Biosynthesis of amino
acids (KO: zma01230)”, “Carbon metabolism (KO: zma01200)” and “Alanine, aspartate and glutamate metabolism (KO: zma00250)”. Finally, ten pathways were found significantly enriched which
include “Fatty acid degradation (KO: zma00071)”, “Tyrosine metabolism (KO: zma00350)”, “Selenocompound metabolism (KO: zma00450)”, “Isoquinoline alkaloid biosynthesis (KO: zma00950)”,
“Starch and sucrose metabolism (KO: zma00500)”, “Biosynthesis of secondary metabolites (KO: zma01110)”, “Tropane, piperidine and pyridine alkaloid biosynthesis (KO: zma00960)”, “Fatty acid
metabolism (KO: zma01212)”, “Peroxisome (KO: zma04146)”, “Carbon metabolism (KO: zma01200)”. Besides, the enriched KEGG pathways also included the photosynthesis and circadian rhythm-plant
pathways (Supplementary Fig. S2), suggesting that proline metabolism affects the photosynthesis and circadian rhythm in switchgrass. Notably, “Phenylpropanoid biosynthesis” pathway was
significantly enriched (_P_-value < 0.05). The expression levels of those DEGs including _PvPAL_, _PvCOMT_, _PvCCoAOMT_, _PvCYP98A3_, _PvCCR_, _PvCAD, PvG6PDH_, _Pv6PGDH_ and _PvTK_ (Fig.
4), were all significantly up-regulated in the group II plants. Especially, the expression level of _PvCCR_ in group II was 12-fold greater than in the WT. In summary, the metabolic network
of enriched pathways included arginne and proline metabolism, flavonoid biosynthesis, phenylpropanoid biosynthesis and pentose phosphate pathway, indicating that proline metabolism is
involved in those metabolism pathways in switchgrass (Supplementary Fig. S3). LIGNIN MONOMER COMPOSITIONS IN THE TWO GROUPS OF TRANSGENIC PLANTS In the data of RNA-seq, the expression level
of differentially expressed genes (DEGs) involved in the phenylpropanoid biosynthesis were significantly up-regulated in the group II plants compared with the group I and WT plants.
Moreover, the lignin monomer composition in the two groups of transgenic and WT plants (Table 1), showed a relatively lower guaiacyl (G) and syringyl (S) contents, as well as hydroxyphnyl
(H) content in group I plants. While group II transgenic plants showed relatively higher guaiacyl (G) and syringyl (S) contents, as well as lower hydroxyphenyl (H) content compared with the
WT plants. Notably, the ratio of S/G was lower in the group I plants, and the lignin content (S + G + H monomer content) showed a significant difference between the two groups of transgenic
and WT plants. Lignin content was lower in the group I plants and higher in the group II plants compared with the WT. In addition, S monomer content of the stem from TG2, TG6 and WT at the
R1 stage was evaluated after staining with the Mäule reagent, we found that the S monomer content (Mäule staining) was clearly reduced in the stem of TG6 (Fig. 5). These results suggest that
proline metabolism affects lignin biosynthesis in switchgrass. DISCUSSION P5CS activity was lower in group II plants, which could led to the decreased proline accumulation. In the present
study, KEGG enrichment included pentose phosphate pathway (PPP), fatty acid synthesis and phenylalanine biosynthesis pathway, and at the same time, NADP+ and NADPH content showed a
significant difference between the two groups of transgenic and WT plants. NADP+ and NADPH are generated during the synthesis and degradation of proline, respectively18. The cycling of
proline substrate is coupled to maintaining the NADP+/NADPH ratio via the pentose phosphate pathway19, and PPP provides NADPH for fatty acid synthesis in plastids6. So it seems that proline
degradation was accelerated in the group II transgenic plants, which led to an increase in the NADPH content. It was reported that antisense _AtP5CS_ transgenic Arabidopsis led to proline
depletion and abnormal leaf formation20. Down-regulation of proline biosynthesis genes, on the other hand, resulted in growth defects21, indicating the importance of proline for plant
development. In our study, the stems were significantly thicker in group I transgenic plants and thinner in group II plants, when compared with the WT plants (Fig. 3D). Furthermore, as the
results of the RNA-seq analysis indicated, the enriched KEGG pathways also include the photosynthesis and circadian rhythm-plant pathways (Supplementary Fig. S2). This leads us to conclude
that proline may play important roles in plant growth and development. Proline metabolism is connected to the pentose phosphate pathway (PPP) and the TCA cycle, and the PPP and Calvin cycle
provide erythrose-4-phosphate (E4P) which together with phosphoenolpyruvate acts as a precursor for phenylalanine biosynthesis through the shikimic acid pathway5. In our result of RNA-seq,
the enriched KEGG pathways were including arginine and proline metabolism, flavonoid biosynthesis, phenylpropanoid biosynthesis and pentose phosphate pathway (Supplementary Fig. S3). To
precisely understand the relationship between proline metabolism and lignin biosynthesis in switchgrass, we summarized the metabolic network that exists between the phenylpropanoid
biosynthesis pathway, pentose phosphate pathway and proline metabolic pathway in Fig. 6. In these metabolism pathways, _PvG6PDH_ and _Pv6PGDH_ are genes encoding key enzymes involved in the
pentose phosphate pathway; _PvPAL_, _PvCOMT_, _PvCCoAOMT_, _PvCCR_ and _PvCAD_ are the genes encoding for important enzymes in lignin biosynthesis, and transketolase (TK) participates in the
Calvin cycle. The proline biosynthesis pathway is linked with the pentose phosphate pathway through consuming the reductants (NADPH), while the pentose phosphate pathway and the Calvin
cycle provide erythrose-4-phosphate (E4P) for phenylalanine biosynthesis. Thus, proline metabolism pathway is coordinated with phenylpropanoid biosynthesis pathways in switchgrass. In
particular, our results showed that the lignin content (S + G + H monomer content) was lower in group I plants and higher in group II plants compared with the WT. So, reducing proline
biosynthesis may induce the increase of lignin content in group II transgenic plants. Many studies showed that lignin content affected plant growth and development. Suppressing _Pt4CL1_
expression can reduce the lignin content and enhance the growth of leaf, root, and stem in aspen11, and RNA interference of _Pv4CL1_ lead to a reduction in lignin content with uncompromised
biomass yields15. Additionally, overexpressing _AtLOV1_ gene increased total lignin content, delayed flowering time and reduce the aboveground biomass in switchgrass12. Besides, extreme
lignin deficiency can also contribute to stunted growth in plants13. In our result, group II transgenic plants which had lower proline and higher lignin content exhibited more stunted
growth. Thus, we speculate that proline affects switchgrass growth and development by coordination with lignin biosynthesis. In conclusion, unlike the previous studies that proline plays an
important role in regulating cyclin genes and affecting general protein synthesis to improve plant growth and development. Our study showed that lacking proline could result in an increased
lignin content, and leading to the stunted growth in switchgrass. However, the regulatory mechanism how proline plays roles in plant growth and development is still unclear, and
understanding the molecular mechanism will help us to prime candidates in crop genetic engineering for improving the biomass of plants. Moreover, appropriate reduction in lignin content and
increase in biomass is one important strategy of bioenergy crop to lower processing costs for biomass fermentation-derived fuels. MATERIALS AND METHODS PLANT MATERIALS AND GROWTH CONDITIONS
Switchgrass callus generated from mature seeds (Alamo) was transformed with a pCAMBIA 1301-_LpP5CS_ overexpression cassette using the Agrobacterium-mediated transformation method16. Group I
consists of two independent overexpression lines (TG4 and TG6) while group II contains TG1 and TG2. The two groups of transgenic switchgrass plants were grown in the greenhouse under a 16 h
light/8 h dark photoperiod at 25 ± 2 °C. The switchgrass plants were used for the measurement of tiller number and stem diameter at reproductive stage (R3), and each line comprises three
biological samples. P5CS AND PRODH ENZYME ACTIVITIES P5CS and ProDH activities in the transgenic and control plants were assayed based on the protocal22,23. Briefly, frozen switchgrass
leaves (0.1 g) were ground in liquid nitrogen and mixed with 1 mL of extraction buffer (0.1 M Tris–HCl, pH 7.2, 10 mM MgCl2, 10 mM 2-mercaptoethanol, and 1 mM PMSF). The homogenate was
centrifuged at 12,000 g for 20 min at 4 °C and the P5CS activity was calculated. Switchgrass leaves (0.1 g) were homogenized into powder and placed in 0.5 mL extraction buffer (100 mM sodium
phosphate, 1 mM cysteine, and 0.1 mM EDTA [pH 8.0]). After centrifugation at 12,000 g for 10 min at 4 °C, the supernatant was used to measure ProDH activity. Each assay included two
technical with three biological replicates. COENZYME II NADP (H) CONTENTS The coenzyme II NADP(H) contents in the two groups of overexpression lines and WT plants were measured according to
the protocol24,25 with some modifications. Briefly, swithgrass leaves (0.1 g) were ground in liquid nitrogen and homogenized with 0.1 M HCl (for NADP assay) or 0.1 M NaOH (for NADPH assay).
Samples were heated at 95 °C for 2 min and cooled in an ice bath. After samples were centrifuged for 10 min at 4 °C, and the supernatants were used for coenzyme assay. Assays were performed
using a reaction volume of 200 mL on 96-well plates, and absorbance was measured at 565 nm from 0 to 30 min after the start of the reaction. Two technical and three biological replicates of
each transgenic line were performed in each experiment. RNA-SEQ ANALYSIS Total RNA was extracted from transgenic and WT leaves at the E5 stage, and RNA samples included three groups: TG1 and
TG2 lines (group II), TG4 and TG6 lines (group I), and WT1 and WT2 plants. The RNA-seq data were downloaded from NCBI, the SRA number is SRP13027516. The paired-end reads were aligned to
the reference genome (https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Pvirgatum_er) using TopHat v2.0.1226. The HTSeq v0.6.127 and DEGSeq R package28 were used to count the
reads numbers mapped to each gene and determine if any differential expression exists. The KOBAS software was performed to test the statistical enrichment of differential expression genes in
KEGG pathways. QUANTITATIVE REAL-TIME RT-PCR ANALYSIS Total RNA from the leaves of the transgenic and WT plants at elongation stage (E5) was isolated by the TRIzol reagent method
(Invitrogen, Carlsbad, CA, USA). Quantitative real-time RT-PCR analysis was performed following the description17, the data were normalized using the level of switchgrass _Ubq_ transcripts
(GenBank accession no. FL955474.1), and the relative expression levels of genes were calculated using the 2−△△CT method29. Two biological with three technical replicates of each line were
performed in each experiment. The primers used for qRT-PCR are listed in Table S1. LIGNIN MONOMER COMPOSITION Lignin monomer levels of stems from the two groups of transgenic and WT lines at
the reproductive stage (R1) were measured following the description30. Three technical replicates of each line were performed in the experiment. HISTOLOGY AND MICROSCOPY The internode 1
(I1) of stem collected from transgenic and control plants at the R1 stage was used to make 50 μm thick cross sections31. This was followed by Mäule staining as described32. Images were taken
under an Olympus BX-51 compound microscope, and the data were analyzed using Image-Pro Plus 6.0. STATISTICAL ANALYSIS Triplicate samples were collected for each transgenic and WT lines.
Data from each trait were subjected to analysis of variance (ANOVA). The significance of the difference between treatments was tested at the _P_ < 0.05 level. Standard errors are provided
in tables and figures as appropriate. All the statistical analyses were performed using the SPSS package (SPSS 20.0, IBM Company, USA). REFERENCES * Szabados, L. & Savouré, A. Proline:
a multifunctional amino acid. _Trends in Plant Science_ 15(2), 89–97 (2010). Article CAS Google Scholar * Wang, G. _et al_. Proline responding1plays a critical role in regulating general
protein synthesis and the cell cycle in maize. _The Plant Cell_ 26, 2582–2600 (2014). Article CAS Google Scholar * Kavi, K. P. B., Hima, K. P., Sunita, M. S. & Sreenivasulu, N. Role
of proline in cell wall synthesis and plant development and its implications in plant ontogeny. _Frontiers in Plant Science_ 6(544), 544 (2015). Google Scholar * Sarkar, D., Bhowmika, P.
C., Kwon, Y. & Shetty, K. The role of proline-associated pentose phosphate pathway in coolseason turfgrasses after UV-B exposure. _Environmental and Experimental Botany_ 70, 251–258
(2011). Article CAS Google Scholar * Kaur, G. & Asthir, B. Proline: a key player in plant abiotic stress tolerance. _Biologia Plantarum_ 59(4), 609–619 (2015). Article CAS Google
Scholar * Hutchings, D., Rawsthorne, S. & Emes, M. J. Fatty acid synthesis and the oxidative pentose phosphate pathway in developing embryos of oilseed rape (Brassica Napus L.).
_Journal of Experimental Botany_ 56(412), 577–585 (2005). Article CAS Google Scholar * Willige, B. C., Kutzer, M., Tebartz, F. & Bartels, D. Subcellular localization and enzymatic
properties of differentially expressed transketolase genes isolated from the desiccation tolerant resurrection plant craterostigma plantagineum. _Planta_ 229(3), 659–666 (2009). Article CAS
Google Scholar * Pascual, M. B. _et al_. Biosynthesis and metabolic fate of phenylalanine in conifers. _Frontiers in Plant Science_ 7, 1030 (2016). Article Google Scholar * Fu, C. _et
al_. Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. _Plant Biotechnology Journal_ 10(4),
443 (2012). Article CAS Google Scholar * Liu, Q., Luo, L. & Zheng, L. Lignins: biosynthesis and biological functions in plants. _International Journal of Molecular Sciences_ 19(2),
335 (2018). Article ADS Google Scholar * Hu, W. J. _et al_. Repression of lignin biosynthesis promotes cellulose accumulation andgrowth in transgenic trees. _Nature Biotechnology_ 17(8),
808–812 (1999). Article CAS Google Scholar * Xu, B. _et al_. Overexpression of atlov1 in switchgrass alters plant architecture, lignin content, and flowering time. _Plos One_ 7(12),
e47399 (2012). Article ADS CAS Google Scholar * Li, X., Bonawitz, N. D., Weng, J. K. & Chapple, C. The growth reduction associated with repressed lignin biosynthesis in arabidopsis
thaliana is independent of flavonoids. _The plant cell_ 22(5), 1620–1632 (2010). Article CAS Google Scholar * Chabannes, M. _et al_. Strong decrease in lignin content without significant
alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. _The Plant Journal_
28(3), 257–270 (2001). Article CAS Google Scholar * Xu, B. _et al_. Silencing of 4-coumarate: coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable
sugar yields for biofuel production. _New phytologist_ 192(3), 611–625 (2011). Article CAS Google Scholar * Guan, C. _et al_. Overexpression of the Lolium perenne L. delta1-pyrroline
5-carboxylate synthase (LpP5CS) gene results in morphological alterations and salinity tolerance in switchgrass (Panicum virgatum L.). _PloS one_ 14(7), e021966 9 (2019). Article CAS
Google Scholar * Huang, Y. H. _et al_. Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. _Scientific reports_ 7(1), 12212
(2017). Article ADS Google Scholar * Giberti, S., Funck, D. & Forlani, G. Δ 1 -pyrroline-5-carboxylate reductase from arabidopsis thaliana: stimulation or inhibition by chloride ions
and feedback regulation by proline depend on whether NADPH or NADH acts as co-substrate. _New Phytologist_ 202(3), 911–919 (2014). Article CAS Google Scholar * Hare, P. D., Cress, W. A.
& Staden, J. V. A regulatory role for proline metabolism in stimulating arabidopsis thaliana, seed germination. _Plant Growth Regulation_ 39(1), 41–50 (2003). Article CAS Google
Scholar * Nanjo, T. _et al_. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. _The Plant Journal_ 18(2), 185–193
(1999). Article CAS Google Scholar * Funck, D., Winter, G., Baumgarten, L. & Forlani, G. Requirement of proline synthesis during Arabidopsis reproductive development. _BMC Plant
Biology_ 12, 191 (2012). Article CAS Google Scholar * Bagdi, D. L., Shaw, B. P., Sahu, B. B. & Purohit, G. K. Real time pcr expression analysis of gene encoding p5cs enzyme and
proline metabolism under naci salinity in rice. _Journal of Environmental Biology_ 36(4), 955–961 (2015). CAS PubMed Google Scholar * García-Ríos, M. _et al_. Cloning of a polycistronic
cDNA from tomato encoding gamma-glutamyl kinase and gamma-glutamyl phosphate reductase. _Proceedings of the National Academy of Sciences_ 94(15), 8249–54 (1997). Article ADS Google Scholar
* Hayashi, M. _et al_. Enhanced dihydroflavonol-4-reductaseactivity and NAD homeostasis leading to cell death tolerance in transgenic rice. _Proc Natl Acad Sci USA_ 102, 7020–7025 (2005).
Article ADS CAS Google Scholar * Hodges, M., Flesch, V., Gálvez, S. & Bismuth, E. Higher plant NADP+-dependent isocitrate dehydrogenases, ammonium assimilation and NADPH production.
_Plant Physiology &_ _Biochemistry_ 41(6–7), 577–585 (2003). Article CAS Google Scholar * Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with
RNA-Seq. _Bioinformatics_ 25(9), 1105–11 (2009). Article CAS Google Scholar * Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data.
_Bioinformatics_ 31(2), 166–9 (2015). Article CAS Google Scholar * Anders, S. & Huber, W. Differential expression analysis for sequence count data. _Genome Biology_ 11(10), R106
(2010). Article CAS Google Scholar * Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
_Methods_ 25(4), 402–408 (2001). Article CAS Google Scholar * Fu, C. _et al_. Genetic manipulation of lignin biosynthesis in switchgrass significantly reduces recalcitrance and improves
biomass ethanol production. _Proceedings of the National Academy of Sciences_ 108, 3803–3808 (2011). Article ADS CAS Google Scholar * Chen, F. & Dixon, R. A. Lignin modification
improves fermentable sugar yields for biofuel production. _Nature Biotechnology_ 25(7), 759–761 (2007). Article CAS Google Scholar * Pomar, F. _et al_. Changes in stem lignins (monomer
composition and crosslinking) and peroxidase are related with the maintenance of leaf photosynthetic integrity during Verticillium wilt in Capsicum annuum. _New Phytologist_ 163(1), 111–123
(2004). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Funding for this work was provided by the Ministry of Science And Technology, China (2014BAD23B03), National
Natural Science Foundation of China (31672478) and Natural Science Foundation of Beijing (6162016). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * College of Grassland Science and Technology,
China Agricultural University, Beijing, China Cong Guan, Hui-Fang Cen, Xin Cui, Dan-Yang Tian & Yun-Wei Zhang * Beijing Key Laboratory for Grassland Science, China Agricultural
University, Beijing, China Yun-Wei Zhang * National Energy R&D Center for Biomass (NECB), Beijing, China Yun-Wei Zhang * Beijing Sure Academy of Biosciences, Beijing, China Yun-Wei Zhang
* Department of Plant and Soil Sciences, Institute for Agricultural Bioscience, Oklahoma State University, Oklahoma, OK, USA Dimiru Tadesse Authors * Cong Guan View author publications You
can also search for this author inPubMed Google Scholar * Hui-Fang Cen View author publications You can also search for this author inPubMed Google Scholar * Xin Cui View author publications
You can also search for this author inPubMed Google Scholar * Dan-Yang Tian View author publications You can also search for this author inPubMed Google Scholar * Dimiru Tadesse View author
publications You can also search for this author inPubMed Google Scholar * Yun-Wei Zhang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
Conceived and designed the experiments: Y.Z. Performed the experiments: C.G., H.C., X.C. and D.T. Analyzed the data: C.G. and Y.Z. Wrote the paper: C.G. and Y.Z. Edited language: T.D. All
authors reviewed and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Yun-Wei Zhang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Guan, C., Cen, HF., Cui, X. _et al._ Proline improves switchgrass growth and development by reduced lignin biosynthesis. _Sci Rep_ 9, 20117 (2019).
https://doi.org/10.1038/s41598-019-56575-9 Download citation * Received: 02 October 2019 * Accepted: 08 December 2019 * Published: 27 December 2019 * DOI:
https://doi.org/10.1038/s41598-019-56575-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
