A cross-reactive mouse monoclonal antibody against rhinovirus mediates phagocytosis in vitro
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Rhinoviruses (RVs) are the main cause of the common cold worldwide. To date, more than 160 types of the virus have been recognized, categorized into three major species - A, B, and
C. There are currently no approved vaccines available to prevent infection with RVs. To elicit antibodies against conserved regions located on capsid proteins of RV A viruses, mice were
sequentially vaccinated with DNA plasmids encoding capsid proteins of different RV A types. After a final boost with whole virus, antibody-expressing hybridomas were generated. After
isotyping, 11 monoclonal antibodies (mAbs) expressing an IgG subtype Fc-domain were selected for further expansion and purification. Three mAbs showed cross-reactivity against multiple
strains of RV A viruses by ELISA, including strains A1A, A1B, A15, A16 and A49. Other mAbs had strain-specific binding patterns, with the majority of mAbs showing reactivity to RV-A15, the
strain used for the final vaccination. We found that the RV-A15-specific mAbs, but not the cross-reactive mAbs, had neutralizing activity against RV-A15. An antibody dependent cellular
phagocytosis (ADCP) assay revealed substantial ADCP activity for one of the cross-reactive mAbs. Epitope mapping of the neutralizing mAbs via escape mutant virus generation revealed a shared
binding epitope on VP1 of RV-A15 for several neutralizing mAbs. The epitope of the ADCP-active, non-neutralizing mAb was determined by microarray analysis of peptides generated from the VP1
capsid protein. VP1-specific, cross-reactive antibodies, especially those with ADCP activity, could contribute to protection against RV infections. SIMILAR CONTENT BEING VIEWED BY OTHERS
PROFILING OF HMPV F-SPECIFIC ANTIBODIES ISOLATED FROM HUMAN MEMORY B CELLS Article Open access 10 May 2022 FUNCTIONAL AND STRUCTURAL BASIS OF HUMAN PARAINFLUENZA VIRUS TYPE 3 NEUTRALIZATION
WITH HUMAN MONOCLONAL ANTIBODIES Article 10 June 2024 A SELF-AMPLIFYING RNA RSV PREFUSION-F VACCINE ELICITS POTENT IMMUNITY IN PRE-EXPOSED AND NAÏVE NON-HUMAN PRIMATES Article Open access 14
November 2024 INTRODUCTION Rhinoviruses (RVs) belong to the family of _Picornaviridae_ and are known as a leading cause of respiratory infections. These viruses can also cause acute
exacerbations of asthma and chronic obstructive pulmonary disease (COPD)1,2. Despite considerable efforts in past decades, no vaccines or therapeutics have yet been approved to combat RV
infection3,4. The major obstacles in RV vaccine development are the large number of types and the lack of an appropriate animal model for preclinical evaluation of vaccine candidates5,6,7.
Currently more than 160 types of RVs have been identified8. Based on genetic diversity and phylogenetic sequence analysis, these types are classified into three species: RV A, B, and C9. So
far, three different cellular membrane glycoproteins have been recognized as binding receptors for RVs. These include the intercellular adhesion molecule 1 (ICAM-1, used by the majority of
RV A, and all RV B types), the low-density lipoprotein receptor family members (LDLR, used by the minority of RV A types), and the cadherin-related family member 3 (CDHR3; used by RV C)10.
The genomic RNA of RVs is surrounded by an icosahedral capsid shell that consists of 60 copies of four proteins: VP1, VP2, VP3, and VP4. The outer surface of this capsid is made up of VP1,
VP2, and VP3, whereas VP4 is localized internally at the interface between the capsid and the viral genome11. These three exterior capsid proteins form a canyon structure that allows RV
viruses which bind to ICAM-1 to engage their receptor on the surface of target host cells12,13,14. Antibodies raised against the capsid proteins of RVs are the primary host defense against
RV infection15. VP1 is the most exposed surface protein, and plays a critical role in viral antigenicity and induction of neutralizing antibodies16. Although neutralizing antibodies elicited
by infection can reduce viral replication, only limited cross-protection against heterologous strains is provided because of the large antigenic diversity of RVs17. Previous attempts to
establish cross-type protection using vaccines containing multiple conserved regions of the virus had some success in eliciting neutralizing responses18,19,20,21. Despite these early
successes, whether or not viable cross-reactive targets for cross-protective vaccines exist remains an open question. To further identify potential future vaccination target epitopes, we
utilized a sequential immunization strategy in mice with heterologous RV A antigens. In this study, we identified three cross-reactive monoclonal antibodies (mAbs). While these mAbs did not
exhibit neutralizing activity, one mAb interestingly showed a high level of activity in an antibody-dependent cellular phagocytosis (ADCP) assay. RESULTS HYBRIDOMA GENERATION AND SCREENING
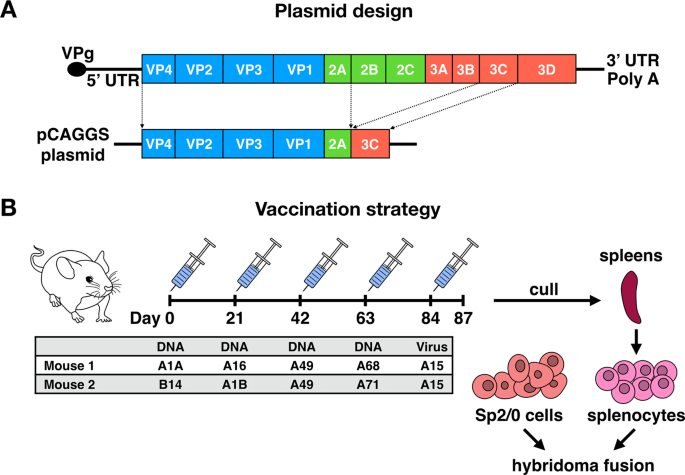
To generate hybridomas, two female BALB/c mice were sequentially vaccinated with recombinant pCAGGS plasmids encoding diverse capsid proteins and two proteases of RVs, to facilitate proper
protein cleavage (Fig. 1A). The immunizations were followed by a final boost with purified whole virus using the RV-A15 strain (Fig. 1B). Each round of vaccination was performed with
plasmids encoding for a single type of RV as illustrated in the table in Fig. 1B. Since RVs cannot bind murine ICAM-1, intramuscular injection of RV-A15 was not expected to result in virus
replication, but solely to provide an antigenic stimulus. Hybridomas were screened for RV reactivity and Ig isotypes. Eleven IgG mAbs were selected for further characterization based on
robust reactivity against at least one of the RV types used during the immunization regimen. Of these mAbs, 3 showed cross-reactivity against multiple types of RV A viruses by ELISA,
including A1A, A1B, A15, A16 and A49 (Fig. 2A,B). Additional mAbs showed strain-specific binding patterns, with the majority showing reactivity to RV-A15, the virus used for the final
vaccination (Fig. 2B, Supplementary Fig. 1). STRAIN-SPECIFIC, BUT NOT CROSS-REACTIVE ANTIBODIES, SHOW NEUTRALIZING ACTIVITY To characterize the functionality of these mAbs, we first explored
their neutralizing activity against a panel of RV types. Using a microneutralization assay, we found that five RV-A15-specific mAbs were highly neutralizing, with IC50 values ranging from
0.827 μg/ml for mAb HRV-18011 to 2.638 μg/ml for mAb HRV-18009. Interestingly, none of the cross-reactive mAbs (HRV-18003, HRV-18004, and HRV-18005), showed neutralizing activity (Fig. 3A,
Supplementary Fig. 2). A CROSS-REACTIVE MAB SHOWS POTENT ADCP ACTIVITY In addition to neutralizing activity, Fc-mediated effector functions are becoming an important topic for antiviral
immunity. Therefore, we investigated the capacity of the RV-A15-reactive mAbs to induce ADCP activity in a mouse macrophage cell line _in vitro_ (Supplementary Fig. 3). This flow-cytometry
based ADCP assay revealed a high degree of ADCP activity for mAb HRV-18003 (on the order of ~30-fold signal over background), one of the cross-reactive mAbs we identified (Fig. 3B). The
other two cross-reactive mAbs, HRV-18004 and HRV-18005, scored markedly lower in ADCP activity (~10-fold and 8-fold signal over background, respectively). Among the RV-A15-specific
neutralizing mAbs, HRV-18009 appears to have the highest ADCP activity (~10-fold signal over background). We observed a similarly strong ADCP activity for HRV-18003 against RV-A1A and RV-A16
(Supplementary Fig. 4). RV-A15-NEUTRALIZING MABS BIND A SHARED EPITOPE ON VP1 To identify the targets of the RV-A15-specific mAbs, the 6 RV-A15-specific and 3 cross-reactive mAbs were
tested against recombinantly expressed VP1-VP4 capsid proteins of RV-A15 (Fig. 4). The ELISA results showed that 2 cross-reactive mAbs and 4 RV-A15-specific, neutralizing mAbs bound to the
recombinant VP1 protein. Only weak binding was observed for cross-reactive mAb HRV-18005, while the other two RV-A15-specific mAbs did not bind to any of the recombinant proteins,
potentially indicating binding sites that span across multiple proteins. The residues critical for RV-A15-neutralizing mAbs binding were identified through the generation of escape mutant
viruses. After serial passage in the presence of mAb and subsequent sequencing, two critical residues were identified for mAbs HRV-18007, HRV-18010, and HRV-18011. The mutations were
observed on the βG strand and the GH loop of VP1 of RV-A15 (A190S-H208L). Only the H208L residue changed in the escape mutants for mAbs HRV-18008 and HRV-18009 (Fig. 5). In order to confirm
that the escape mutants had truly “escaped” mAb binding, microneutralization assays were performed on the five escape mutant viruses against all five mAbs. The results revealed no
neutralization of escape mutant viruses by the tested mAbs, further confirming a shared binding epitope of the neutralizing mAbs on the VP1 capsid protein (Supplementary Fig. 5).
CROSS-REACTIVE MABS HRV-18003 AND HRV-18004 BIND A CONSERVED EPITOPE ON VP1 We next aimed to characterize the binding of the cross-reactive mAbs. In addition to the ELISA against recombinant
VP proteins, a reducing SDS-PAGE under denaturing conditions, followed by Western blotting was performed. Both mAbs HRV-18003 and HRV-18004 showed reactivity against a protein with the
approximate size of 37 KDa for multiple RV types, consistent with the size of VP1 (Supplementary Fig. 6). The other cross-reactive mAb, HRV-18005, only showed reactivity to whole RV
particles under non-denaturing conditions, suggesting that the epitope for that mAb was disrupted under denaturing conditions (data not shown). The epitope of the strongly ADCP-active,
non-neutralizing mAb HRV-18003, was further investigated by a microarray analysis using overlapping cyclic peptides with lengths of 7, 10 or 13 amino acids (aa) derived from the VP1 capsid
protein. A moderate to strong response profile at moderate signal-to-noise ratios was observed against peptides with the consensus motifs HISDLKIHYE, YYMFYD and PRPPRAVE (Fig. 6A). Despite
showing similar peak intensities, visual assessment showed the clearest spot morphologies for peptides with the consensus motif PRPPRAVE, which was therefore regarded as the most likely
epitope of mAb HRV-18003. The binding of peptides with the consensus motifs HISDLKIHYE and YYMFYD presumably resulted from non-specific interaction with multiple aromatic and acidic amino
acids. By aligning the VP1 sequences of RV-A15 (bound by mAb HRV-18003) and RV68 (not bound by mAb HRV-18003), we identified a single amino acid difference in the putative epitope PRPPRAVE
(P253A). We therefore generated a mutant RV-A15 VP1 introducing the alanine of RV-A68. Further, we produced both a wildtype RV-A68 VP1 protein and a mutant protein in which the alanine was
replaced with a proline (Fig. 6B). We then tested binding of both cross-reactive mAbs HRV-18003 and HRV-18004 by ELISA against these recombinant proteins. Both mAbs recognized wildtype
RV-A15 VP1, but lost binding to the RV-A15 mutant VP1. RV-A15-specific mAb HRV-18008 - which binds a different epitope as described above – showed binding against both wildtype and mutant
VP1 of RV-A15 (Fig. 6C,D). Furthermore, when the point mutation was introduced into RV-A68 VP1, binding of mAbs HRV-18003 and HRV-18004 was observed (Fig. 6E,F). These data suggest that mAbs
HRV-18003 and HRV-18004 both bind to the epitope PRPPRAVE on VP1. Interestingly, based on a mapping of the epitope on the structure of RV-A1A (utilizing PDB accession no. 1R1A), the P253A
mutation is not exposed on the surface of the virion (Supplementary Fig. 7). This could indicate that the epitope is only exposed during virion “breathing”22, which could help explain the
lack of neutralizing activity by the cross-reactive antibodies. DISCUSSION In the present study, we utilized a sequential DNA vaccination approach expressing the capsid proteins of
heterologous types of RV, followed by a final whole virus boost with RV-A15 to elicit cross-reactive antibodies. Interestingly, a large proportion of mAbs isolated from the spleens of
vaccinated mice were specific to the final vaccine strain, which was administered only 3 days prior to splenectomy. Four of the neutralizing RV-A15-specific mAbs were isolated from the same
mouse and likely were clonally related, based on their binding of a shared epitope on VP1. However, mAb HRV-18011 was isolated from a different mouse, but bound the same epitope. Based on
the rapid response, it is possible that the epitope is immunodominant in BALB/c mice. We also isolated 3 cross-reactive mAbs that did not show neutralizing activity. Two of these mAbs bound
a shared epitope on VP1, which was identified using a peptide microarray and further confirmed by testing binding to mutant recombinant VP1 proteins. Based on mapping of the identified
epitope, it is not exposed on the virion surface. It is therefore possible that the mAbs can only bind to the epitope during viral “breathing”, which could expose epitopes otherwise buried
within the capsid23,24,25,26. The third antibody did not bind well to the tested recombinant protein and showed binding by Western blot only under non-denaturing conditions, which could
indicate binding to a conformational epitope spanning multiple proteins or to a non-capsid protein. Importantly, since these antibodies do not seem to interfere with viruses binding to the
host cells, it is possible that their protective effect would be infection permissive, similar to Fc-mediated protection by antibodies against other viral infections27. The binding of the
cross-reactive antibodies did not span all types of RV A. It would be therefore important to identify additional epitopes that could broaden cross-reactive responses against all types of RV
A. Since RV C viruses especially are a major cause of asthma, antibodies targeting similar conserved epitopes in these viruses could fill an important therapeutic gap. Interestingly, a
recent editorial by Stepanova _et al_. identified a number of conserved epitopes (including epitopes in the C-terminal region of VP1, similar to the epitope bound by mAb HRV-18003) that
could enable the generation of additional cross-reactive antibodies28. Further, systematic vaccination experiments focusing on specific RV clusters could reveal more detailed information on
the cross-reactive potential of mAbs. Importantly, one of the cross-reactive mAbs (HRV-18003) showed strong activity in an _in vitro_ ADCP assay. Recent advances in understanding the
mechanisms of antibody functions have revealed the critical importance of Fc-mediated effector activities such as ADCP and ADCC in clearance of virus and virus-infected cells29,30. ADCC
would likely not contribute to protection against RV, since the capsid proteins are not presented on the surface of infected cells until the cell is lysed and virus released. However, ADCP
could increase the uptake of RV virions by immune cells, which could reduce viral load, facilitate antigen presentation, and stimulate the secretion of inflammatory mediators31,32. It is
important to note that the ADCP assay in this study does not assess the functional reduction in infectious viral titers, which will require further investigation. It is possible that during
the epitope bound by mAb HRV-18003 is preferentially exposed upon conjugation to beads, contributing to the observed effect. An earlier study has shown that human polyclonal antibody
responses target an epitope distinct from the one identified here, that is also on VP1 and similarly not exposed on the virion surface33. It would be interesting to test the ability of these
antibodies to mediate ADCP, to investigate if they may contribute to protection through non-traditional mechanisms. If future studies indicate a potential protective role for these
antibodies, ADCP may be an interesting mechanism to be explored in addition to neutralization for vaccine development against RV. Importantly, Fc-mediated effector functions such as ADCP are
substantially affected by the IgG subtype34. All mAbs used in this study (including the negative control), with the exception of HRV-18009 (IgG2b) were IgG2a – which have both been shown to
mediate similar levels of Fc-receptor engagement. The observed differences were therefore likely mediated by the affinity and/or specificity of the antibody Fab-domain. The differences
observed between mAbs HRV-18003 and HRV-18004 were most likely due to differences in affinity since they both bind to the same epitope and express the same Fc-domain. However, the
differences observed compared to some of the strong binding RV-A15-specific mAbs could be epitope-specific. It has been previously shown for other viruses that Fc-mediated effector functions
can be epitope-specific, which remains to be explored for RVs35. In summary, we have discovered cross-reactive mAbs that bind a conserved epitope on VP1. Further, one cross-reactive mAb
(HRV-18003) demonstrated a high level of ADCP activity _in vitro_, which may be a mechanism that could be explored for future vaccine development against RV. METHODS CELLS AND VIRUSES HeLa
H1 cells (ATCC CRL-1958) were grown in complete Dulbecco’s modified Eagle medium (DMEM; Life Technologies) supplemented with antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin
[Pen-Strep]; Gibco), and 10% fetal bovine serum (FBS; HyClone). Hybridoma cell culture was performed as previously described36. Briefly, SP2/0 mouse myeloma cells were cultured in complete
DMEM supplemented with antibiotics (Pen-Step; cDMEM) prior to fusion with primary mouse splenocytes. Monoclonal hybridoma cells were initially grown in Clonacell-HY Medium E (Stemcell
Technologies) and subsequently switched to serum-free hybridoma medium (Hybridoma-SFM; Life Technologies) for antibody production. RAW 264.7 macrophages (ATCC TIB-71) were cultured in RPMI
1640 containing 10% FBS. RVs 1A, 1B, 14, 15, 16, 49, 68, and 71 were purchased from ATCC and passaged in HeLa H1 cells. To create purified viruses, infected cell cultures were lysed by
freeze/thawing and clarified supernatants harvested after low-speed centrifugation (at 3,000 g for 30 min at 4 °C) to remove cellular debris. Viruses were pelleted through a 30% sucrose
cushion (30% sucrose in NTE buffer (100 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA), pH 7.4) by ultracentrifugation (Beckman L7-65 ultracentrifuge with SW-28 rotor at 25,000 rpm for 5 h). Once all
of the supernatant was aspirated, virus pellets were resuspended in phosphate-buffered saline (PBS; Life Technologies). CONSTRUCTION OF PLASMIDS The polyprotein coding region of P1-2A (2.3
kb) and 3C viral protease (639 bp) of RVs A1A, A1B, B14, A16, A49, A68, and A71 were amplified using corresponding primers. The PCR products of P1-2A and 3C were cloned into a pCAGGS vector
plasmid using In-Fusion cloning (Takara Bio; Fig. 1A). Positive clones were selected by colony PCR and the plasmids confirmed by nucleotide sequencing. To generate recombinant capsid
proteins (VP1-4) of RV-A15 and mutant VP1 of RV-A15 and RV-A68 wt and mutant VP1 (sequences of mutant VP1s are shown in Fig. 6B), the corresponding fragments were amplified by PCR. A
C-terminal His6 tag was further added to the amplicons, as previously described20,37. The PCR products were cloned into the pQE30 expression vector (Qiagen, Hilden, Germany), and the
constructs were screened by sequencing. Protein expression in _Escherichia coli_ SG13009 (pREP4) bacteria containing the pQE30 recombinant vector was induced by addition of
isopropyl-β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM. Soluble recombinant protein was purified on Ni-nitrilotriacetic acid agarose columns using Ni-NTA Fast Start
Kit (Qiagen, Hilden, Germany) under native conditions according to the manufacturer’s instructions. The purified proteins were verified by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and confirmed with Western blot analysis using anti-His antibody (Qiagen, Hilden, Germany) (Supplementary Fig. 8). The concentrations of the purified VPs were
determined by Bradford protein assay testing (Bio-Rad, Hercules, CA). GENERATION AND SCREENING OF MABS Six- to eight-week-old female BALB/c mice (n = 2) were immunized with 75 μg DNA
encoding P1-2A and 3C of different strains of RVs in pCAGGS plasmids (Fig. 1B). Injections were performed bilaterally with 50 μl per leg (total volume of 100 μl). Each animal received four
vaccinations, 3 weeks apart, via intramuscular electroporation in the medial thigh using a TriGridDelivery System from Ichor Medical Systems. Mice were bled 4 weeks post each vaccination to
monitor antibody responses against RVs (Supplementary Fig. 9). Three days prior to hybridoma fusion, mice were boosted with 50 μg of purified RV-A15 (Fig. 1B). The final boost followed a
short interval before spleen harvest aims to boost B cells, but to elicited only limited _de novo_ responses. B cell hybridomas were generated as previously described36. All animal
procedures were approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee (IACUC) and performed in accordance with the guidelines set by the
committee. SCREENING OF HYBRIDOMA SUPERNATANTS Hybridoma supernatants were screened by ELISA (Enzyme-linked immunosorbent assay) for reactivity with multiple strains of RVs (purified, whole
virus) including 1A, 1B, 14, 15, 16, 49, 68, and 71. All wells that had activity (defined as signal over background) against any virus were then isotyped using a Pierce rapid antibody
isotyping kit (Life Technologies). The isotyped mAbs expressing IgG heavy-chain subclasses were selected for further expansion and purification, as previously described38. All mAbs, except
for HRV-18009 (IgG2b) were IgG2a. BINDING CHARACTERIZATION (ELISA) ELISAs were performed as previously described39. Briefly, Microtiter 96-well plates (Immulon 4HBX; Thermo Fisher
Scientific) were coated with 5 μg/ml purified RVs or 2 μg/well of recombinant VP1-VP4 proteins (50 µl/well) from RV-A15 or mutant or wt. of RV-A15 or RV-A68 diluted in coating solution (KPL,
Gaithersburg, MD), and were incubated at 4 °C overnight. Plates were washed 3x with phosphate-buffered saline (PBS; Gibco) containing 0.1% Tween 20 (PBS-T; Fisher) and then blocked with
PBS-T containing 3% goat serum (Life Technologies, Inc.) and 0.5% milk powder (blocking solution) for 1 h. Three-fold antibody dilutions were performed in blocking solution at a
concentration of 30 μg/mL in a final well volume of 100 μL. After 2 h plates were washed 3x with PBS-T, followed by 1 h staining with goat anti-mouse IgG Fc-chain horse radish peroxidase
secondary antibody (abcam, ab98717) diluted 1:10,000 in blocking solution at a final well volume of 50 μl. Plates were washed 4x with PBS-T, followed by development with SigmaFast
o-phenylenediamine dihydrochloride (OPD; Sigma). The reaction was stopped with 3 M hydrochloric acid. Binding of antibodies to the antigens was detected at an optical density (OD) of 490 nm
using by a Synergy H1 hybrid multimode microplate reader (BioTek). MICRONEUTRALIZATION (MN) ASSAY To evaluate the functionality of the mAbs against different types of RVs, a MN assay based
on measuring viability of HeLa cells using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was used40. Using MTS over crystal violet solution
allowed for a more incremental quantification using OD measurements. First, TCID50s (50% tissue culture infectious dose) of each RV strain were measured on Hela cells. The cells were seeded
at the concentration of 3.5*105 /mL 24 hours prior to the assay in 96-well plates. At the time of the assay, the viruses were serially diluted (half-log10 dilutions) in Hela infectious media
(10X MEM supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin [Pen-Strep]; Gibco), and 10% fetal bovine serum (FBS; HyClone), 1% Non-essential amino acids (NEAA; Gibco), and 1% 3 M
MgCl2), added to the cells (100 μl per well), and incubated at 33 °C for 1 h. After incubation, the cells were washed and overlaid with 100 μl of infectious media and incubated at 33 °C for
72 hours. After an incubation period, virus replication was visualized by measuring the viability of cells with an MTS assay kit (abcam, ab197010), as described previously40. All the tests
were done in triplicate. TCID50/ml was calculated according to the Reed-Muench method. To perform the MN assay, 100× TCID50 (50% tissue culture infectious dose) of each RV strain were mixed
with 2-fold serial dilutions of each mAb (starting at the concentration of 120 μg/mL, 2-fold dilution) in HeLa infectious media and incubated to allow binding of the antibodies to the
viruses for 1 h at room temperature (RT). The virus-mAb mixture was then added onto HeLa cells and incubated at 33 °C for 1 h. After the incubation period, the virus-mAb mixture was removed
and replaced with diluted mAbs at the previous concentration. After an incubation period of 72 h, virus replication was measuring as described above using an MTS assay kit. Plates were read
at an OD of 490 nm and the IC50 of each mAb for each virus was calculated in GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA). ANTIBODY-DEPENDENT CELLULAR PHAGOCYTOSIS (ADCP)
ASSAY The assay was performed according to previously described methods41,42.To measure ADCP, viruses (RV-A15, RV-A1A or RV-A16) were biotinylated using an EZ-Link NHS-PEG4-Biotin kit
(Thermo Fisher Scientific) and conjugated to FluoSpheres NeutrAvidin-Labeled Microspheres beads (0.2 μm, yellow-green, Life Technologies) according to manufacturer’s instructions. Antibodies
(2-fold diluted from 200 μg/ml to 25 μg/ml) were incubated with beads for 2 h at 37 °C. RAW cells were added at a concentration of 1.0 × 105 cells/well and incubated for 1 h at 37 °C. Cells
were analyzed by flow cytometry on a Gallios flow cytometer (Beckman Coulter), and fold-increase compared to cells with RV-conjugated beads only was calculated based on the gating strategy
shown in Supplementary Fig. 3. All mAb samples were tested in duplicate. WESTERN BLOTTING Binding of mAbs to RV protein was analyzed on SDS-PAGE (4–20% polyacrylamide; Mini Protean TGX gels;
Bio-Rad) under reducing conditions. Briefly, the protein samples were boiled for 5 minutes at 100 °C in 2× Laemmli sample buffer (Bio-Rad) containing 5% β-mercaptoethanol and subjected to
SDS-PAGE electrophoresis, and then transferred onto nitrocellulose membranes using the iBlot 2 dry blot system (Thermo Fisher) at 20 V for 7 min. Following blocking with 3% skim milk for 2 h
at RT, the membrane was incubated with primary antibody overnight at 4 °C. After three washes with 1×PBST buffer (1× phosphate-buffered saline, 0.05% Tween 20), HRP-conjugated goat
anti-mouse IgG (abcam) was added to the membrane and incubated at RT for 1 h. The positive bands were visualized by electrochemiluminescence (ECL) reagent (Life Technologies) and developed
using1-Step Ultra TMB-Blotting Solution (Life Technologies). GENERATION OF RV ESCAPE MUTANTS AGAINST NEUTRALIZING MABS Monoclonal antibody escape mutants of RV-A15 were generated as
previously described with some modifications36,43. Initially, HeLa cells in 6-well tissue culture plates (Sigma) were infected with RV-A15 at a multiplicity of infection (MOI) of 1 in the
presence of 1×IC50 of mAb (performed in duplicate for each mAb) in Minimum Essential Medium (MEM; Life Technologies). After incubation for 72 h at 33 °C, 100 μl of supernatant was collected
and used to directly inoculate a fresh monolayer of HeLa cells in the presence of a 2-fold increase in the mAb concentration. This process was repeated for seven passages, until the final
concentration of mAb was 128×IC50. Throughout serial passaging, successful infection was confirmed by the presence of cytopathic effect. To control for mutants obtained from passaging alone,
the virus was also passaged in the presence of an irrelevant mouse mAb, and without mAb. Escape mutants were plaque purified once serial passaging was completed to create stocks for
sequencing. EPITOPE MAPPING OF HRV-18003 USING A PEPTIDE MICROARRAY The epitope of mAb HRV-18003 in the VP1 capsid protein of RV-A15 was mapped using peptide microarray technology performed
by PEPperPRINT (PEPperCHIP Platform Technology, Heidelberg, Germany). Briefly, the sequence of VP1 capsid protein of RV-A15 was translated into 7, 10 and 13 amino acid peptides with a
peptide-peptide overlap of 6, 9 and 12 amino acids, respectively. The resulting peptide microarrays contained 885 different cyclic peptides printed in duplicate (1,770 peptide spots), and
were framed by additional HA (YPYDVPDYA) control peptides. Blocking, washing, and incubation procedures were performed using Rockland blocking buffer MB-070 (VWR International Frankfurt, DE)
(PBS plus 0.05% Tween 20, PBS-Tween plus 10% Rockland blocking buffer, respectively). Mouse mAb HRV-18003 was incubated at a concentration of 0.1 µg/ml in incubation buffer followed by
staining with the secondary antibody (Goat anti-mouse IgG-DyLight680, New England Biolabs, Frankfurt, DE). DATA AVAILABILITY The datasets generated during the current study are available
from the corresponding author upon request. REFERENCES * Mikhail, I. & Grayson, M. H. Asthma and viral infections: An intricate relationship. _Annals of Allergy, Asthma & Immunology_
123, 352–358, https://doi.org/10.1016/j.anai.2019.06.020 (2019). Article Google Scholar * Blaas, D. & Fuchs, R. Mechanism of human rhinovirus infections. _Molecular and cellular
pediatrics_ 3, 1–4, https://doi.org/10.1186/s40348-016-0049-3 (2016). Article Google Scholar * Papi, A. & Contoli, M. Rhinovirus vaccination: the case against. _Eur Respir J_ 37, 5–7,
https://doi.org/10.1183/09031936.00145710 (2011). Article CAS PubMed Google Scholar * Makris, S. & Johnston, S. Recent advances in understanding rhinovirus immunity. _F1000Research_
7, https://doi.org/10.12688/f1000research.15337.1 (2018). * Bartlett, N. W. _et al_. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. _Nature
medicine_ 14, 199, https://doi.org/10.1038/nm1713 (2008). Article CAS PubMed PubMed Central Google Scholar * Han, M. _et al_. Small Animal Models of Respiratory Viral Infection Related
to Asthma. _Viruses_ 10, 682, https://doi.org/10.3390/v10120682 (2018). Article CAS PubMed Central Google Scholar * Glanville, N. & Johnston, S. L. Challenges in developing a
cross-serotype rhinovirus vaccine. _Current opinion in virology_ 11, 83–88, https://doi.org/10.1016/j.coviro.2015.03.004 (2015). Article CAS PubMed Google Scholar * Palmenberg, A. C.
& Gern, J. E. In _Rhinoviruses_ 1–10 (Springer, 2015). * Palmenberg, A. C. _et al_. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution.
_Science_ 324, 55–59, https://doi.org/10.1126/science.1165557 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Basnet, S., Palmenberg, A. C. & Gern, J. E.
Rhinoviruses and their receptors. _Chest_ 155, 1018–1025, https://doi.org/10.1016/j.chest.2018.12.012 (2019). Article PubMed PubMed Central Google Scholar * Arnold, E. & Rossmann, M.
G. Analysis of the structure of a common cold virus, human rhinovirus 14, refined at a resolution of 3.0 Å. _J Mol Biol_ 211, 763–801, https://doi.org/10.1016/0022-2836(90)90076-X (1990).
Article CAS PubMed Google Scholar * Rossmann, M. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. _Journal of Biological
Chemistry_ 264, 14587–14590 (1989). CAS PubMed Google Scholar * Plevka, P., Perera, R., Cardosa, J., Kuhn, R. J. & Rossmann, M. G. Structure determination of enterovirus 71. _Acta
Crystallographica Section D: Biological Crystallography_ 68, 1217–1222, https://doi.org/10.1107/S0907444912025772 (2012). Article CAS Google Scholar * Fuchs, R. & Blaas, D. Uncoating
of human rhinoviruses. _Reviews in medical virology_ 20, 281–297, https://doi.org/10.1002/rmv.654 (2010). Article CAS PubMed Google Scholar * Peltola, V., Waris, M., Kainulainen, L.,
Kero, J. & Ruuskanen, O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. _Clinical Microbiology and Infection_ 19,
E322–E327, https://doi.org/10.1111/1469-0691.12193 (2013). Article CAS PubMed Google Scholar * Smith, T. J. In _Molecular Biology of Picornavirus_ (eds. Bert Semler & Eckard Wimmer)
Ch. 4, 39–49 (American Society of Microbiology, 2002). * McLean, G. R. Vaccine strategies to induce broadly protective immunity to rhinoviruses. _Human vaccines & immunotherapeutics_,
1–3, https://doi.org/10.1080/21645515.2019.1661207 (2019). * Hamory, B. H., Hamparian, V. V., Conant, R. M. & Gwaltney, J. M. Jr. Human Responses to Two Decavalent Rhinovims Vaccines.
_Journal of Infectious Diseases_ 132, 623–629 (1975). Article CAS Google Scholar * Lee, S. _et al_. A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques.
_Nat Commun_ 7, 12838, ARTN 12838, https://doi.org/10.1038/ncomms12838 (2016). * Edlmayr, J. _et al_. Antibodies induced with recombinant VP1 from human rhinovirus exhibit
cross-neutralisation. _European Respiratory Journal_ 37, 44–52, https://doi.org/10.1183/09031936.00149109 (2011). Article CAS PubMed Google Scholar * Glanville, N. _et al_.
Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. _PLoS pathogens_ 9, e1003669, https://doi.org/10.1371/journal.ppat.1003669 (2013). Article CAS
PubMed PubMed Central Google Scholar * Roy, A. & Post, C. B. Long-distance correlations of rhinovirus capsid dynamics contribute to uncoating and antiviral activity. _Proceedings of
the National Academy of Sciences_ 109, 5271–5276 (2012). Article ADS CAS Google Scholar * Katpally, U., Fu, T.-M., Freed, D. C., Casimiro, D. R. & Smith, T. J. Antibodies to the
buried N terminus of rhinovirus VP4 exhibit cross-serotypic neutralization. _Journal of virology_ 83, 7040–7048, https://doi.org/10.1128/JVI.00557-09 (2009). Article CAS PubMed PubMed
Central Google Scholar * Valbuena, A., Rodríguez-Huete, A. & Mateu, M. G. Mechanical stiffening of human rhinovirus by cavity-filling antiviral drugs. _Nanoscale_ 10, 1440–1452,
https://doi.org/10.1039/c7nr08704g (2018). Article CAS PubMed Google Scholar * Reisdorph, N. _et al_. Human rhinovirus capsid dynamics is controlled by canyon flexibility. _Virology_
314, 34–44, https://doi.org/10.1016/S0042-6822(03)00452-5 (2003). Article CAS PubMed Google Scholar * Katpally, U. & Smith, T. J. Pocket factors are unlikely to play a major role in
the life cycle of human rhinovirus. _Journal of virology_ 81, 6307–6315, https://doi.org/10.1128/JVI.00441-07 (2007). Article CAS PubMed PubMed Central Google Scholar * Schotsaert, M.
_et al_. Long-Lasting Cross-Protection Against Influenza A by Neuraminidase and M2e-based immunization strategies. _Sci Rep_ 6, 24402, https://doi.org/10.1038/srep24402 (2016). Article ADS
CAS PubMed PubMed Central Google Scholar * Stepanova, E., Isakova-Sivak, I. & Rudenko, L. Overview of human rhinovirus immunogenic epitopes for rational vaccine design. _Expert Rev
Vaccines_ 18, 877–880, https://doi.org/10.1080/14760584.2019.1657014 (2019). Article CAS PubMed Google Scholar * Pollara, J. & Tay, M. Z. Antibody-Dependent Cellular Phagocytosis in
Antiviral Immune Responses. _Front Immunol_ 10, 332, ARTN 332, https://doi.org/10.3389/fimmu.2019.00332 (2019). * Bournazos, S., DiLillo, D. J. & Ravetch, J. V. The role of Fc–FcγR
interactions in IgG-mediated microbial neutralization. _Journal of Experimental Medicine_ 212, 1361–1369, https://doi.org/10.1084/jem.20151267 (2015). Article CAS PubMed Google Scholar *
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors as regulators of immune responses. _Nature Reviews Immunology_ 8, 34 (2008). Article CAS Google Scholar * Pincetic, A. _et al_. Type I
and type II Fc receptors regulate innate and adaptive immunity. _Nature immunology_ 15, 707, https://doi.org/10.1038/ni.2939 (2014). Article CAS PubMed Google Scholar * Niespodziana, K.
_et al_. Misdirected antibody responses against an N-terminal epitope on human rhinovirus VP1 as explanation for recurrent RV infections. _The FASEB journal_ 26, 1001–1008 (2012). Article
CAS Google Scholar * Michaelsen, T. E., Kolberg, J., Aase, A., Herstad, T. K. & Hoiby, E. A. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic
activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. _Scand J Immunol_ 59, 34–39, https://doi.org/10.1111/j.0300-9475.2004.01362.x
(2004). Article CAS PubMed Google Scholar * He, W. _et al_. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A
virus. _Proc Natl Acad Sci USA_ 113, 11931–11936, https://doi.org/10.1073/pnas.1609316113 (2016). Article CAS PubMed Google Scholar * Wohlbold, T. J. _et al_. Broadly protective murine
monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. _Nature microbiology_ 2, 1415, https://doi.org/10.1038/s41564-017-0011-8 (2017). Article CAS
PubMed PubMed Central Google Scholar * Coughlan, L. _et al_. _In vivo_ retargeting of adenovirus type 5 to αvβ6 integrin results in reduced hepatotoxicity and improved tumor uptake
following systemic delivery. _Journal of virology_ 83, 6416–6428 (2009). Article CAS Google Scholar * Wohlbold, T. J. _et al_. Hemagglutinin stalk-and neuraminidase-specific monoclonal
antibodies protect against lethal H10N8 influenza virus infection in mice. _Journal of virology_ 90, 851–861, https://doi.org/10.1128/Jvi.02275-15 (2016). Article CAS PubMed Google
Scholar * Rajendran, M. _et al_. Analysis of anti-influenza virus neuraminidase antibodies in children, adults, and the elderly by ELISA and enzyme inhibition: evidence for original
antigenic sin. _MBio_ 8, e02281–02216, https://doi.org/10.1128/mBio.02281-16 (2017). Article CAS PubMed PubMed Central Google Scholar * Zheng, H. _et al_. A novel neutralizing antibody
specific to the DE loop of VP1 can inhibit EV-D68 infection in mice. _The Journal of Immunology_ 201, 2557–2569 (2018). Article CAS Google Scholar * Ackerman, M. E. _et al_. A robust,
high-throughput assay to determine the phagocytic activity of clinical antibody samples. _J Immunol Methods_ 366, 8–19, https://doi.org/10.1016/j.jim.2010.12.016 (2011). Article CAS PubMed
Google Scholar * Hioe, C. E. _et al_. Modulation of Antibody Responses to the V1V2 and V3 Regions of HIV-1 Envelope by Immune Complex Vaccines. _Front Immunol_ 9, 2441,
https://doi.org/10.3389/fimmu.2018.02441 (2018). Article CAS PubMed PubMed Central Google Scholar * Duehr, J. _et al_. Novel cross-reactive monoclonal antibodies against Ebolavirus
glycoproteins show protection in a murine challenge model. _Journal of virology_ 91, e00652–00617 (2017). Article CAS Google Scholar Download references AUTHOR INFORMATION Author notes *
James Duehr Present address: University of Pittsburgh School of Medicine, Pittsburgh, PA, USA AUTHORS AND AFFILIATIONS * Department of Microbiology, Icahn School of Medicine at Mount Sinai,
New York, NY, USA Mohammad Amin Behzadi, Angela Choi, James Duehr, Michael Schotsaert, Peter Palese & Raffael Nachbagauer * Global Health and Emerging Pathogens Institute, Division of
Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, USA Angela Choi & Michael Schotsaert * Graduate School of Biomedical Sciences, Icahn School of Medicine at
Mount Sinai, New York, NY, USA Angela Choi * Department of Medicine, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, USA Roya Feyznezhad, Chitra
Upadhyay & Peter Palese Authors * Mohammad Amin Behzadi View author publications You can also search for this author inPubMed Google Scholar * Angela Choi View author publications You
can also search for this author inPubMed Google Scholar * James Duehr View author publications You can also search for this author inPubMed Google Scholar * Roya Feyznezhad View author
publications You can also search for this author inPubMed Google Scholar * Chitra Upadhyay View author publications You can also search for this author inPubMed Google Scholar * Michael
Schotsaert View author publications You can also search for this author inPubMed Google Scholar * Peter Palese View author publications You can also search for this author inPubMed Google
Scholar * Raffael Nachbagauer View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.A.B., P.P. and R.N. conceived the study. M.A.B., C.U., M.S.
and R.N. designed experiments. M.A.B., A.C., R.F. and J.D. performed experiments. M.A.B., A.C., M.S. and R.N. reviewed and interpreted data. M.A.B., A.C. and R.N. drafted the manuscript and
all authors contributed to the finalization of the manuscript. All authors had full access to the data and gave final approval before submission. CORRESPONDING AUTHOR Correspondence to
Raffael Nachbagauer. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard
to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Behzadi, M.A., Choi, A., Duehr, J. _et al._ A cross-reactive mouse
monoclonal antibody against rhinovirus mediates phagocytosis _in vitro_. _Sci Rep_ 10, 9750 (2020). https://doi.org/10.1038/s41598-020-66600-x Download citation * Received: 23 December 2019
* Accepted: 22 May 2020 * Published: 16 June 2020 * DOI: https://doi.org/10.1038/s41598-020-66600-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
