Trisk 95 as a novel skin mirror for normal and diabetic systemic glucose level
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Developing trustworthy, cost effective, minimally or non-invasive glucose sensing strategies is of great need for diabetic patients. In this study, we used an experimental type I diabetic
mouse model to examine whether the skin would provide novel means for identifying biomarkers associated with blood glucose level. We first showed that skin glucose levels are rapidly
influenced by blood glucose concentrations. We then conducted a proteomic screen of murine skin using an experimental in vivo model of type I diabetes and wild-type controls. Among the
proteins that increased expression in response to high blood glucose, Trisk 95 expression was significantly induced independently of insulin signalling. A luciferase reporter assay
demonstrated that the induction of Trisk 95 expression occurs at a transcriptional level and is associated with a marked elevation in the Fluo-4AM signal, suggesting a role for intracellular
calcium changes in the signalling cascade. Strikingly, these changes lead concurrently to fragmentation of the mitochondria. Moreover, Trisk 95 knockout abolishes both the calcium flux and
the mitochondrial phenotype changes indicating dependency of glucose flux in the skin on Trisk 95 function. The data demonstrate that the skin reacts robustly to systemic blood changes, and
that Trisk 95 is a promising biomarker for a glucose monitoring assembly.
In view of its dynamic behaviour in the presence of multiples stress, the human skin is widely used to test cellular and molecular responses to specific treatments. Mirroring the biological
and physiological internal changes in healthy and disease environments via the skin is the new revolutionary approach. The paradigm is based on the skin ultrastructure, consisting a sensory
neuron network in tight connection with the skin keratinocytes1. Moreover, there are numerous clinical studies describing the skin features related to pancreas diseases2,3, rheumatic
disorders4 and lung diseases5. Recent reports have revealed the presence of specific biomarkers in the skin that may serve for early diagnosis of neuronal conditions such as Alzheimer’s and
Parkinson’s diseases6. In addition, acute or chronic ulcers, which are the most widely described skin features related to type I diabetes, are associated with reduced skin wound healing and
an increased risk of infections7,8,9,10. These facts have meant that new biomarkers and non-invasive devices for glucose monitoring in the skin have emerged on the market in the last
decade11,12,13,14. In fact, the currently most widely used devices require skin penetration as far as the interstitial fluid, and are thereby invasive, although a new generation of devices
based on the measurement of various metabolites in sweat such as sodium, lactate, potassium and glucose has been proposed recently15,16,17,18. One major drawback with such devices, however,
is the amount of sweat required, resulting in secondary effects such as skin irritation. In view of all the devices and the skin features to high glucose, we propose a new paradigm that
operates by converting the skin keratinocytes cells to serve as biosensors for monitoring blood glucose.
Keratinocytes are the main cell type (≈ 90%) constituting the epidermis19,20, and with respect to their key roles in wound healing21, they have been extensively studied, notably in cases of
diabetes22,23,24. Reports have demonstrated that impaired keratinocytes function can result in delayed wound healing, and that multiple physiological processes in keratinocytes, such as
proliferation25, migration26, apoptosis27 and differentiation28, may be affected by a hyperglycaemic condition. Moreover, the skin barrier dysfunction29,30 and increased inflammation31,32,33
brought about by a hyperglycaemic environment will prevent the keratinocytes from healing; probably leading to continuously infected severe skin lesions34,35. In view of the literature,
skin keratinocytes could be used as indicators of the status of normal and diabetic skin.
We set out here to investigate whether blood glucose concentration could be monitored by means of keratinocytes and whether this sensing activates specific biomarkers and their signalling
pathways. Our results show that Trisk 95 expression is increased in the skin and its primary keratinocytes following an increase in blood glucose concentration. In turn, upregulation of
Trisk 95 is associated with an increase in the intracellular calcium level, which then triggers a particular modification in the morphology of the mitochondrial network.
Our data suggest that keratinocytes could serve as promising biological sensors for developing a new generation of blood glucose monitoring devices, especially for use with diabetic
patients.
Little is known about the responses of epidermal cells to increased blood glucose concentrations or whether a particular signalling network is activated in these cells. To test this
hypothesis, we first injected glucose into healthy and type I diabetic mice, the latter having been prepared as previously described36. Briefly, C57BL/6 mice were divided into two groups:
(healthy) controls, which were injected intraperitoneally with citrate buffer (pH 4.5), and type I diabetic mice, which received one single dose of 150 mg/kg streptozotocin (STZ). Blood
glucose was monitored every 48 h and the mice were considered diabetic when its level was ≥ 15 mmol/l (data not shown). The first step was to select a suitable end-point at which the blood
glucose level in the healthy mice reaches that found in the diabetic mice (≥ 15 mmol/l), as this can be used for collecting the skin samples. For this purpose, we performed a glucose
tolerance test (GTT) on the healthy mice. After fasting for 12 h, the mice were divided into two groups: a control group in which PBS was injected intraperitoneally, and a treatment group
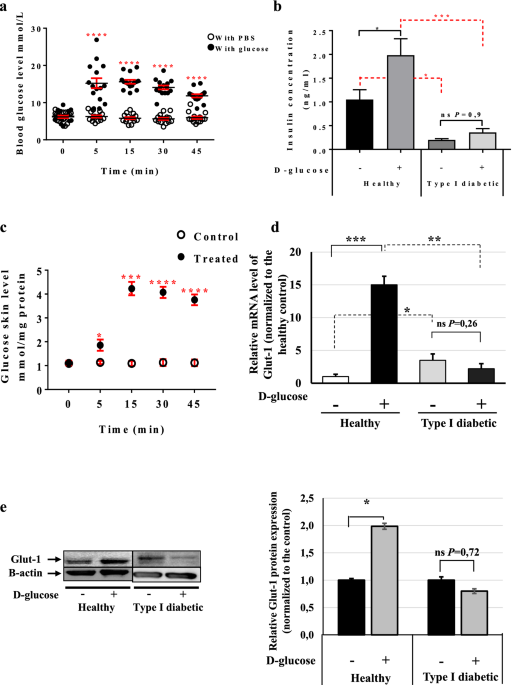
that received 2 g/kg d-glucose. Serum was collected from both groups (15 animals/group) at time-points 0, 5, 15, 30 and 45 min and blood glucose levels were monitored. The results showed a
significant increase in blood glucose at 5 min post-injection in the glucose-injected mice (15.20 mmol/l ± 1.36) (Fig. 1a, full circle) as compared with the placebo-treated mice (6.27 mmol/l
± 0.31) (Fig. 1a, empty circles). The glucose concentration remained at this level up to the 45 min time-point and then started to drop (Fig. 1a). However, no changes in the blood glucose
were observed in the glucose- and placebo-injected type I diabetic mice (Supplementary Fig. 1a and Table 1).
Skin rapidly senses any increase in blood glucose. (a) GTT assay using C57BL/6 mice. The mice were divided into two groups: the control group (empty circle) was injected IP with PBS, and the
treated group (full circle) with 2 g/kg d-glucose diluted in PBS. Blood glucose levels were monitored at the given time points (n = 15 mice/group). (b) Insulin concentrations in healthy and
type I diabetic mice. Insulin levels at 45 min post-injection were measured using the Insulin Rodent Chemiluminescence ELISA technique (n = 6 mice/group). (c) Skin glucose levels in vivo.
The GTT assay was performed and skin biopsies were collected at 0, 5, 15, 30 and 45 min post-injection (n = 7 mice/group). Glucose levels were measured using the YSI 2,950 Biochemistry
Analyzer (YSI Life Sciences) and normalized to the total concentration of proteins (mmol/mg protein). (d) Glut-1 mRNA levels at 45 min post-injection in the skin of both healthy and type I
diabetic mice was quantified by qRT-PCR. The results are shown as averages after normalization to the controls ± SD (n = 6/group). (e) Glut-1 protein expression level at 45 min
post-injection was determined then quantified by western blotting and b-actin was used as a loading control. Two-way ANOVA analysis was performed in a using GraphPad Prism software, ****P
