Sublingual indocyanine green films for non-invasive swallowing assessment and inflammation detection through NIR/SWIR optical imaging
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Indocyanine green (ICG) is the most commonly used FDA-approved agent for clinical optical imaging, administered through injections only, due to its poor membrane permeability. Although ICG
has vast potential for non-invasive non-radioactive imaging in patients, the clinical applications are limited by the invasive administration and short half-life in blood circulation. To
expand the clinical value of ICG, non-toxic chitosan-based ICG-loaded films were designed for sublingual administration for near-infrared (NIR) and short-wave infrared (SWIR) optical
imaging. Two film formulations were developed with different ICG release rates. Mold-casted self-emulsifying films rapidly released ICG (80% in 4 h) in the form of nanosized droplets, which
were mostly swallowed and produced significant contrast of upper digestive tract to enable in vivo swallowing evaluations using NIR/SWIR imaging. Regular films released ICG slowly (80% in 25
h), allowing for steady absorption of ICG to systemic circulation. Inflammation in mouse feet was detected within 30 min after sublingual administration with a 1.43-fold fluorescence
increase within 1 h at the inflammation sites, comparable to a 1.76-fold increase through intravenous injection. Administering ICG using sublingual films displayed notable potential for
non-invasive diagnosis and monitoring of inflammatory conditions and swallowing disorders, addressing a current need for alternatives to ICG parenteral administration.
Fluorescence imaging within the near infrared window (NIR, 700–1,000 nm) has enabled new technologies for preclinical and clinical applications1. Compared with conventional diagnostic
imaging techniques (e.g. X-ray computed tomography, positron emission tomography, magnetic resonance imaging), NIR imaging provides low cost and allows for real-time high-sensitivity
molecular imaging, without ionizing radiation2. A variety of NIR fluorophores are commercially available, including indocyanine green (ICG) which has been approved by the U.S. Food and Drug
Administration (FDA) for human clinical use in angiography, blood flow evaluation and hepatic function assessment3,4. ICG is remarkably promising as an optical imaging contrast as it can
also be detected under short-wave infrared wavelengths (SWIR, 1,000–2000 nm)5,6, conferring significant improvement in imaging sensitivity and light penetration depth, while substantially
reducing tissue autofluorescence5,7.
The administration of ICG in the clinical setting is currently performed either intravenously (i.v.) or intradermally, requiring invasive procedures which limits utilization in sensitive
populations, such as pediatric patients8,9,10. Furthermore, this approach requires immediate imaging post contrast administration or multiple contrast dosing for monitoring due to the rapid
clearance of ICG in systemic circulation11. Yet, there is an absence of formulations on the market that allow for the administration of ICG in a more convenient and patient-friendly manner,
without need for multiple and invasive administrations. The choice of route of administration in this case is limited by the biopharmaceutical characteristics of ICG. The oral route,
non-invasive and usually preferred from a patient compliance perspective, cannot be reasonably considered for ICG administration due to its perceived poor oral bioavailability12, directly
associated with the dye’s low membrane permeability, gastric instability, first-pass hepatic metabolism and fast excretion from liver to bile11,13. When administered orally, ICG is absorbed
in the digestive system, accessing the hepatic portal system and being carried to the liver via portal vein before it reaches systemic circulation. Molecules displaying high liver extraction
rates such as ICG (hepatic extraction rate in healthy humans ≈ 70%14,15) have their blood concentrations greatly reduced prior to reaching systemic circulation, and therefore greatly
reducing bioavailability. In this sense, the sublingual route has many advantages when compared with oral that can assist increasing systemic exposure of ICG: (1) Sublingual rate of
absorption is higher due to thinner membrane thickness16,17, allowing for easier and faster mass transfer; (2) low degree of keratinization17, facilitating permeability; and most-importantly
(3) molecules absorbed from the buccal cavity have direct access to the systemic circulation via jugular vein, by-passing first-pass hepatic metabolism associated with oral absorption and
increasing bioavailability18,19.
In order to address the current gap in novel technologies for patient-friendly administration of ICG, our group developed ICG film formulations for sublingual administration with
mucoadhesive properties20. We hypothesized sublingual films could promote enhanced systemic exposure of ICG by promoting sublingual absorption while steadily releasing the dye in a sustained
manner. Furthermore, due to the intended sustained release capabilities of these sublingual films, their potential use for optical imaging-guided dysphagia assessment was also explored as
excess dye (not absorbed from the sublingual space) would likely promote optical contrast within the upper gastrointestinal tract as it is swallowed with saliva.
Considering ICG displays high aqueous solubility3, absorption is mainly limited by the absorption rate rather than dye dissolution. In that sense, an immediate release oral dosage form (e.g.
conventional tablet) would not promote adequate systemic delivery of ICG as the entire dose is available for absorption at once but kinetically limited by slow absorption of ICG crossing
the gastrointestinal wall into the blood circulation21,22. Sublingual films containing ICG may foster systemic exposure by steadily releasing smaller doses of ICG into the sublingual
vasculature over a prolonged period of time, partially bypassing first-pass metabolism with direct absorption. Portion of the released ICG dose may be swallowed, allowing for diagnosing
dysphagia and other esophageal disorders via optical imaging. Systemically absorbed ICG will facilitate constant monitoring of the vasculature and can be used to monitor inflammation and
disease progression due to the prolonged release of the film formulations. Chitosan-based mucoadhesive films were selected as platform of choice due to flexibility of use, easy of
application and potential of being employed for controlled release purposes. Chitosan has been selected as film-forming mucoadhesive polymer for this platform as it has been reported to be
non-toxic for oral applications23 and it is capable of fostering mucoadhesion via electrostatic interactions with negatively charged mucin proteins present in mucus23,24,25.
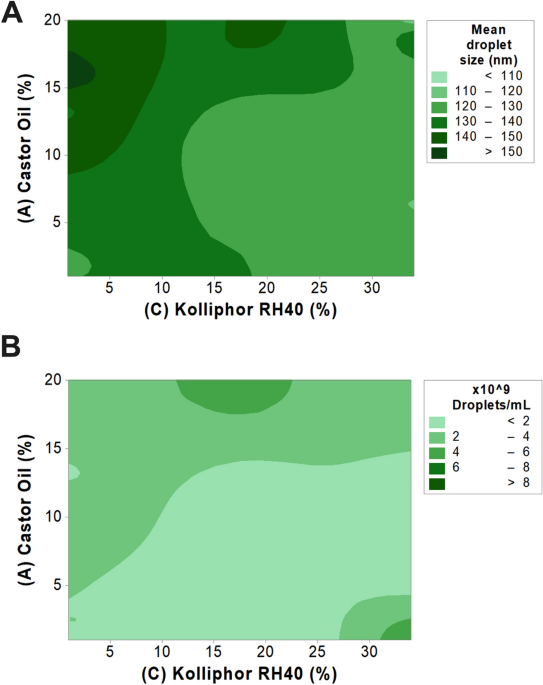
A Quality by Design (QbD) approach was taken to investigate the impact of critical SEDDS components on droplet size and concentration. SEDDS components and concentration ranges were
determined based on previous studies conducted by our group26. A mathematical model was developed by means of a Response Surface Methodology design to describe changes in responses as a
function of the composition of the microemulsion. The final experimental matrix, with the respective factors, levels and responses found, is provided in Table 1.
The mathematical model describing the mean droplet size was found significant (p = 0.0006), whereas two main factors showed relevant influence: (A) castor oil (p = 0.0481) and (C) Kolliphor
RH 40 (p
