Capabilities of multi-pinhole spect with two stationary detectors for in vivo rat imaging
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We aimed to investigate the image quality of the U-SPECT5/CT E-Class a micro single-photon emission computed tomography (SPECT) system with two large stationary detectors for
visualization of rat hearts and bones using clinically available 99mTc-labelled tracers. Sensitivity, spatial resolution, uniformity and contrast-to-noise ratio (CNR) of the small-animal
SPECT scanner were investigated in phantom studies using an ultra-high-resolution rat and mouse multi-pinhole collimator (UHR-RM). Point source, hot-rod, and uniform phantoms with
99mTc-solution were scanned for high-count performance assessment and count levels equal to animal scans, respectively. Reconstruction was performed using the similarity-regulated
ordered-subsets expectation maximization (SROSEM) algorithm with Gaussian smoothing. Rats were injected with ~ 100 MBq [99mTc]Tc-MIBI or ~ 150 MBq [99mTc]Tc-HMDP and received multi-frame
micro-SPECT imaging after tracer distribution. Animal scans were reconstructed for three different acquisition times and post-processed with different sized Gaussian filters. Following
reconstruction, CNR was calculated and image quality evaluated by three independent readers on a five-point scale from 1 = “very poor” to 5 = “very good”. Point source sensitivity was 567
cps/MBq and radioactive rods as small as 1.2 mm were resolved with the UHR-RM collimator. Collimator-dependent uniformity was 55.5%. Phantom CNR improved with increasing rod size, filter
size and activity concentration. Left ventricle and bone structures were successfully visualized in rat experiments. Image quality was strongly affected by the extent of post-filtering,
whereas scan time did not have substantial influence on visual assessment. Good image quality was achieved for resolution range greater than 1.8 mm in bone and 2.8 mm in heart. The recently
introduced small animal SPECT system with two stationary detectors and UHR-RM collimator is capable to provide excellent image quality in heart and bone scans in a rat using standardized
reconstruction parameters and appropriate post-filtering. However, there are still challenges in achieving maximum system resolution in the sub-millimeter range with in vivo settings under
limited injection dose and acquisition time. SIMILAR CONTENT BEING VIEWED BY OTHERS PERFORMANCE EVALUATION OF A PRECLINICAL SPECT/CT SYSTEM FOR MULTI-ANIMAL AND MULTI-ISOTOPE QUANTITATIVE
EXPERIMENTS Article Open access 28 October 2022 THE USEFULNESS OF SWIFTSCAN TECHNOLOGY FOR BONE SCINTIGRAPHY USING A NOVEL ANTHROPOMORPHIC PHANTOM Article Open access 29 January 2021
EVALUATION OF RADIOMICS FEATURE STABILITY IN ABDOMINAL MONOENERGETIC PHOTON COUNTING CT RECONSTRUCTIONS Article Open access 15 November 2022 INTRODUCTION Preclinical single-photon emission
computed tomography (SPECT) imaging is an evolving field full of challenges. The introduction of pinhole collimation opened up new applications for small-animal SPECT imaging1,2,3 and with
the development of multi-pinhole collimation, high spatial resolution in the sub-millimeter range with acceptable sensitivity was possible3,4,5,6. Combining sub-millimeter precision imaging
with a plethora of easily accessible radioisotopes and the option of detecting multiple radioisotopes simultaneously set SPECT apart from its competitor positron emission tomography (PET)7.
Further, the use of stationary detectors covering 360° of a fixed field of view (FOV) enables dynamic studies with increased precision8,9, while reducing mechanical issues and complex system
maintenance compared to a setup with moving parts10. The previous generations of these ultra-high-resolution SPECT systems usually have three large stationary detectors in a triangular
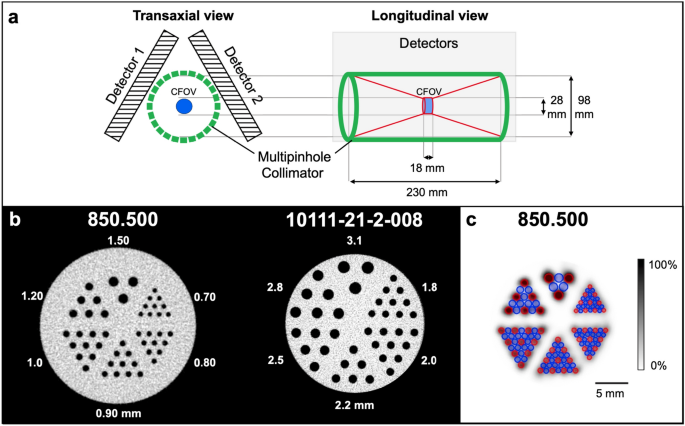
configuration10,11. For this work, however, we assessed the image quality of a cost-efficient scanner that omits the bottom detector. High spatial resolution can improve the diagnostic value
of SPECT scans if sufficient image quality is achieved, which is particularly affected by the choice of collimator12, reconstruction algorithm5,13, post-reconstruction filter13 and
injection dose14. Hence, we evaluated the influence of acquisition time and Gaussian post-filtering, using a novel iterative reconstruction algorithm with similarity-regulated
ordered-subsets expectation maximization (SROSEM), that enables constant reconstruction parameters for a wide range of activity concentrations15. Basic and translational researchers have
been mainly using small-animal models such as mice or rats to reduce housing and maintenance costs, to explore pathophysiology and to develop new drugs. Rats have certain potential
advantages over mice16. It is easier to develop suitable invasive devices for surgical procedures and hemodynamic measurements and offers larger tissue mass for histological and biological
analyses. Furthermore, recent transfer of functional genomics technology into rats reemphasizes the potential of rat models16. For animal SPECT imaging, although bigger size of the organs is
an advantage of rats over mice, the higher soft tissue attenuation and scattering, as well as requirement of larger bore and transaxial size, might have negative impact on precise imaging.
This study aims to examine the preclinical applicability and image quality of the recently introduced micro-SPECT system for rat imaging under in vivo conditions using [99mTc]Tc-MIBI17 and
[99mTc]Tc-HMDP18,19 with a pre-set SROSEM reconstruction algorithm and Gaussian post-filtering. MATERIALS AND METHODS SYSTEM DESCRIPTION U-SPECT5/CT E-Class (referred to as “U-SPECT5-E”;
MILabs, Utrecht, The Netherlands) is an ultra-high-resolution SPECT system for preclinical imaging of small- to medium-sized animals. While the scanner architecture is based on previous
generations of micro-SPECT systems (U-SPECT-II; U-SPECT+; MILabs)10,11, the conventional U-SPECT5 system features three stationary detectors with a size of 472 mm × 595 mm and 9.5 mm thick
thallium-doped sodium iodide (NaI(Tl)) scintillation crystals arranged in a triangular format around the FOV. Striving for cost effectiveness, the U-SPECT5-E is built without the bottom
detector as illustrated in Fig. 1a. A centrally located collimator with multi-pinhole configuration allows the acquisition of SPECT images in a spiral step mode using the XYZ stage20. In
this study, an ultra-high resolution rat/mouse (UHR-RM) collimator made of tungsten was used with 75 pinholes (diameter of 1.0 mm) in 5 rows, all pointing towards a center FOV of 28 mm
diameter and 18 mm length. Due to the missing bottom detector only the upper 50 pinholes contribute to the imaging process. DATA ACQUISITION AND PROCESSING All images were acquired in
list-mode and reconstructed with the SROSEM algorithm. For simplifying the reconstruction process, we used four iterations with 128 subsets and a voxel size of 0.4 mm3, subsequently applying
Gaussian post-filtering as advised by Vaissier et al.15. This approach showed no perceptible decrease in image quality compared to the reference standard of maximum likelihood expectation
maximization (MLEM)21. For scatter correction, we applied the triple energy window method22 with a photopeak window of 126 keV to 154 keV, a lower background window of 120.4 keV to 126 keV
and an upper one of 154 keV to 159.6 keV. The obtained SPECT images were transferred to a workstation for further Gaussian post-filtering and analysis using AMIDE (version 1.0.5 for MacOS;
open source)23. PERFORMANCE MEASUREMENTS Sensitivity was examined by using a point source with a 99mTc-solution of 2.7 MBq. Scan time was 5 min with 1 bed position (BP). Calculation was
based on the National Electrical Manufacturers Association (NEMA)24 with \({R}_{i}\) representing the detected photopeak countrate and \({A}_{cal}\) standing for the total activity of the
point source determined by a dose calibrator (ISOMED 2010, NUVIA Instruments, Dresden, Germany). $$ Sensitivity = \frac{{R_{i} }}{{A_{cal} }} $$ Figure 1b shows the mini Derenzo hot-rod
phantom (850.500) that was used to investigate the spatial resolution. It was filled with a 99mTc-solution with an activity concentration of 285.2 MBq/mL. Scan time was 45 min with 9 BP. In
the visual analysis of the reconstructed data, maximum resolution was assessed as the smallest distinguishable rod size. Uniformity was investigated by using the cylindrical container of the
mini Derenzo phantom. The filling volume was 10.6 mL with a 99mTc-solution of 315.0 MBq and the scan time was 45 min with 9 BP. Gaussian post-filtering was applied with a full width at half
maximum (FWHM) of 1.2 mm representing the maximum resolution. For calculation, a cylindrical region of interest (ROI) was placed centrally in the phantom, measuring 18 mm in diameter and 10
mm in length. Collimator-dependent system uniformity was calculated as recommended by NEMA24. $$ Uniformity \left( \% \right) = 100 \times \frac{Max\;count - Min\; count}{{Max\;count + Min
\;count}} $$ PHANTOM IMAGE QUALITY To evaluate the in vitro image quality, a contrast-to-noise ratio (CNR) analysis was carried out by using two mini Derenzo phantoms. This technique, as
shown in Fig. 1c, was described initially by Walker et al.25. Using a high-resolution computed tomography (CT) image as a template, ROIs of 6 mm length were placed in the center of the rods.
All ROIs have a diameter of 0.9 times the size of the respective radioactive rod. In addition, ROIs of the same size were placed in the non-radioactive regions in-between two radioactive
rods. The contrast \({C}_{d}\) was defined as: $$ C_{d} = \frac{{\overline{{R_{d} }} - \overline{{B_{d} }} }}{{\overline{{R_{d} }} }} $$ \(\stackrel{-}{{R}_{d}}\) is the mean value of all
radioactive ROIs and \(\stackrel{-}{{B}_{d}}\) is the mean value of all non-radioactive ROIs for the rod size \(d\). The noise \({N}_{d}\) was defined as: $$ N_{d} = \frac{{\sqrt
{\sigma_{{R_{d} }}^{2} + \sigma_{{B_{d} }}^{2} } }}{{\overline{{ROIs_{d} }} }} $$ \({\sigma }_{{R}_{d}}\) and \({\sigma }_{{B}_{d}}\) are the standard deviations in radioactive and
non-radioactive ROIs, while \(\stackrel{-}{{ROIs}_{d}}\) is the mean value of all ROIs of a rod size \(d\), no matter if they are radioactive or non-radioactive. These two formulas are used
to calculate the CNR: $$ CNR_{d} = \frac{{C_{d} }}{{N_{d} }} $$ This image quality analysis was performed for the smaller 850.500 and the larger 10111-21-2-008 phantom with a rod size range
of 0.7–1.5 mm and 1.8–3.1 mm, respectively. In addition to high-count performance measurements with ~ 290.0 MBq/mL, low-count measurements were also conducted (~ 1.0 MBq/mL, ~ 0.5 MBq/mL, ~
0.1 MBq/mL). To minimize the loss of resolution, images were optimized by a Gaussian post-filter for each rod size. The kernel size of the filter always corresponded to FWHM = rod size.
ANIMAL STUDIES Animal protocols were approved by the local Animal Care and Use Committee (Regierung von Unterfranken, Germany) and conducted according to the Guide for the Care and Use of
Laboratory Animals26. [99MTC]TC-HMDP BONE RAT IMAGING One healthy female Wistar rat (Charles River Laboratories, Sulzfeld, Germany) with 217.0 g body weight was injected 154.86 MBq of
[99mTc]Tc-HMDP18,19 via tail vein. Acquisition started at 1 h post-injection with a total scan time of 90 min containing 18 5-min-frames with 20 s time per bed position (TPB) and 15 BP.
During acquisition, the rat underwent an inhalation anesthesia (2.0% isoflurane, 1.5 L O2/min). To determine the CNR in the reconstructed bone images, one radioactive ROI was placed
centrally in the pelvic bone on each side and a corresponding non-radioactive ROI was placed in the background at 4 mm distance. All ROIs in the bone scan were box-shaped and had a size of
0.8 × 8.0 × 1.2 mm3 (Fig. 2a). This analysis was applied to the focused bone scan with reconstructed frame lengths of 5, 30 and 90 min. The three reconstructions were analyzed unfiltered and
for five different Gaussian post-filters (1.2, 1.8, 2.2, 2.8 and 3.5 mm). [99MTC]TC-MIBI CARDIAC RAT IMAGING Another healthy female Wistar rat (Charles River Laboratories), weighing 231.5
g, was injected 108.51 MBq of [99mTc]Tc-MIBI17 into the tail vein. Twenty-five minutes after injection, a total-body scan was performed with an acquisition time of 60 min, divided into six
10-min-frames (TPB 15 s, BP 40). While scanning, an inhalation anesthesia (2.0% isoflurane, 1.5 L O2/min) was carried out. By using the heart uptake of [99mTc]Tc-MIBI described in the
literature with 1.71 ± 0.63%ID/g for the myocardium of the rat27, we calculated the expected activity in the rat heart to match it with the phantom studies, taking into account the different
TPB. For the reconstructed data, three radioactive ROIs were placed centrally in the wall of the left ventricle and three non-radioactive ROIs were each positioned 4 mm away from the
corresponding radioactive ROI in the background outside of the heart. All ROIs in the heart scan were box-shaped and had a size of 4.0 × 0.8 × 2.0 mm3 (Fig. 2b). This analysis was applied to
the whole-body heart scan with frame lengths of 10, 30 and 60 min. Again, all three images were analyzed unfiltered and for five different Gaussian post-filters (1.2, 2.2, 2.8, 3.5 and 4.0
mm). IN VIVO IMAGE QUALITY CONTRAST-TO-NOISE RATIO The CNR was calculated in the same way as described above for the mini Derenzo phantoms. However, instead of using the ROIs for one
specific rod diameter \(d\), the ROIs for the respective animal scan were employed. VISUAL IMAGE QUALITY ASSESSMENT All images were sent to three independent readers for visual assessment of
image quality as vertical long axis view (heart) and coronal view (bone). The images were doubled and randomized. Observers were blinded to the acquisition and post-processing protocol and
were asked to rate the overall image quality on a five-point scale (1 = “very poor”, 2 = “poor”, 3 = “moderate”, 4 = “good”, 5 = “very good”). STATISTICS Statistical analysis was carried out
with specialized software (SPSS Statistics Version 27 for MacOS, IBM, Amonk, New York, USA). Kolmogorov–Smirnov tests were applied to assess normal distribution of continuous variables.
Categorical variables are presented as percentages, frequencies and median values with interquartile range (IQR), e.g. for image quality scale results. RESULTS PERFORMANCE MEASUREMENTS Point
source sensitivity examined with technetium-99m for the UHR-RM collimator was 567 cps/MBq (0.057%). In visual analysis of the hot-rod phantoms’ rod sections, a minimum diameter of 1.2 mm
could be discriminated, what was assumed to represent the maximum resolution (Fig. 3a). Uniformity for the UHR-RM collimator was 55.5% in accordance with the NEMA protocol. Figure 3b,c
illustrate reconstructed image and corresponding line profile. IN VITRO IMAGE QUALITY Figure 4a provides an illustration of the phantom images used for CNR analysis. For the lower activity
an increased image noise is visible, while contrast remains fairly constant. Figure 4b shows the dependency of the CNR on the investigated rod size for four different activity
concentrations. While measurements in rods smaller than 1.2 mm mostly resulted in very low, partly negative CNR values (− 0.31 to 0.48), only 300 MBq/mL achieved a higher value below the
maximum resolution (1.41 at 1.0 mm). CNR of 1.50 was achieved for 300 MBq/mL, 1.0 MBq/mL, 0.5 MBq/mL and 0.1 MBq/mL at rod sizes of 1.2 mm, 1.8 mm, 2.0 mm and 2.8 mm, respectively. For all
activity concentrations, CNR improved continuously with increasing rod size and Gaussian filtering. IN VIVO IMAGE QUALITY [99MTC]TC-MIBI CARDIAC RAT IMAGING SPECT images of the investigated
healthy rat heart for different acquisition times (10, 30 and 60 min) and various post-reconstruction Gaussian filters (FWHM of 1.2, 2.2, 2.8, 3.5 and 4.0 mm) are displayed in vertical long
axis view in Fig. 5a. The images from all three perspectives (horizontal long axis, short axis, vertical long axis) are shown in supplemental Fig. 1. No artifacts were detected. Left
ventricle of the heart is clearly visualized as the myocardium shows sufficient uptake of [99mTc]Tc-MIBI. Images of each scan time without any filtering result in poor image quality and high
noise. Gaussian post-filtering clearly improved image quality for each scan. The results for the in vivo contrast-to-noise ratio and image quality assessment of the healthy rat heart are
summarized in Fig. 5b,c. Increasing the FWHM up to 3.5 mm of the Gaussian filtering results in improved CNR. The highest values were achieved for the 10-min scan with 4.0 mm and for the
30-min and 60-min scan with 3.5 mm kernel size, respectively (Fig. 5b). Comparing the three different acquisition times and therefore different count levels, peak values for CNR increased
substantially from 10 min (6.3) to 60 min (12.2). Peak image quality scores for the investigated scan times were 5.00 (IQR = 0.00) for 10 min and 30 min with FWHM of 3.5 and 4.0 mm, 5.00
(IQR = 0.75) for 60 min with FWHM of 4.0 mm (Fig. 6c). All images without filtering received the lowest rating “very poor”. FWHM = 1.2 mm scored in the range of “very poor” to “moderate”,
2.2 mm “poor” to “good” and 2.8 mm “moderate” to “very good”. [99MTC]TC-HMDP BONE RAT IMAGING Reconstructed images of the healthy rat’s lower spine and pelvis region with various Gaussian
filters (FWHM = 1.2, 1.8, 2.2, 2.8 and 3.5 mm) and three different scan times (5, 30 and 90 min) are shown from the coronal view in Fig. 6a. The images from all three perspectives
(transverse, coronal, sagittal) are shown in supplemental Fig. 2. No artifacts were detected. Differentiation of vertebra and intervertebral discs was feasible, the pelvic bones were
visualized in detail. Images without any filtering appeared noisy and provided poor detail. Nevertheless, higher count levels and longer acquisition time led to an overall improvement of
image quality. By increasing the post-reconstruction Gaussian filter, image quality was further enhanced. High filtering resulted in homogenous and smooth images but provided less detail and
seemed overly blurred. Figure 6b depicts results of the in vivo CNR analysis. Irrespective of acquisition time, Gaussian filtering of 3.5 mm was associated with the highest CNR values.
However, no clear difference in absolute CNR values could be determined by extending the acquisition time. Figure 6c plots the image quality scores of images shown in Fig. 6a as a function
of Gaussian kernel size. Peak image quality scores of 5.00 for the investigated scan times were achieved for 5 min and FWHM of 2.8 and 3.5 mm, for 30 min and FWHM of 2.2, 2.8 and 3.5 mm and
for 90 min and FWHM ≥ 1.8 mm. Quality of unfiltered images scored 1.00 (IQR = 0.00) for every acquisition time by all readers. Ratings for kernel size 1.2 mm ranged from “poor” to
“moderate”. Image quality for FWHM of 1.8 mm was considered “moderate” to “good”. The images filtered with a kernel size of 2.2, 2.8 and 3.5 mm were all rated “moderate” to “very good”.
DISCUSSION We investigated to what extent the recently introduced two-detector U-SPECT5-E with UHR-RM collimator and the novel SROSEM algorithm is suitable for ultra-high-resolution rat
imaging in a realistic in vivo setting. In the performance evaluation, the SPECT system achieved a sensitivity of 567 cps/MBq, a resolution of 1.2 mm and a uniformity of 55.5%. This was
comparable to the previous generation with three detectors and a similar multi-pinhole rat collimator, which achieved a sensitivity of 700 cps/MBq and a resolution of 0.8 mm. It should be
noted that an activity concentration of 600 MBq/mL was used10. No direct comparison was made between the two-detector system and the three-detector system regarding the rat imaging
capabilities. Such comparison might reveal the impact of the missing lower detector in more detail. Boisson et al. evaluated a system with rotating detectors and three different rat
collimators with maximum resolutions in the range of 1.1–2.0 mm but sensitivities below 300 cps/MBq. They only used either one or three pinholes28. For another established system with four
detectors, three rat collimators were reported with maximum resolutions in the range of 1.1–1.9 mm and sensitivities of up to more than 2000 cps/MBq29, but more detailed methodological
information is lacking and the recently published study on the new generation of the system contains mainly information on the mouse collimators30. However, it should be noted that the
activity concentrations in these performance evaluations using phantoms are much higher than the activity concentrations under realistic in vivo conditions. In the in vitro CNR analysis,
values were considerably lower for small rod sizes and lower activity concentrations and increased with larger rod size and post-filtering. Similar observations were made for pinhole PET by
Walker et al.25. The Gaussian post-filtering results in a maximum resolution (FWHM) limited by the kernel size of the filter. The CNR values in phantom measurements (Fig. 4) were
substantially lower at low activities compared to high activities. To achieve a CNR similar to values in rods of 1.2 mm at 300 MBq/mL, larger rods and filters were required in the in vivo
study count range. Hence, reasonable CNR values were achieved for 1.0 MBq/mL with ≥ 1.8 mm, for 0.5 MBq/mL with ≥ 2.0 mm and for 0.1 MBq/mL with ≥ 2.8 mm rod size. Multi-observer analysis of
the longest in vivo scans (60 and 90 min) gives a count range comparable to approximately 1.0 MBq/mL in phantom studies. Good CNR and image quality scores were found for the SPECT images
with Gaussian kernels bigger than 1.8 mm in bone and 2.8 mm in the rat heart. This implies that the maximum resolution that can realistically be achieved for in vivo imaging is slightly
lower than the system resolution of 1.2 mm. Analysis of in vivo heart studies revealed a substantial increase in CNR between 10 and 30 min, while extension of acquisition time to 60 min
resulted in only a minor increase. A similar tendency was seen in the visual image analysis, where the scan time did not lead to a relevant image quality increase and the longest scan time
of 60 min even resulted in inferior image quality ratings. This loss of image quality could be the result of slightly more intensive scaling of the 60 min images. In contrast to acquisition
time, the choice of filter size had considerably more effect on subjective ratings. While 4.0 mm led to the best results for short scan times in the heart, the best CNR value could be
achieved with a longer scan time and a filter of 3.5 mm. The filters of 3.5 mm or 4.0 mm also provided the best subjective image quality for professional readers. Nonetheless, even a filter
of 2.2 mm delivered acceptable image quality and can be suitable if the additional resolution is required for certain imaging tasks. Mizutani et al. also investigated the influence of
injection dose and post-filtering on image quality in the rat heart. A SPECT system with cadmium-zinc telluride detectors and two different five-pinhole collimators was used (sensitivity =
321 cps/MBq, 139 cps/MBq; resolution = 1.5 mm, 1.2 mm). The evaluation by two readers showed an increase in image quality by higher injection dose (25–200 MBq) and stronger filters (no
filter; FWHM = 1.5 mm, 2.5 mm)14. The injection dose had more impact on the image quality score than we found for the scan time, but the results are still largely consistent with ours. In
the case of bone imaging, stronger filtering increased CNR without restriction, although the anatomical structures appeared blurry throughout. The bone structure in the area of the lumbar
spine and pelvis is very precise and complex, which might lead to a supposedly high noise level in the calculation. On the other hand, observer analysis showed no considerable increase
between the different scan times of 5, 30 and 90 min, while increasing the Gaussian filter from 1.2 mm to 1.8 mm leads to a noticeably higher image quality score. The positive effect of
post-filtering on CNR and image quality can most likely be attributed to the improvements in uniformity and noise13. The study is limited by the use of only one rat per tracer. A certain
inter-animal variance could be expected, since we focused on the impact of scan time and post filter on the image quality and not the quantitative capabilities, we decided to use only two
examples with well-established tracers for reasons of good animal practice. CONCLUSION We analyzed the performance of a recently introduced ultra-high-resolution micro-SPECT system with two
stationary detectors for preclinical rat imaging. Providing good image quality with a multi-pinhole UHR-RM collimator, the scanner is suitable for heart and bone scans using standardized
reconstruction parameters and appropriate post-filtering. Although the system demonstrates excellent performance in rat imaging as compared to conventional systems, there are still
challenges to achieve sub-millimeter system resolution in rats where there are safety limits on injection dose and acquisition time. DATA AVAILABILITY The datasets generated and/or analyzed
during the current study are available from the corresponding author on reasonable request. REFERENCES * King, M. A., Pretorius, P. H., Farncombe, T. & Beekman, F. J. Introduction to the
physics of molecular imaging with radioactive tracers in small animals. _J. Cell Biochem. Suppl._ 39, 221–230. https://doi.org/10.1002/jcb.10447 (2002). Article CAS PubMed Google Scholar
* Meikle, S. R., Kench, P., Kassiou, M. & Banati, R. B. Small animal SPECT and its place in the matrix of molecular imaging technologies. _Phys. Med. Biol._ 50, R45-61.
https://doi.org/10.1088/0031-9155/50/22/R01 (2005). Article ADS CAS PubMed Google Scholar * Beekman, F. & van der Have, F. The pinhole: Gateway to ultra-high-resolution
three-dimensional radionuclide imaging. _Eur. J. Nucl. Med. Mol. Imaging_ 34, 151–161. https://doi.org/10.1007/s00259-006-0248-6 (2007). Article PubMed Google Scholar * Magota, K. _et
al._ Performance characterization of the Inveon preclinical small-animal PET/SPECT/CT system for multimodality imaging. _Eur. J. Nucl. Med. Mol. Imaging_ 38, 742–752.
https://doi.org/10.1007/s00259-010-1683-y (2011). Article PubMed Google Scholar * Matsunari, I. _et al._ Performance evaluation of the eXplore speCZT preclinical imaging system. _Ann.
Nucl. Med._ 28, 484–497. https://doi.org/10.1007/s12149-014-0828-7 (2014). Article CAS PubMed Google Scholar * Ivashchenko, O., van der Have, F., Goorden, M. C., Ramakers, R. M. &
Beekman, F. J. Ultra-high-sensitivity submillimeter mouse SPECT. _J. Nucl. Med._ 56, 470–475. https://doi.org/10.2967/jnumed.114.147140 (2015). Article PubMed Google Scholar * Franc, B.
L., Acton, P. D., Mari, C. & Hasegawa, B. H. Small-animal SPECT and SPECT/CT: Important tools for preclinical investigation. _J. Nucl. Med._ 49, 1651–1663.
https://doi.org/10.2967/jnumed.108.055442 (2008). Article PubMed Google Scholar * Furenlid, L. R. _et al._ FastSPECT II: A second-generation high-resolution dynamic SPECT imager. _IEEE
Trans. Nucl. Sci._ 51, 631–635. https://doi.org/10.1109/TNS.2004.830975 (2004). Article ADS PubMed PubMed Central Google Scholar * Vastenhouw, B. & Beekman, F. Submillimeter
total-body murine imaging with U-SPECT-I. _J. Nucl. Med._ 48, 487–493 (2007). PubMed Google Scholar * van der Have, F. _et al._ U-SPECT-II: An ultra-high-resolution device for molecular
small-animal imaging. _J. Nucl. Med._ 50, 599–605. https://doi.org/10.2967/jnumed.108.056606 (2009). Article Google Scholar * Ivashchenko, O. _et al._ Quarter-millimeter-resolution
molecular mouse imaging with U-SPECT(+). _Mol.S Imaging_ 13, 10. https://doi.org/10.2310/7290.2014.00053 (2014). Article CAS Google Scholar * Higaki, Y. _et al._ Appropriate collimators
in a small animal SPECT scanner with CZT detector. _Ann. Nucl. Med._ 27, 271–278. https://doi.org/10.1007/s12149-012-0681-5 (2013). Article PubMed Google Scholar * Harteveld, A. A. _et
al._ Using the NEMA NU 4 PET image quality phantom in multipinhole small-animal SPECT. _J. Nucl. Med._ 52, 1646–1653. https://doi.org/10.2967/jnumed.110.087114 (2011). Article PubMed
Google Scholar * Mizutani, A. _et al._ Impact of injection dose, post-reconstruction filtering, and collimator choice on image quality of myocardial perfusion SPECT using cadmium-zinc
telluride detectors in the rat. _EJNMMI Phys._ 2, 7. https://doi.org/10.1186/s40658-015-0111-6 (2015). Article PubMed PubMed Central Google Scholar * Vaissier, P. E., Beekman, F. J.
& Goorden, M. C. Similarity-regulation of OS-EM for accelerated SPECT reconstruction. _Phys. Med. Biol._ 61, 4300–4315. https://doi.org/10.1088/0031-9155/61/11/4300 (2016). Article CAS
PubMed Google Scholar * Iannaccone, P. M. & Jacob, H. J. Rats!. _Dis. Model Mech._ 2, 206–210. https://doi.org/10.1242/dmm.002733 (2009). Article PubMed PubMed Central Google
Scholar * Jones, A. G. _et al._ Biological studies of a new class of technetium complexes: The hexakis(alkylisonitrile)technetium(I) cations. _Int. J. Nucl. Med. Biol._ 11, 225–234.
https://doi.org/10.1016/0047-0740(84)90004-4 (1984). Article CAS PubMed Google Scholar * Bevan, J. A., Tofe, A. J., Benedict, J. J., Francis, M. D. & Barnett, B. L. Tc-99m HMDP
(hydroxymethylene diphosphonate): A radiopharmaceutical for skeletal and acute myocardial infarct imaging. I. Synthesis and distribution in animals. _J. Nucl. Med._ 21, 961–966 (1980). CAS
PubMed Google Scholar * Bevan, J. A., Tofe, A. J., Benedict, J. J., Francis, M. D. & Barnett, B. L. Tc-99m HMDP (hydroxymethylene diphosphonate): A radiopharmaceutical for skeletal and
acute myocardial infarct imaging. II. Comparison of Tc-99m hydroxymethylene diphosphonate (HMDP) with other technetium-labeled bone-imaging agents in a canine model. _J. Nucl. Med._ 21,
967–970 (1980). CAS PubMed Google Scholar * Vaissier, P. E. _et al._ Fast spiral SPECT with stationary gamma-cameras and focusing pinholes. _J. Nucl. Med._ 53, 1292–1299.
https://doi.org/10.2967/jnumed.111.101899 (2012). Article PubMed Google Scholar * Shepp, L. A. & Vardi, Y. Maximum likelihood reconstruction for emission tomography. _IEEE Trans Med.
Imaging_ 1, 113–122. https://doi.org/10.1109/TMI.1982.4307558 (1982). Article CAS PubMed Google Scholar * Ogawa, K., Harata, Y., Ichihara, T., Kubo, A. & Hashimoto, S. A practical
method for position-dependent Compton-Scatter correction in single photon emission CT. _IEEE Trans. Med. Imaging_ 10, 408–412. https://doi.org/10.1109/42.97591 (1991). Article CAS PubMed
Google Scholar * Loening, A. M. & Gambhir, S. S. AMIDE: A free software tool for multimodality medical image analysis. _Mol. Imaging_ 2, 131–137.
https://doi.org/10.1162/153535003322556877 (2003). Article PubMed Google Scholar * National Electrical Manufacturers Association in _NEMA Standards Publication NU 4–2008: Performance
Measurements of Small Animal Positron Emission Tomographs_ (Rosslyn, VA, 2008). * Walker, M. D. _et al._ Performance assessment of a preclinical PET scanner with pinhole collimation by
comparison to a coincidence-based small-animal PET scanner. _J. Nucl. Med._ 55, 1368–1374. https://doi.org/10.2967/jnumed.113.136663 (2014). Article Google Scholar * Bayne, K. Revised
guide for the care and use of laboratory animals available. American Physiological Society. _Physiologist_ 39(199), 208–111 (1996). Google Scholar * Arsos, G. _et al._ (99m)Tc-sestamibi
uptake in rat skeletal muscle and heart: Physiological determinants and correlations. _Physiol. Res._ 58, 21–28 (2009). CAS PubMed Google Scholar * Boisson, F. _et al._ Imaging
capabilities of the Inveon SPECT system using single-and multipinhole collimators. _J. Nucl. Med._ 54, 1833–1840. https://doi.org/10.2967/jnumed.112.117572 (2013). Article PubMed Google
Scholar * Schramm, N. _et al._ Improving resolution, sensitivity and applications for the NanoSPECT/CT: A high-performance SPECT/CT imager for small-animal research. _J. Nucl. Med._ 48,
436P (2007). Google Scholar * Lukas, M., Kluge, A., Beindorff, N. & Brenner, W. Multi-isotope capabilities of a small-animal multi-pinhole SPECT system. _J. Nucl. Med._ 61, 152–161.
https://doi.org/10.2967/jnumed.119.226027 (2020). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the Department of Nuclear Medicine of the
University Hospital Würzburg for providing the tracers. Jan Vincent Hoffmann and Jan Paul Janssen were supported by a doctoral fellowship of the Faculty of Medicine, University of Würzburg
in the framework of the Graduate School of Life Sciences. Takayuki Kanno was supported by the Japan Public–Private Partnership Student Study Abroad Program (TOBITATE! Young Ambassador
Program). The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. FUNDING Open Access
funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION Author notes * These authors contributed equally: Jan P. Janssen and Jan V. Hoffmann. AUTHORS AND AFFILIATIONS * Department
of Nuclear Medicine, University Hospital Würzburg, Oberdürrbacher Strasse 6, 97080, Würzburg, Germany Jan P. Janssen, Jan V. Hoffmann, Andreas K. Buck & Takahiro Higuchi * Comprehensive
Heart Failure Centre, University Hospital Würzburg, Würzburg, Germany Jan P. Janssen, Jan V. Hoffmann, Takayuki Kanno, Xinyu Chen & Takahiro Higuchi * Department of Quantum Medical
Technology, Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Japan Takayuki Kanno & Masahisa Onoguchi * Graduate School of Medicine, Dentistry and Pharmaceutical
Sciences, Okayama University, Okayama, Japan Naoko Nose & Takahiro Higuchi * Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany
Jan-Peter Grunz * Nuclear Medicine, Medical Faculty, University of Augsburg, Augsburg, Germany Xinyu Chen & Constantin Lapa Authors * Jan P. Janssen View author publications You can also
search for this author inPubMed Google Scholar * Jan V. Hoffmann View author publications You can also search for this author inPubMed Google Scholar * Takayuki Kanno View author
publications You can also search for this author inPubMed Google Scholar * Naoko Nose View author publications You can also search for this author inPubMed Google Scholar * Jan-Peter Grunz
View author publications You can also search for this author inPubMed Google Scholar * Masahisa Onoguchi View author publications You can also search for this author inPubMed Google Scholar
* Xinyu Chen View author publications You can also search for this author inPubMed Google Scholar * Constantin Lapa View author publications You can also search for this author inPubMed
Google Scholar * Andreas K. Buck View author publications You can also search for this author inPubMed Google Scholar * Takahiro Higuchi View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS Conception: J.P.J., J.V.H., T.H.; Acquisition, analysis and interpretation: J.P.J., J.V.H., T.K.; Writing—original draft preparation: J.P.J.,
J.V.H.; Writing—review and editing: J.P.J., J.V.H., T.K., N.N., J.G., M.O., X.C., C.L., A.B., T.H.; Funding acquisition: J.P.J., J.V.H., T.K., C. L., T.H., A.B.; Supervision: T.H., A.B.;
Validation: N.N., J.G., M.O., X.C., C.L., A.B., T.H.; Visualization: J.P.J., J.V.H. CORRESPONDING AUTHOR Correspondence to Takahiro Higuchi. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link
to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence,
unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Janssen, J.P., Hoffmann, J.V., Kanno, T. _et al._ Capabilities of multi-pinhole
SPECT with two stationary detectors for in vivo rat imaging. _Sci Rep_ 10, 18616 (2020). https://doi.org/10.1038/s41598-020-75696-0 Download citation * Received: 27 July 2020 * Accepted: 07
October 2020 * Published: 29 October 2020 * DOI: https://doi.org/10.1038/s41598-020-75696-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
