Serum angiopoietin-like 3 levels are elevated in obese non diabetic men but are unaffected during an oral glucose tolerance test
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT This study aimed to determine ANGPTL3 serum levels in healthy young lean and obese non-diabetic men during an oral glucose tolerance test (OGTT) and correlate them with
anthropometric, biochemical and hormonal parameters. A case–control study was carried out and 30 young obese non-diabetic (23.90 ± 3.84 years and BMI 37.92 ± 4.85 kg/m2) and 28 age-matched
healthy lean (24.56 ± 3.50 years and BMI of 22.10 ± 1.72 kg/m2) men were included in this study. The primary outcome measures were serum basal ANGPTL3 and ANGPTL3–area under the curve (AUC)
levels. The percentage of body fat was measured by dual-energy X-ray absorptiometry and biochemical, hormonal and insulin resistance indices were determined. Basal ANGPTL3 and ANGPTL3–AUC
levels were significantly elevated (p < 0.05) in young obese subjects compared with lean subjects and were positively and significantly associated with different anthropometric
measurements. Fasting ANGPTL3 serum levels were positively correlated with fasting insulin, leptin, Leptin/Adiponectin index and triglyceride—glucose index. Moreover, ANGPTL3–AUC was
negatively correlated with Matsuda index. In this regard, chronically high ANGPTL3 levels in young obese subjects might favor triglyceride-rich lipoprotein clearance to replenish
triglyceride stores by white adipose tissue rather than oxidative tissues. SIMILAR CONTENT BEING VIEWED BY OTHERS SERUM OMENTIN-1 LEVELS IN HYPERTENSIVE PATIENTS Article 25 September 2020
CIRCULATING SPEXINS IN CHILDREN WITH OBESITY: RELATION TO CARDIOMETABOLIC RISK Article 13 April 2021 THE ROLE OF HIGH FAT DIET ON SERUM URIC ACID LEVEL AMONG HEALTHY MALE FIRST DEGREE
RELATIVES OF TYPE 2 DIABETES MELLITUS Article Open access 16 October 2023 INTRODUCTION Elevated plasma triglyceride levels are associated with increased risk of atherosclerosis and
cardiovascular disease, a major cause of morbidity and mortality worldwide1. Lipoprotein lipase (LPL) plays a fundamental role in the normal lipid metabolism and energy balance, by
catalyzing the hydrolysis of the triglyceride (TG) component of circulating chylomicrons and very-low-density lipoprotein (VLDL) at the luminal surface of endothelial cells in extrahepatic
tissues, to release fatty acids that can be used or stored2,3,4. LPL is mainly expressed in adipose tissue and striated muscle (skeletal and cardiac) and its activity is regulated in a
tissue-specific manner5,6. Different members of the angiopoietin-like (ANGPTL) family of proteins participate in the regulation of LPL activity, including ANGPTL3, ANGPTL4 and ANGPTL87,8.
ANGPTL3 is a secreted glycoprotein mainly expressed in the liver and acts as a negative regulator of LPL and endothelial lipase activity in oxidative tissues such as brown adipose tissue
(BAT) and skeletal muscle9. Elevated circulating levels of this hepatokine are known to increase plasma levels of triglycerides, high-density lipoprotein cholesterol (HDL-c) and low-density
lipoprotein cholesterol (LDL-c)10. In addition, Muniyappa et al., reported higher plasmatic levels of ANGPTL3 in leptin-deficient patients with lipodystrophy when compared to healthy control
subjects11. Furthermore, leptin replacement therapy (metreleptin) in patients with lipodystrophy decreased significantly serum ANGPTL3 levels, total cholesterol and triglyceride
concentrations11. Consistent with clinical data, preclinical studies showed that the overexpression of both ANGPTL3 and ANGPTL8 in mice resulted in hypertriglyceridemia12. Moreover, plasma
levels and hepatic gene expression of ANGPTL3 are increased in leptin-deficient _ob/ob_ and leptin-resistant _db/db_ mice and were associated with an increase in plasma triglycerides and
free fatty acids13. Additionally, leptin-deficient _ob/ob_ mice supplemented with recombinant leptin, showed lower gene expression and plasmatic levels of ANGPTL3 and normalization of
plasmatic triglyceride levels13. Despite ANGPTL3 was proposed as an important regulator of plasmatic lipoprotein metabolism through LPL inhibition, to date, contradictory results have been
reported on ANGPTL3 circulating levels in obese subjects2,3,14,15,16. Furthermore, some studies have shown that ANGPTL3 can be involved in carbohydrate metabolism, but the evaluation of
ANGPTL3 response to oral glucose stimulation has not been clearly established yet2,17,18,19,20. Therefore, the aim of this case–control study was to determine serum ANGPTL3 levels in young
healthy lean and obese non-diabetic men in response to the oral glucose tolerance test (OGTT), a physiological imitator of meal stimulation. In addition, we studied the relationship between
serum ANGPTL3 levels with anthropometric, biochemical, hormonal and insulin resistance indices. MATERIALS AND METHODS ETHICAL ASPECTS All experimental protocols were reviewed and approved by
the Ethics Review Board of the School of Medicine of the Universidad Nacional de Colombia (permission number 012-204-18, August 27—2018) and a written informed consent was obtained from all
study participants. All protocols were performed in accordance with the Declaration of Helsinki for Medical Research involving Human Subjects and approved methods. STUDY POPULATION The
present investigation was conducted as a case–control study at the Department of Internal Medicine—Division of Endocrinology School of Medicine Universidad Nacional de Colombia. According to
the World health organization (WHO) body mass index (BMI = kg/m2) criteria, 30 young non-diabetic obese men (BMI ≥ 30 kg/m2) (23.90 ± 3.84 years and BMI 37.92 ± 4.85 kg/m2) and 28 young
healthy lean men (BMI 18.50–24.99 kg/m2) (24.56 ± 3.50 years and BMI of 22.10 ± 1.72 kg/m2) were included in this study4. Healthy lean individuals in the study were normotensive, euglycemic
and with triglycerides and cholesterol levels within the normal range21. Subjects who met any of the following criteria were excluded from the study: current smoking, alcoholism, mental
illness, subjects diagnosed with type 2 diabetes mellitus (T2DM), chronic kidney disease, cardiac failure, hepatic failure, thyroid diseases, infectious diseases among other diseases, use of
approved weight lowering pharmacotherapy or patients with a history of gastric bypass and other bariatric surgery. Also, subjects who had taken drugs that affect energy metabolism, such as
metformin, levothyroxine or steroids, within the last 12 months, were excluded. CLINICAL EVALUATION Subjects underwent clinical and physical examination. Weight and height were determined
and BMI (kg/m2) was calculated. Anthropometric data included height, hip circumference (HC), waist circumference (WC) and estimated measuring of body fat percent (BF%) was determined by
Dual-Energy X-ray absorptiometry (DXA) (GE Lunar Prodigy Advance). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in all subjects and mean arterial pressure
(MAP) were calculated. BIOCHEMICAL AND HORMONAL ANALYSIS All experiments started in the morning between 7:00 and 9:00 AM after an overnight fast. All subjects were given 75 g of anhydrous
glucose, dissolved in 200 mL of water, after overnight fasting. The subjects underwent an oral glucose tolerance test (OGTT) and blood samples were collected at fasting and 30, 60 and 120
min after glucose ingestion. The serum samples were separated by centrifugation at 1000×_g_ for 15 min and stored at − 80 °C until the respective assays. Serum levels of glucose (basal and
postprandial), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), high-sensitivity C-reactive protein (hs-CRP) and
triglycerides (TG) were determined. Additionally, serum levels of ANGPTL3 (basal and postprandial), serum insulin levels (basal and postprandial), serum levels of adiponectin and leptin,
were analyzed. Serum ANGPTL3 levels were measured using a commercial ELISA kit (Catalog Number DANL30—R&D Systems, Inc. USA). The intra- and inter-assay coefficients of variation (CVs)
were < 4.1% and < 8.5%, respectively. Serum human leptin levels were measured with a commercial ELISA kit (Catalog Number KAC2281-ThermoFisher Scientic Inc.). The intra- and
inter-assay coefficients of variation for leptin concentrations were < 3.9% and < 5.3%, respectively. Likewise, serum levels of adiponectin were determined by an ELISA KIT (Catalog
Number KHP0041-ThermoFisher Scientific Inc.) and the coefficients of intra- and inter-assay variation were < 3.8% and < 5.5% respectively. All samples were analyzed in duplicates.
Insulin–Area Under the Curve (AUC) (µUI–mL/2 h), glucose–Area Under the Curve (AUC) (mg–dL/2 h) and ANGPTL3–Area Under the Curve (AUC) (ng–mL/2 h) during OGTT were calculated by trapezoidal
approximation rule as described elsewhere22,23. DEFINITIONS All subjects underwent a full physical examination and measurements of anthropometric parameters were performed with a
standardized technique. Anthropometric measurements were taken while the participants dressed in light clothing, without shoes. Body Mass Index (BMI = kg/m2) was calculated as weight (kg)
divided by height (m) squared. Furthermore, we calculated the waist circumference (WC) to height ratio (WHtR), an anthropometric index for central adiposity, determined by dividing the WC by
height (cm)24. Additionally, we measured the waist-to-hip ratio (WHipR) as described elsewhere21. Metabolic syndrome was defined according to the International Diabetes Federation (IDF)
criteria, to which central obesity (defined by WC) and ethnicity is an essential component for diagnosis of the syndrome and required the presence of two of the following elements: fasting
plasma glucose (FPG) ≥ 100 mg/dL, systolic blood pressure (SBP) ≥ 130 mm Hg and diastolic blood pressure (DBP) ≥ 85 mm Hg, or on antihypertensive medication, TG ≥ 150 mg/dL or on treatment,
HDLc < 40 mg/dL in men and < 50 mg/dL in women, or on treatment21. Additionally, borderline metabolic abnormalities for fasting glucose and high normal blood pressure (BP), as
described by Baden et al.25. Moreover, borderline lipid profile for metabolic syndrome factor, among these, TC, LDLc, HDLc and TG are described elsewhere26. Insulin resistance was determined
according to the homeostatic insulin resistance assessment model (HOMA-IR) as described by the Matthews et al.27. Likewise, the quantitative insulin sensitivity check index (QUICKI) and
Matsuda index for hepatic and muscle resistance/sensitivity were estimated as described elsewhere28,29,30. Triglyceride-glucose (TyG) and TG/HDL-c indices were calculated as described
elsewhere31,32. Values of the leptin/adiponectin ratio (LAR) were made as Finucane et al. described in individuals with non-diabetic insulin resistance33. Leptin resistance is a term
commonly used to define states in which hyperleptinemia, as seen in obesity-related condition, results in a decreased response to leptin34. STATISTICAL ANALYSIS Descriptive data were
presented as mean ± SD. Variables with normal distribution were compared by unpaired Student’s t-test and one-way ANOVA and repeated-measured ANOVA. Additionally, a post hoc analysis was
made among the groups. Mann–Whitney U test, Kruskal–Wallis one-way analysis of variance and Friedman test were performed for non-normal distribution variables. Pearson’s correlation
coefficient was used to assess of linear relationship between fasting ANGPTL3 ng/mL and ANGPTL3–AUC (ng–mL/2 h) with different metabolic, anthropometrics and hormonal variables. A p value
< 0.05 was considered to be statistically significant (*p-value < 0.05, **p-value < 0.01, ***p-value < 0.001). All statistical analyses were done using R version 3.4.0.
Software35. Line plots and Boxplots were generated with the R BiocGenerics package36. RESULTS EXPERIMENTAL RESULTS Characteristics of the study population are shown in Table 1. Young obese
subjects showed a borderline increase of SBP, DBP and MAP25. Additionally, obese subjects presented borderline risk factors for metabolic syndrome, including TC, LDLc, HDLc and TG26. During
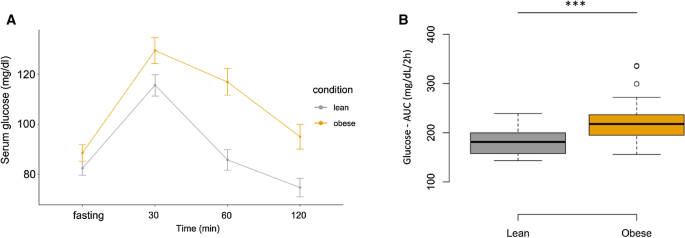
the OGTT, serum glucose and insulin levels were significantly higher in the obese group compare with lean group at fasting, 30, 60, and 120 min (Figs. 1, 2 respectively, Table 2).
Glucose—AUC and insulin—AUC were significantly higher in obese compared with lean subjects (Figs. 1, 2, Table 2). Hyperinsulinemia and hyperleptinemia were confirmed in the obese subjects by
the surrogated indices HOMA-IR, QUICKI, Matsuda, TyG, TG/HDL-c and LAR indices (Table 1). Serum adiponectin levels were significantly lower (p = 0.001) in obese non-diabetic men compared to
the healthy lean men (Table 1). In contrast, serum leptin concentrations were notably higher (p = 0.001) in obese non-diabetic men compared to the healthy lean men (Table 1). Mean ANGPTL3
serum levels at 0, 30, 60, and 120 min and ANGPTL3–AUC, in response to an oral glucose tolerance test, were significantly higher (p = 0.000) in obese compared to healthy lean subjects (Table
2, Fig. 3). No significant difference was seen over time in serum ANGPTL3 levels at baseline (0), 30, 60 and 120 min during the OGTT in healthy young lean and obese non-diabetic men (Fig.
3). At baseline, ANGPTL3 serum levels were significantly (but weakly) positively correlated with fasting insulin (r = 0.267; p = 0.043) (Table 3). Furthermore, leptin (r = 0.381; p = 0.003),
LAR (r = 0.381; p = 0.003) and TyG indices (r = 0.285; p = 0.030) were positively correlated with fasting ANGPTL3 serum levels (Table 3, Supplementary Fig. S1A–C respectively). Fasting
serum ANGPTL3 levels were positively correlated with anthropometric parameters such as BMI (r = 0.413; p = 0.001), WC (r = 0.453; p = 0.000), WHtR (r = 0.435; p = 0.001), AFM (%) (r = 0.395;
p = 0.002), TBF (%) (r = 0.395; p = 0.002), WHipR (r = 0.417; p = 0.001) and GFM (%) (r = 0.350; p = 0.007) (Table 3). There was no statistically significant correlation between basal serum
ANGPTL3 levels and triglyceride levels, HOMA–IR and QUICKI indices (Table 3). Finally, ANGPTL3-AUC was negatively correlated with Matsuda Index (r = − 0.315; p = 0.016) and positively
correlated with Insulin-AUC (r = 0.324; p = 0.013) (Supplementary Fig. S3A,B respectively). Similar correlations between ANGPTL3–AUC and anthropometric parameters such as BMI (r = 0.417; p =
0.001), WC (r = 0.459; p = 0.000), WHtR (r = 0.418; p = 0.001), WHipR (r = 0.418; p = 0.009), AFM (%) (r = 0.476; p = 0.000), GFM (%) (r = 0.428; p = 0.000) and TBF (%) (r = 0.460; p =
0.000) (Supplementary Fig. S2A–D,F respectively), were seen with fasting ANGPTL3 serum levels (Table 3). DISCUSSION This study has demonstrated that serum ANGPTL3 levels were significantly
elevated in young obese non-diabetic subjects compared to healthy control subjects. However, short‐term changes of serum ANGPTL3 levels at fasting and OGTT (30, 60, and 120 min) remained
unchanged in both groups. Thus, the present study suggests that serum ANGPTL3 levels do not seem to be involved in short-term regulation of carbohydrate metabolism. However, previous studies
have shown that ANGPTL3 levels are abnormally increased in insulin-resistant state and obesity, with worsening glucose metabolism and enhancing lipolysis in adipose tissues19,37. This could
indicate the possible role of ANGPTL3 in regulating glucose metabolism at long-term, which could progressively lead to type 2 diabetes and other metabolic lifelong diseases19,37. Basal
serum ANGPTL3 levels were positively correlated with anthropometric parameters such as WC, BMI, WHipR, TBF (%), AFM (%) and GFM (%). However we did not find a significantly correlation with
TG levels, which is in line with previously reported results3,14, but not with others showing that circulating ANGPTL3 was negatively correlated with TG levels38. The controversial
relationship between ANGPTL3 levels and TG suggests that there are other factors affecting the interaction between ANGPTL3 levels and TGs, and further studies are needed to better understand
this relationship3,14,38,39. In this regard, it is worth pointing out that ANGPTL3 levels are significantly higher in patients with advanced forms of NAFLD or nonalcoholic steatohepatitis
(NASH), but no significant differences were detected in individuals with simple fatty liver (NAFL) when compared to healthy and normal weight individuals20. Thus, the results obtained in our
study showing that ANGPTL3 levels were significantly higher in young obese non-diabetic individuals and could be at least partially, affected by the presence of NASH. This is relevant
because the development of NASH reduces insulin clearance and could hinder the interpretation of plasma insulin data and derived indices. Additionally, in this study, results showed serum
ANGPTL3 levels at baseline and ANGPTL3–AUC significantly higher in obese subjects compared to lean subjects. These data are in agreement with previous results reported in human obese
subjects and animal models2,13,17. In addition, plasmatic levels of ANGPTL3 were elevated in patients with generalized lipodystrophy and significantly reduced after metreleptin therapy11.
Our data indicating that fasting serum ANGPTL3 levels were positively correlated with serum leptin levels and leptin/adiponectin index would suggest that hyperleptinemia might lead to
increased serum ANGPTL3 levels in young obese subjects. However, different to ours, Cinkajzlová et al. have found serum ANGPTL3 levels were lower in obese subjects with and without T2DM when
compared to healthy control subjects16. It is important to highlight that those results were obtained in obese subjects with metabolic syndrome, while our cohort showed borderline metabolic
syndrome criteria risk factors. On the other hand, other studies demonstrated that ANGPTL3 levels did not differ significantly between overweight or obese subjects compared to normal weight
subjects14,15. It is important to highlight that Shimamura et al. also demonstrated the negative regulation of ANGPTL3 by insulin action13. In STZ-treated insulin-deficient mice, plasmatic
levels and hepatic expression of ANGPTL3, TG and FFA levels were higher compared with control mice13. Of note, in the current study we observed ANGPTL3–AUC was positively correlated with
insulin–AUC and negatively correlated with the Matsuda index, which would imply that insulin resistance might contribute to the increased serum ANGPTL3 levels in obese subjects. On the other
hand, the associations of basal serum levels of ANGPTL3 with fasting insulin levels and TyG index was found to be weak as evidenced by their low correlation coefficients which were smaller
than 0.29. Therefore, it is evident that assessing ANGPTL3 in obese subjects will require further investigation, in particular performing large and well conducted clinical and
epidemiological studies, stratified by age, sex, ethnicity and geographic region. Recent Genome-Wide Association Studies (GWAS) and whole-exome sequencing have identified sequence variants
associated with loss of function in the ANGPTL3 gene across populations from Europe, Asia, Africa and the Americas40,41,42,43,44,45,46. In this way, ANGPTL3 genetic variation across
populations from Europe, Asia, Africa and the Americas, has been studied to identify Single Nucleotide Polymorphism (SNPs) specific to each population. Therefore, sequence variant in the
ANGPTL3 gene might have important implications for the diagnosis and therapeutic interventions of diseases such as combined hypolipidemia that consists of extremely low plasma levels of
HDL-c, LDL cholesterol and triglycerides40,41,42,43,44,45,46. From a genetic perspective on diversity, the population of Latin American is descended from indigenous American, European, and
African populations. SNPs and structural variants that may be unique or specific to each population for ANGPTL3 have not been studied extensively and it may have important consequences for
health and wellbeing. This paper is the first study of ANGPTL3 status in young obese non-diabetic and age-matched healthy non-obese men control in the Latino American population. However, a
limitation of the study is that the possible prevalence of NAFLD in obese subjects, which may have an important influence in the ANGPTL3 levels that was not considered. In addition, only
young men were enrolled in the study, with the purpose to reduce the effects of possible confounding variables, such as the influences of sex hormones in healthy women during normal
menstrual cycle and in obese women with polycystic ovary syndrome (PCOS). CONCLUSIONS In conclusion, our results indicate that in Latin American young obese individuals, circulating ANGPTL3
levels are higher than those non-obese healthy controls at the basal state, but these levels remained unchanged during an OGTT. Thus, these findings support an important role of ANGPTL3 in
obesity. REFERENCES * Toth, P. P. _et al._ High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims
analysis of statin-treated patients with high residual cardiovascular risk. _J. Am. Heart Assoc._ 7, e008740 (2018). Article PubMed PubMed Central CAS Google Scholar * Abu-Farha, M. _et
al._ Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. _Lipids Health Dis._ 15, 181 (2016). Article PubMed PubMed Central CAS Google Scholar * Mehta,
N. _et al._ Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. _Arterioscler. Thromb. Vasc. Biol._ 34, 1057–1063 (2014). Article PubMed
PubMed Central CAS Google Scholar * World Health Organization. _Obesity and Overweight_ (WHO, Geneva, 2020). Google Scholar * Kersten, S. Physiological regulation of lipoprotein lipase.
_Biochim. Biophys. Acta Mol. Cell Biol. Lipids_ 1841, 919–933 (2014). Article CAS Google Scholar * Wang, H. & Eckel, R. H. Lipoprotein lipase: from gene to obesity. _Am. J.
Physiol.-Endocrinol. Metab._ 297, E271–E288 (2009). Article PubMed CAS Google Scholar * Zhang, R. The ANGPTL3-4-8 model, a molecular mechanism for triglyceride trafficking. _Open Biol._
6, 150272 (2016). Article PubMed PubMed Central CAS Google Scholar * Kovrov, O., Kristensen, K. K., Larsson, E., Ploug, M. & Olivecrona, G. On the mechanism of angiopoietin-like
protein 8 for control of lipoprotein lipase activity. _J. Lipid Res._ 60, 783–793 (2019). Article PubMed PubMed Central CAS Google Scholar * Koishi, R. _et al._ Angptl3 regulates lipid
metabolism in mice. _Nat. Genet._ 30, 151–157 (2002). Article PubMed CAS Google Scholar * Kersten, S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular
disease risk. _Curr. Opin. Lipidol._ 30, 205–211 (2019). Article PubMed CAS Google Scholar * Muniyappa, R. _et al._ Metreleptin therapy lowers plasma angiopoietin-like protein 3 in
patients with generalized lipodystrophy. _J. Clin. Lipidol._ 11, 543–550 (2017). Article PubMed PubMed Central Google Scholar * Chi, X. _et al._ ANGPTL8 promotes the ability of ANGPTL3
to bind and inhibit lipoprotein lipase. _Mol. Metab._ 6, 1137–1149 (2017). Article PubMed PubMed Central CAS Google Scholar * Shimamura, M. _et al._ Leptin and insulin down-regulate
angiopoietin-like protein 3, a plasma triglyceride-increasing factor. _Biochem. Biophys. Res. Commun._ 322, 1080–1085 (2004). Article PubMed CAS Google Scholar * Chung, H. S. _et al._
Circulating angiopoietin-like protein 8 (ANGPTL8) and ANGPTL3 concentrations in relation to anthropometric and metabolic profiles in Korean children: a prospective cohort study. _Cardiovasc.
Diabetol._ 15, 1 (2016). Article PubMed PubMed Central CAS Google Scholar * Guo, W., Che, Z., Xu, A. & Zhou, Z. Angiopoietin-like protein 3 and adiponectin levels in patients with
metabolic syndrome. _Zhong Nan Da Xue Xue Bao Yi Xue Ban_ 35, 203–208 (2010). PubMed CAS Google Scholar * Cinkajzlová, A. _et al._ Angiopoietin-like protein 3 and 4 in obesity, type 2
diabetes mellitus, and malnutrition: the effect of weight reduction and realimentation. _Nutr. Diabetes_ 8, 21 (2018). Article PubMed PubMed Central CAS Google Scholar * Inukai, K. _et
al._ ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. _Biochem. Biophys. Res. Commun._ 317, 1075–1079 (2004). Article PubMed CAS Google Scholar * Robciuc,
M. R. _et al._ Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. _Arterioscler. Thromb. Vasc. Biol._ 33,
1706–1713 (2013). Article PubMed CAS Google Scholar * Christopoulou, E., Elisaf, M. & Filippatos, T. Effects of angiopoietin-like 3 on triglyceride regulation, glucose homeostasis,
and diabetes. _Dis. Mark._ 2019, 1–8 (2019). Article CAS Google Scholar * Yilmaz, Y., Ulukaya, E., Atug, O. & Dolar, E. Serum concentrations of human angiopoietin-like protein 3 in
patients with nonalcoholic fatty liver disease: association with insulin resistance. _Eur. J. Gastroenterol. Hepatol._ 21, 1247–1251 (2009). Article PubMed CAS Google Scholar * Alberti,
K. G. M., Zimmet, P. & Shaw, J. The metabolic syndrome—a new worldwide definition. _The Lancet_ 366, 1059–1062 (2005). Article Google Scholar * Huda, M. S. B. _et al._ Plasma obestatin
levels are lower in obese and post-gastrectomy subjects, but do not change in response to a meal. _Int. J. Obes._ 32, 129–135 (2008). Article CAS Google Scholar * Sakaguchi, K. _et al._
Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. _Diabetol. Int._ 7, 53–58 (2016). Article PubMed Google Scholar * Amato, M. C. _et al._
Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. _Diabetes Care_ 33, 920–922 (2010). Article PubMed PubMed Central Google
Scholar * Baden, M. Y. _et al._ Serum adiponectin level is not only decreased in metabolic syndrome but also in borderline metabolic abnormalities. _Nutr. Diabetes_ 1, e18 (2011). Article
PubMed PubMed Central CAS Google Scholar * Mayo Clinic. _High Cholesterol_ (2019). https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/diagnosis-treatment/drc-20350806.
* Matthews, D. R. _et al._ Homeostasis model assessment: insulin resistance and ?-cell function from fasting plasma glucose and insulin concentrations in man. _Diabetologia_ 28, 412–419
(1985). Article PubMed CAS Google Scholar * Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic
insulin clamp. _Diabetes Care_ 22, 1462–1470 (1999). Article PubMed CAS Google Scholar * Keskin, M., Kurtoglu, S., Kendirci, M., Atabek, M. E. & Yazici, C. Homeostasis model
assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents.
_Pediatrics_ 115, e500–e503 (2005). Article PubMed Google Scholar * Katz, A. _et al._ Quantitative Insulin sensitivity check index: a simple, accurate method for assessing insulin
sensitivity in humans. _J. Clin. Endocrinol. Metab._ 85, 2402–2410 (2000). Article PubMed CAS Google Scholar * Guerrero-Romero, F. _et al._ The product of triglycerides and glucose, a
simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. _J. Clin. Endocrinol. Metab._ 95, 3347–3351 (2010). Article PubMed CAS Google Scholar *
Salazar, M. R. _et al._ Use of the triglyceride/high-density lipoprotein cholesterol ratio to identify cardiometabolic risk: impact of obesity?. _J. Investig. Med._ 65, 323–327 (2017).
Article PubMed CAS Google Scholar * Finucane, F. M. _et al._ Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. _Diabetologia_
52, 2345–2349 (2009). Article PubMed PubMed Central CAS Google Scholar * Kusminski, C. M. & Scherer, P. E. Leptin beyond the lipostat: key component of blood pressure regulation.
_Circ. Res._ 116, 1293–1295 (2015). Article PubMed PubMed Central CAS Google Scholar * R Core Team. _R: A Language and Environment for Statistical Computing._ (R Foundation for
Statistical Computing, 2020). * Huber, W. _et al._ Orchestrating high-throughput genomic analysis with bioconductor. _Nat. Methods_ 12, 115–121 (2015). Article PubMed PubMed Central CAS
Google Scholar * Shimamura, M. _et al._ Angiopoietin-like protein 3, a hepatic secretory factor, activates lipolysis in adipocytes. _Biochem. Biophys. Res. Commun._ 301, 604–609 (2003).
Article PubMed CAS Google Scholar * Robciuc, M. R., Tahvanainen, E., Jauhiainen, M. & Ehnholm, C. Quantitation of serum angiopoietin-like proteins 3 and 4 in a Finnish population
sample. _J. Lipid Res._ 51, 824–831 (2010). Article PubMed PubMed Central CAS Google Scholar * Tarugi, P., Bertolini, S. & Calandra, S. Angiopoietin-like protein 3 (ANGPTL3)
deficiency and familial combined hypolipidemia. _J. Biomed. Res._ 33, 73–81 (2019). PubMed PubMed Central Google Scholar * Willer, C. J. _et al._ Newly identified loci that influence
lipid concentrations and risk of coronary artery disease. _Nat. Genet._ 40, 161–169 (2008). Article PubMed PubMed Central CAS Google Scholar * Musunuru, K. _et al._ Exome sequencing,
_ANGPTL3_ mutations, and familial combined hypolipidemia. _N. Engl. J. Med._ 363, 2220–2227 (2010). Article PubMed PubMed Central CAS Google Scholar * Romeo, S. _et al._ Rare
loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. _J. Clin. Investig._ https://doi.org/10.1172/JCI37118 (2008). Article PubMed Google
Scholar * Martín-Campos, J. M. _et al._ Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. _Clin.
Chim. Acta_ 413, 552–555 (2012). Article PubMed CAS Google Scholar * Noto, D. _et al._ Prevalence of ANGPTL3 and APOB gene mutations in subjects with Combined Hypolipidemia.
_Arterioscler. Thromb. Vasc. Biol._ 32, 805–809 (2012). Article PubMed CAS Google Scholar * Pisciotta, L. _et al._ Characterization of three kindreds with familial combined hypolipidemia
caused by loss-of-function mutations of ANGPTL3. _Circ. Cardiovasc. Genet._ 5, 42–50 (2012). Article PubMed CAS Google Scholar * Wang, X. & Musunuru, K. Angiopoietin-like 3 from
discovery to therapeutic gene editing. _JACC Basic Transl. Sci._ 4, 755–762 (2019). Article PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors
thank the men whose participation made this study possible. FUNDING This study was supported by government grants to the Universidad Nacional de Colombia (Dirección de investigaciones de
Sede Bogotá) and School of Medicine (código Hermes: 41439) and Colciencias (Departamento Administrativo de Ciencia, Tecnología e Innovación—Cod. 110154531660). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Physiology, School of Medicine, Universidad Nacional de Colombia, Bogotá, Colombia Maria Fernanda Garcés, Julieth Daniela Buell-Acosta, Haiver Antonio
Rodríguez-Navarro, Estefania Pulido-Sánchez, Juan José Rincon-Ramírez, Elizabeth Sanchez & Jorge Eduardo Caminos * Endocrine Unit - Department of Internal Medicine, School of Medicine,
Universidad Nacional de Colombia, Bogotá, Colombia Diana Carolina Moreno-Ordóñez & Roberto Franco-Vega * Department of Internal Medicine, School of Medicine, Universidad Nacional de
Colombia, Bogotá, Colombia Jhoan Sebastian Roncancio-Muñoz & Alvaro Javier Burgos-Cardenas * CINIBA, Facultad de Ciencias Médicas, Universidad Nacional de La Plata, La Plata, Argentina
Ezequiel Lacunza * Maimonides Institute of Biomedical Research of Cordoba (IMIBIC), Córdoba, Spain Justo P. Castaño * Department of Cell Biology, Physiology and Immunology, Universidad de
Córdoba, Córdoba, Spain Justo P. Castaño * Reina Sofia University Hospital, Córdoba, Spain Justo P. Castaño * CIBER Physiopathology of Obesity and Nutrition (CIBERobn), Córdoba, Spain Justo
P. Castaño, Carlos Diéguez & Rubén Nogueiras * Department of Physiology (CIMUS), School of Medicine, Instituto de Investigaciones Sanitarias (IDIS), Universidad de Santiago de
Compostela, Santiago, Spain Carlos Diéguez & Rubén Nogueiras * Department of Physiology, School of Medicine, Universidad Nacional de Colombia, Carrera 30 No. 45-03, Edificio 471 Piso 4
Oficina 406, Bogotá, Colombia Jorge Eduardo Caminos Authors * Maria Fernanda Garcés View author publications You can also search for this author inPubMed Google Scholar * Julieth Daniela
Buell-Acosta View author publications You can also search for this author inPubMed Google Scholar * Haiver Antonio Rodríguez-Navarro View author publications You can also search for this
author inPubMed Google Scholar * Estefania Pulido-Sánchez View author publications You can also search for this author inPubMed Google Scholar * Juan José Rincon-Ramírez View author
publications You can also search for this author inPubMed Google Scholar * Diana Carolina Moreno-Ordóñez View author publications You can also search for this author inPubMed Google Scholar
* Roberto Franco-Vega View author publications You can also search for this author inPubMed Google Scholar * Jhoan Sebastian Roncancio-Muñoz View author publications You can also search for
this author inPubMed Google Scholar * Alvaro Javier Burgos-Cardenas View author publications You can also search for this author inPubMed Google Scholar * Ezequiel Lacunza View author
publications You can also search for this author inPubMed Google Scholar * Justo P. Castaño View author publications You can also search for this author inPubMed Google Scholar * Carlos
Diéguez View author publications You can also search for this author inPubMed Google Scholar * Rubén Nogueiras View author publications You can also search for this author inPubMed Google
Scholar * Elizabeth Sanchez View author publications You can also search for this author inPubMed Google Scholar * Jorge Eduardo Caminos View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS Additionally, all authors have contributed to the intellectual content of this manuscript: (a) J.E.C., M.F.G., J.P.C., C.D., R.N. and R.F.V.
conceived and designed the experiments, (b) J.E.C., M.F.G., H.A.R.N. and J.D.B.A. performed the experiments, (c) E.L., J.E.C., H.A.R.N., J.D.B.A., J.J.R.R., E.P.S. and D.C.M.O. analyzed the
data and (d) J.E.C., C.D., R.N., J.P.C., M.F.G., E.S., A.J.B.C., J.S.R.M., H.A.R.N., J.D.B.A. and R.F.V. prepared the original draft and edited manuscript. CORRESPONDING AUTHOR
Correspondence to Jorge Eduardo Caminos. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Garcés, M.F., Buell-Acosta,
J.D., Rodríguez-Navarro, H.A. _et al._ Serum angiopoietin-like 3 levels are elevated in obese non diabetic men but are unaffected during an oral glucose tolerance test. _Sci Rep_ 10, 21118
(2020). https://doi.org/10.1038/s41598-020-77961-8 Download citation * Received: 21 May 2020 * Accepted: 29 September 2020 * Published: 03 December 2020 * DOI:
https://doi.org/10.1038/s41598-020-77961-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
