Enhancement of campylobacter hepaticus culturing to facilitate downstream applications
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT _Campylobacter hepaticus_ causes Spotty Liver Disease (SLD) in chickens. _C. hepaticus_ is fastidious and slow-growing, presenting difficulties when growing this bacterium for the
preparation of bacterin vaccines and experimental disease challenge trials. This study applied genomic analysis and in vitro experiments to develop an enhanced _C. hepaticus_ liquid culture
method. In silico analysis of the anabolic pathways encoded by _C. hepaticus_ revealed that the bacterium is unable to biosynthesise l-cysteine, l-lysine and l-arginine. It was found that
l-cysteine added to Brucella broth, significantly enhanced the growth of _C. hepaticus_, but l-lysine or l-arginine addition did not enhance growth. Brucella broth supplemented with
l-cysteine (0.4 mM), l-glutamine (4 mM), and sodium pyruvate (10 mM) gave high-density growth of _C. hepaticus_ and resulted in an almost tenfold increase in culture density compared to the
growth in Brucella broth alone (log10 = 9.3 vs 8.4 CFU/mL). The type of culture flask used also significantly affected _C. hepaticus_ culture density. An SLD challenge trial demonstrated
that _C. hepaticus_ grown in the enhanced culture conditions retained full virulence. The enhanced liquid culture method developed in this study enables the efficient production of bacterial
biomass and therefore facilitates further studies of SLD biology and vaccine development. SIMILAR CONTENT BEING VIEWED BY OTHERS _BACILLUS ARYABHATTAI_ CKNJH11 AS A PROMISING PROBIOTIC
IMPROVES GROWTH PERFORMANCE AND EGG QUALITY IN LAYING HENS Article Open access 21 April 2025 DRUG-INDEPENDENT CONTROL STRATEGY OF CLOSTRIDIAL INFECTION IN BROILER CHICKENS USING ANTI-TOXIN
ENVIRONMENTALLY FRIENDLY MULTIENZYMES Article Open access 06 April 2023 MICROBIOTA ATTENUATES CHICKEN TRANSMISSION-EXACERBATED CAMPYLOBACTERIOSIS IN _IL10__−/−_ MICE Article Open access 30
November 2020 INTRODUCTION _Campylobacter hepaticus_ has been identified as the causative agent of Spotty Liver Disease (SLD) in laying hens1. _C. hepaticus_ is a fastidious bacterium that
requires microaerobic conditions (a mix of 5–10% oxygen, 5–10% CO2, and 80–85% N2), a narrow temperature range (growth at 37 and 42 °C, but not 25 °C), and rich nutrient media for
growth1,2,3,4. The incubation time needed to form _C. hepaticus_ colonies on agar plates varies from 3 to 7 days, depending on the strain cultivated1,2,5. The incubation time for other
_Campylobacter_ species, such as _C. jejuni, C. coli, C. lari_, and _C. concisus_ ranges from 24 to 48 h on plates and 18–24 h in liquid media6,7,8,9. In previous studies_,_ when a large _C.
hepaticus_ biomass was required, such as for SLD induction experiments, _C. hepaticus_ was cultured on Brucella agar supplemented with 5% defibrinated horse blood (HBA), incubated for 3
days and harvested. _C. hepaticus_ had to be harvested from dozens of Petri dishes to produce sufficient biomass for a modestly sized animal trial10. This methodology is time–consuming, uses
a lot of resources, and is prone to contamination. It also presents difficulties with scaling up to produce sufficient biomass to produce challenge material for large animal trials or for
production of killed vaccines_._ Although Brucella broth, the standard media for growing _C. hepaticus_ cultures, is a rich medium, it may not supply all the nutrients required to support
optimal growth of _C. hepaticus_. An in silico approach was used to identify nutrients that may need to be supplemented to improve culture productivity. The availability of _C. hepaticus_
whole-genome sequences11 and tools such as Metagenomic Rapid Annotations using Subsystem Technology (MG-RAST)12 and Kyoto Encyclopedia of Genes and Genomes (KEGG)13 to annotate genomes and
analyse metabolic pathways, allows the identification of growth-supporting compounds for _C. hepaticus_. KEGG analysis has been used to predict the nutritional requirements of _C. jejuni_
NCTC 1116814. The study found that l-cysteine, l-leucine, l-methionine, and l-aspartic acid are essential amino acids that need to be exogenously supplied for the growth of _C. jejuni_. The
addition of pyruvate or lactate and niacinamide as carbon sources have previously been shown to improve the growth of _C. jejuni_ NCTC 1116815. Similarly, necessary substrates for the growth
of _Bukholderia glumae_ were defined using the Pathcomp tool in KEGG16_._ The objective of this project was to identify and evaluate compounds that are required or that could enhance the
growth of _C. hepaticus_ in liquid culture_,_ by analysing the metabolic pathways of this species. Also, culture conditions, including temperature, pH, mixing, and culture vessel types were
assessed to characterise and improve the growth of _C hepaticus_ in liquid culture. A reliable liquid culture method that resulted in high culture biomass would aid in the design of
reproducible assays to investigate stress resistance, virulence mechanisms, vaccine development, and survival in the environment of _C. hepaticus_. This study applied genomic analysis and in
vitro experiments to develop an enhanced _C. hepaticus_ liquid culture method. RESULTS IN SILICO PATHWAY ANALYSIS OF _C. HEPATICUS_ KEGG pathway analysis of three _C. hepaticus_ strains
showed that this bacterium harbours a complete tricarboxylic acid (TCA) cycle. Therefore, _C. hepaticus_ can use all substrates in the citric pathways including pyruvate, succinate,
oxaloacetate, fumarate, 2-oxoglutarate, malate and citrate as energy sources. The genome of _C. hepaticus_ also contains genes encoding the enzymes for complete metabolic pathways for many
amino acids, such as l-methionine, l-histidine, l-alanine, l-glycine, l-valine, l-leucine and l-threonine. In contrast, _C. hepaticus_ lacks a complete pathway for the biosynthesis of
l-arginine from l-glutamate. The _argE_ gene, encoding acetylcitrulline deacetylase is absent. l-lysine cannot be biosynthesised from l-aspartate as the _dapA_ and _dapB_ genes are not
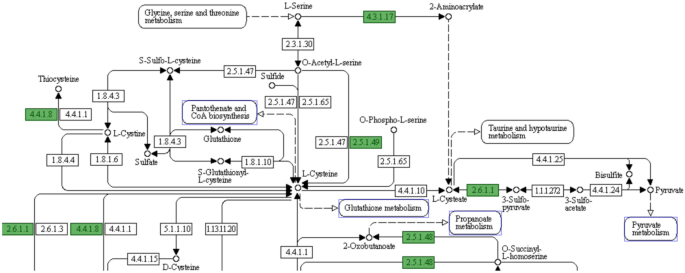
present. Similarly, it is predicted that l-cysteine cannot be biosynthesised, as the _cysE, cysM_ and _cysK_ genes required to synthesise l-cysteine from l-serine and l-methionine, are
absent (Fig. 1). Consequently, _C. hepaticus_ was predicted to be unable to biosynthesise l-cysteine, l-lysine and l-arginine. Adding these amino acids to the culture media may improve the
growth of _C. hepaticus._ This prediction was tested in the following in vitro experiments. Sodium pyruvate was also added into Brucella broth to check the growth of _C. hepaticus,_ because
it has been used as a carbon source14 and scavenges hydrogen peroxide17, an oxidative stress factor that is generated via the use of oxygen of bacteria18. THE EFFECT OF CULTURE VESSEL TYPE,
PH, AND INOCULUM LEVEL ON THE YIELD OF _C. HEPATICUS_ Before testing the effect of amino acids and sodium pyruvate on the growth of _C. hepaticus_, three factors, culture vessel types, pH,
and inoculum level were examined. Figure 2A shows that _C. hepaticus_ grew to a higher final density when growth in 24-well cell culture plates (CCP24) and 75 cm2 cell culture flasks (TCF75)
(log10 CFU/mL = 8.54–8.62 (P ≤ 0.05) compared to that seen in 50 mL tubes (CT50) and 250 mL Erlenmeyer flasks (EF) (log CFU/mL = 7.75–8.0). The CCP24 culture plates (1 mL of culture
added/well) were therefore used to test the effect of amino acid and sodium pyruvate supplementation on the growth of _C. hepaticus,_ and TCF75 flasks (25 mL of media/flask) were used to
scale-up the production of biomass of _C. hepaticus._ _C. hepaticus_ was monitored for growth in Brucella broth adjusted to a pH ranging from 6.0 to 8.0 to evaluate the pH tolerance. There
was no statistically significant difference in the growth of _C. hepaticus_, indicating little or no effect of pH within this range (Fig. 2B). Therefore, Brucella broth without pH adjustment
was used for the further culturing of _C. hepaticus,_ as it has a neutral pH value (7.0 ± 0.2)_._ The growth kinetics of _C. hepaticus_ in Brucella broth was investigated using initial
inocula ranging from 101 to 107 CFU/mL (Fig. 2C). Viable cells counts were carried out after 0, 24, 48, 60, 72 and 96 h of incubation. The two highest inoculum levels (106 and 107 CFU/ml)
produced maximum growth to 108 CFU/ml after 24 h of incubation. With the lower inoculum levels (105 and 104 CFU/mL), _C. hepaticus_ achieved maximum growth after 48 h of incubation and for
low inoculum levels of 101 to 103 CFU/ml, the maximum growth of _C. hepaticus_ was reached after 60 h. THE EFFECT OF AMINO ACIDS AND SODIUM PYRUVATE ON THE GROWTH OF _C. HEPATICUS_ _C.
hepaticus_ cultured in Brucella broth supplemented with 0.4 mM l-cysteine, 4 mM l- glutamine, 0.8 mM l-valine, 0.4 mM l-serine and 10 mM sodium pyruvate exhibited a significantly greater
growth compared to a culture grown in Brucella broth only (log10 = 8.66–9.26 vs 8.31 CFU/mL) (Fig. 3). l-cysteine (0.4 mM) showed highest growth enhancement to log10 = 9.26 CFU/mL,
significantly higher than the density obtained with the other supplements (p ≤ 0.005). No significant difference was observed (p > 0.05, Fig. 3) when _C. hepaticus_ was grown in Brucella
broth supplemented with other amino acids including l-lysine, l-methionine, l-histidine, l-glycine, l- arginine, l-leucine, and l-threonine compared to a culture grown in Brucella broth
only. The growth of _C. hepaticus_ was then investigated in Brucella broth supplemented with different combinations of each compound that could enhance the growth of _C. hepaticus,_ as
demonstrated above_,_ including l-cysteine, l-glutamine, l-valine, l-serine, and sodium pyruvate. In general, all combinations of supplements in Brucella broth significantly enhanced the
growth of _C. hepaticus_ compared to Brucella broth alone. The highest growth of _C. hepaticus_ observed was in Brucella broth supplemented with a mixture of l-cysteine (0.4 mM), l-glutamine
(4 mM) and sodium pyruvate (10 mM) (log10 = 9.34), followed by a combination of l-cysteine (0.4 mM) and sodium pyruvate (10 mM) (log10 = 9.17), and only l-cysteine (0.4 mM) (log10 = 9.11)
(Table 1). Brucella broth supplemented with l-cysteine (0.4 mM), and l-glutamine (4 mM) and sodium pyruvate (10 mM) was then used to grow _C. hepaticus_ in TCF75 flasks in the following
experiments. EFFECT OF TEMPERATURE AND INCUBATING CONDITIONS ON GROWTH OF _C. HEPATICUS_ Because the culture vessels that provided the largest surface to volume ratio produced the highest
density cultures it was proposed that gas exchange between the liquid medium and the microaerophilic atmosphere might be important. Therefore, the effects of agitation of the cultures by
shaking were investigated. In the modified Brucella broth developed in this study, after 48 h of incubation _C. hepaticus_ grew significantly better (p ≤ 0.05) in static conditions, with
log10 = 9.25 CFU/mL compared to log10 = 8.76 CFU/mL when shaken. It was also found that _C. hepaticus_ exhibited significantly better growth at 37 °C than 42 °C (p ≤ 0.05), log10 = 9.28
CFU/mL compared to log10 = 8.09 CFU/mL. GROWTH OF DIFFERENT _C. HEPATICUS_ STRAINS IN MODIFIED BRUCELLA BROTH The medium development experiments described above were carried out on a single
strain of _C. hepaticus_. To determine if the improved media composition could also enhance the growth of other _C. hepaticus_ isolates, the growth of different isolates in the modified
Brucella broth, at 37 °C, under microaerobic conditions, in static TCF75 flasks was examined. All tested strains of _C. hepaticus_ (HV10T, 19L. VICOCT18, WESTERN3, NSWJUNE19, SAJULY18 and
DALE3) reached densities of log10 = 9.25–9.49 CFU/mL after 48 h, significantly higher (p ≤ 0.05) than non-supplemented Brucella broth (log10 = 8.24–8.66 CFU/mL) Fig. 4). No significant
difference in growth was observed among all _C. hepaticus_ isolates in the modified Brucella broth. EXPERIMENTAL INFECTION OF LAYING HENS WITH _C. HEPATICUS_ CULTURE GROWN IN MODIFIED
BRUCELLA BROTH One of the principal reasons to develop improved culturing conditions for _C. hepaticus_ was to make the preparation of challenge inocula easier and more reproducible for
experimental infection trials used to investigate the biology of infections and assess the efficacy of various SLD treatment protocols. Experience with preparing challenge inocula using the
previously described plate harvesting method had suggested that the state of the inoculum was critical to the success of infection10. Therefore, it was important to establish that the
cultures grown in the modified Brucella liquid medium were capable of eliciting disease. Hens entering peak lay were challenged with, either _C. hepaticus_ HV10T harvested from Brucella agar
plates, or bacteria grown in the newly devised liquid media conditions and the induction of SLD lesions on the liver were scored. Birds in the control group, inoculated with fresh modified
Brucella broth, showed no lesion on livers, whereas 12 out of 12 hens inoculated with the agar plate derived bacteria had lesions on the liver and 11 out of 12 hens inoculated with bacteria
grown in the modified Brucella liquid had liver lesions. Based on disease scores, there was no significant difference between the degree of disease elicited by bacteria grown under the two
conditions (Fig. 5), demonstrating that _C. hepaticus_ grown in the modified Brucella broth developed in this study had equal levels of virulence as those grown by the previously described
plate culture method. The group sizes were sufficient to detect a one-point difference in mean scores with an alpha of 0.05 and 80% power. DISCUSSION In silico pathway analysis of the _C.
hepaticus_ genome predicted that the bacterium lacks some of the genes encoding enzymes required for the biosynthesis of l-cysteine, l-lysine and l-arginine. _C. hepaticus_ lacks the genes
_cysE_ and _cysK, cysM_ to synthesise l-cysteine from l-serine and sulphur. _cysE_ encodes serine acetyltransferase and _cysK_ encodes cysteine synthase. These enzymes are required for the
synthesis of l-cysteine from l-serine in bacteria and plants19. CysE synthesises l-cysteine from l-serine by catalysing an acyl transfer from acetyl-CoA. CysK catalyses O-acetyl-l-serine
combining with hydrogen sulphide to yield l-cysteine19. The addition of l-cysteine to Brucella broth improved the growth of _C. hepaticus_ significantly, to more than 109 CFU/mL compared to
Brucella broth only. This indicates that even though Brucella broth is a rich nutrient source the l-cysteine level is insufficient to support maximal growth of _C. hepaticus_. Growth support
of l-cysteine was also reported in other _Campylobacter_ species. l-cysteine was defined as a vital source of sulphur for _C. jejuni_20. _C. jejuni_ grown in minimal medium with l-cysteine
added was better than their growth in the medium without cysteine14,21. l-cysteine has been identified as a chemotactic attractants of _C. jejuni_22,23. In contrast, although _C. hepaticus_
was also predicted to be unable to biosynthesise l-lysine and l-arginine, the addition of these amino acids to Brucella broth did not significantly increase the culture densities that could
be achieved for _C. hepaticus_ compared to densities supported by unmodified Brucella broth. Similarly, the addition of l-arginine and l-lysine to minimal media did not enhance the growth of
_C. jejuni_ because _C. jejuni_ may have genes that support the biosynthesis of l-arginine and l-lysine14. Better growth of _C. hepaticus_ in Brucella broth supplemented with l-glutamine,
l-valine or l-serine was observed although no missing genes that are responsible for the biosynthesis of these amino acids by _C. hepaticus_ were detected. It was demonstrated that
l-glutamine and l-serine are chemoattractants for _C. jejuni_24. In chickens, l-cysteine, l-glutamine, and l-serine are abundant in chicken liver. The chicken gut also has sufficent
quantities of necessary amino acids such as l-cysteine, l-glutamine, l-valine and l-serine for the growth of many bacteria25 and this likely explains why, despite its inability to synthesise
a number of amino acids, _C. hepaticus_ can colonise the intestinal tract of laying hens10. It was found that the reduction of amino acids such as l-cysteine, l-glutamine and l-serine in
the chicken diet contributed to the reduction of _Campylobacters_ in chickens26. The authors explained that these amino acid are involved in the formation of the mucin production of the
intestinal mucus layer and essential for the survival and growth of _Campylobacter_s27. Thus, an increase or decrease in the concentration of these amino acids results in changes in the
number of _Campylobacters_ in the chicken gut26. For other amino acids including l-methionine, l-histidine, l-glycine, l-leucine, and l-threonine, there was no significant difference in the
growth of _C. hepaticus._ These results agree with the bioinformatic pathway analysis. This study found that the _C. hepaticus_ showed maximum growth in Brucella broth supplemented with a
mixture of l-cysteine (0.4 mM), l-glutamine (4 mM) and sodium pyruvate (10 mM) and reached 109.34 CFU/mL. Sodium pyruvate provides an additional carbon source to support the growth of _C.
hepaticus_ in Brucella broth. _C. hepaticus_ may heavily depend on the Kreb's cycle to generate energy. Many studies have demonstrated the important role of pyruvate in the growth of
campylobacters. Sodium pyruvate is one of the compounds in the _Campylobacter_ growth supplement product (FBP supplement)28 and _Campylobacter_ selective supplement29. Pyruvate was found to
promote the growth of _C. jejuni_ NCTC 11168 in MEM medium14. This compound plays a central role in _C. jejuni_ metabolism and can be fermented to various products such as acetate, formate,
lactate and succinate. It is linked to carbohydrate and amino acid catabolism to produce energy. The synthesis of l-leucine, l-valine, l-alanine and l-isoleucine by campylobacters use
pathways in which pyruvate can play a key role30. Pyruvate also acts as an electron acceptor and can decrease the concentration of hydrogen peroxide to reduce the damage caused by oxygen to
bacteria31. _C. hepaticus_ grew to higher densities in CCP24 and TCF75 than CT50 and EF, suggesting that the surface-area-to-volume ratio (S:V) may be important, possibly for gas exchange,
as mentioned in a study of improvement of culturing of _C. jejuni_32. These authors demonstrated that TCF75 has more S:V ratio than EF and CT50 and therefore TCF75 provided better
atmospheric exchange than EF and CT50. Also, tissue culture flasks were recommended to grow and study standard growth curves of _C. jejuni_33. In a study of the growth of _Helicobacter
pylori_, Gas–Permeable Lifecell tissue culture flasks gave improved growth in Brucella broth supplemented with fetal bovine serum34. These authors also mentioned the effect of surface area
on the growth of _H. pylori_ and suggested that a small surface area resulted in poorer growth of _H. pylori_. Tissue culture flasks were used early in the history of the culture of _C.
jejuni_ and _C. pylori_35,36 and have been employed in many studies of _Campylobacter_ species. It may show that _C. hepaticus_ requires a larger S:V ratio to grow due to better gas
exchange, although it is then unexpected that agitation had a negative effect. A low level of oxygen may be an obligatory requirement for _C. hepaticus_ growth. Under anaerobic conditions
and higher oxygen tensions (21%), _C. hepaticus_ failed to grow1. Similarly, _C. jejuni_ does not grow under anaerobic conditions37,38 and oxygen is considered a requirement for DNA
synthesis in _C. jejuni_37. _Campylobacter_ spp. are sensitive to high oxygen tensions, but still need an optimal oxygen concentration (2%-10%) to grow38. _C. hepaticus_ contains genes for
oxidative phosphorylation11 that need oxygen as an acceptor to generate ATP. _C. hepaticus_ achieved maximum growth after 48–72 h, depending on the initial inoculum, showing slower growth in
comparison to other _Campylobacters_. The maximum growth of _C. jejuni_ was at around 30 h in both Nutrient Broth Number 2 and Mueller Hinton broth39. _C. jejuni_ reached the highest
densities after 24 h of growth in Brain Heart Infusion broth40. _C. hepaticus_ can grow in a range of pH from 6.0 to 8.0. It has been reported that other _Campylobacter_ species also grow
well in this pH range41. Chickens normally have a pH of around 6.3 in the liver42, 6.4 in the small and large intestine43, 6.6—6.7 for caecum43,44, and 6.0 for bile45. These are all tissues
and environments in which it has been shown that _C. hepaticus_ can survive and colonise. _C. hepaticus_ showed better growth at 37 °C than 42 °C while this microorganism has only been
isolated from chickens, which have a body temperature of 40–42°oC46. The growth of _C. hepaticus_ was reported to be somewhat slow at 42 °C, taking 7 days to form colonies in sheep blood
agar3. Temperature differently affects _Campylobacter_ species regarding the growth, motility, and ability to invade the host cells. A study showed that _C. jejuni_ grew at both 37 °C and 42
°C but showed differences in motility and invasion. _C. coli_ grew and moved better at 42 °C. _C. fetus,_ a bacterium that is frequently detected in poultry, showed greater growth and
invasion at 37 °C47. Using the modified Brucella broth developed in this study, together with growth conditions including the use of a large surface area culture vessel, at 37 °C, in
microaerophilic and static conditions, _C. hepaticus_ cultures could grow to 109 CFU/mL and showed virulence in laying hens. This culturing method is time-saving and more cost-effective than
the previously used plate harvesting method to obtain the large biomass required for SLD animal induction experiments and bacterin vaccine production. It also reduces the amount of
subculturing needed, possibly minimising the effect of repeated subculture on the virulence of _C. hepaticus._ A study has shown that the repeated subculturing of the somewhat related
organism, _H. pylori,_ could result in a decrease of adhesion, motility, gastric inflammation and cytotoxicity, and repeated culturing is a recognised way that bacteria have been attenuated
to produce live vaccines48. Thus, the method described in this study can facilitate further studies on _C. hepaticus_ biology and SLD. MATERIALS AND METHODS IN SILICO ANALYSIS OF _C.
HEPATICUS_ METABOLISM The metabolic pathways encoded within the genomes of _C. hepaticus_ strains HV10T, 19L and VICOCT18, were analysed using (1) RAST (Rapid Annotation using Subsystem
Technology)12 and the SEED49 (http://rast.nmpdr.org/rast.cgi) for annotating the genomes and metabolic pathways prediction of _C. hepaticus_; and (2) BLAST50 (Basic Local Alignment Search
Tool) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for comparing nucleotides and protein sequences of _C. hepaticus_ with available sequences in the gene bank. _C. hepaticus_ HV10T is the type
strain for _C. hepaticus_, 19L is a representative of a clade that is distinct form HV10T and VICOCT18 is a more recent isolate. BACTERIAL STRAINS AND CULTURE CONDITIONS The _C. hepaticus_
strains HV10T, 19L11, VICOCT18, WESTERN3, NSWJUNE19, SAJULY18 and DALE351 were used in the study. These strains are representative isolates from independent SLD outbreaks from widely
separated geographical locations in Australia. All _C. hepaticus_ strains were stored at − 80 °C in 70% Brucella broth (BD BBL™) and 30% glycerol. _C. hepaticus_ was routinely cultured on
Brucella agar plates (Brucella broth (BD BBL™) + 1.5% agar (BD BBL™)) supplemented with 5% defibrinated horse blood (Equicel) (HBA) and cultured at 37 °C under microaerobic conditions
(created using Campygen 3.5L gas generation packs (Oxoid)) in an anaerobic jar, for 96 h to recover _C. hepaticus_ cells from − 80 °C stock or for 72 h if they were subcultured from HBA
plates. EFFECT OF TYPE OF CULTURE VESSEL ON THE GROWTH OF _C. HEPATICUS_ Costar® 24-well cell culture plates (CCP24), Corning® 50 mL centrifuge tubes (CT50) with a vented cap (0.2 μm pore
size), Corning® 75cm2 cell culture flask (TCF75) with a vented cap (0.2 μm pore size) and Erlenmeyer flasks (250 mL) (EF) were used to compare the growth of _C. hepaticus_ in Brucella broth
(BD BBL™). The volume of culture media used in CCP24 was 1 mL, 25 mL (CT50 and TCF) and 40 mL (EF). All vessels were placed in BD GasPak™ EZ container, charged with CampyGen 3.5 L (Oxoid) to
produce microaerobic conditions and then incubated at 37 °C for 48 h. The growth rate of _C. hepaticus_ HV10 was determined by the plate count method on HBA plates. EFFECT OF PH AND INITIAL
INOCULUM ON _C. HEPATICUS_ GROWTH The pH of Brucella broth was adjusted from 6.0 to 8.0 in increments of 0.5 units using 1 M NaOH or 1 M HCl. _C. hepaticus_ was cultured in CCP24 plates to
examine growth at different pH levels. Each well of CCP24 was inoculated with 106 CFU/mL of _C. hepaticus_ HV10T. Plates were incubated under microaerobic conditions at 37 °C. Growth was
enumerated after 48 h of incubation by plating serial dilution on HBA. The experiment was performed in triplicate. The effect of initial inoculum size on the growth kinetics and final
culture yields was analysed using seven initial inoculum levels (101 to 107 CFU/ml) of _C. hepaticus_ HV10T in a CCP24 plate. The plates were incubated at 37 °C under microaerobic
conditions. The growth of _C. hepaticus_ HV10T was examined after 24, 48, 60, 72, and 96 h of incubation using the plate count method on HBA. GROWTH OF _C. HEPATICUS_ IN STATIC AND SHAKING
CONDITIONS _C. hepaticus_ HV10T was suspended into Brucella broth to achieve acell density of 105 CFU/ml and then 1 mL of bacterial suspension was incubated into each well of CCP24 at static
condition. For shaking conditions, the anaerobic jars were shaken in a shaking incubator with a speed of 100 rpm. After 48 h viable _C. hepaticus_ cells were enumerated on HBA plates. The
shaking conditions were only used for this test; static growth conditions were used at all other times. EFFECTS OF SODIUM PYRUVATE AND AMINO ACIDS ON THE GROWTH OF _C. HEPATICUS_ Based on
the results of in silico pathway analysis of _C. hepaticus_ and results from Alazzam et al.14, the following supplements were added to Brucella broth to examine the growth of this bacterium:
amino acids (l-cysteine, l-lysine, l-methionine, and l-leucine, l-glutamine, l-valine, l-histidine); carbon source (sodium pyruvate) (Sigma). Each compound was completely dissolved in
Milli-Q water at a concentration recommended in MCLMAN, a new minimal medium for _C. jejuni_14. The chemical solution was passed through a 0.22 µm syringe filter and stored at − 20 °C if not
used immediately. On the day of the experiment, each substrate was added into a bacterial culture and then 1 mL transferred to each well of CCP plates. The plates were incubated under
microaerobic conditions at 37 °C for 48 h. EFFECT OF TEMPERATURE ON GROWTH OF _C. HEPATICUS_ Growth of _C. hepaticus_ was tested at 37 °C and 42 °C in Brucella broth. Briefly, _C. hepaticus_
cells were harvested from an HBA plate and suspended into Brucella broth to obtain an OD of 0.01–0.03. The bacterial suspension was supplemented with sodium pyruvate (10 mM), l-cysteine
(0.4 mM), and l-glutamine (4 mM) (based on the results from the experiments described above). Bacterial culture (1 mL) was placed in CCP plates and incubated under microaerobic conditions
for 48 h at 37 °C and 42 °C. ANIMAL TRIAL A _C. hepaticus_ chicken challenge trial was carried out to compare the virulence between _C. hepaticus_ HV10T cultures grown from modified Brucella
broth developed in this study (liquid method) and the HBA plates (plate method). The plate method was described by Van et al.10 in which _C. hepaticus_ HV10T stored at − 80 °C in glycerol
stocks was first streaked on to HBA plates, then further subcultured to HBA plates and cells were harvested and resuspended in Brucella broth to obtain 1 × 109 CFU/mL. For the liquid method,
_C. hepaticus_ was grown in Brucella broth supplemented with l-cysteine (0.4 mM), and l-glutamine (4 mM) and sodium pyruvate (10 mM) in TCF75 at 37 °C for 48 h in microaerophilic conditions
and used directly for the challenge. The animal experimentation was approved by the Wildlife and Small Institutions Animal Ethics Committee of the Victorian Department of Economic
Development, Jobs, Transport and Resources (approval number 14.16). A total of 36 Hy-Line brown laying hens were used in the experiment. Only healthy birds laying eggs regularly were
included in the study. Chickens were housed in groups of 4 birds per pen, with 3 pens per group: a total of 12 birds in each group (n = 12). Birds were randomly allocated to groups and cages
by stratified rank order based on weight. Unchallenged control birds were orally inoculated with 1 ml of Brucella broth only whereas 1 ml of Brucella broth containing 1 × 109 CFU of _C.
hepaticus_ HV10T was orally administered to the birds for the plate and broth methods groups. Birds were sacrificed after 5 days and SLD lesions on the surface of the liver were counted to
measure the severity of the induced disease. The experienced and trained scorers were blinded to the treatment groups. Scores were based on a logarithmic scale from 0 to 4, where 0 = no
visible lesions, 1 = 1–9 lesions, 2 = 10–99 lesions, 3 = 100–999 lesions and 4 = more than 1000 lesions. STATISTICAL ANALYSIS All experiments to study the growth of _C. hepaticus_ HV10 in
different conditions and supplements were repeated a minimum of three times with biological replicates. _Campylobacter_ cell counts in all tests were converted to log10 CFU/mL. Statistical
comparison of all parameters was performed by t-test, one-way ANOVA using Graphpad Prism version 8 for Windows, GraphPad Software (San Diego California USA, www.graphpad.com). The
significance level was set at 5% (p ≤ 0.05). Sample size calculation for the SLD animal trial was performed using the online calculator at https://clincalc.com/stats/samplesize.aspx.
DECLARATIONS All the methods were carried out in accordance with relevant guidelines and regulations and the animal trial is reported in accordance with the ARRIVE Essential 10 guidelines.
REFERENCES * Van, T.T.H., Elshagmani, E., Gor, M.C., Scott, P.C. & Moore, R.J. _Campylobacter hepaticus_ sp. nov., isolated from chickens with spotty liver disease. _Int. J. Syst. Evol.
Microbiol._ 66, 4518–4524. https://doi.org/10.1099/ijsem.0.001383 (2016). * Crawshaw, T. R. _et al._ Isolation of a novel thermophilic _Campylobacter_ from cases of spotty liver disease in
laying hens and experimental reproduction of infection and microscopic pathology. _Vet. Microbiol._ 179, 315–321. https://doi.org/10.1016/j.vetmic.2015.06.008 (2015). Article PubMed Google
Scholar * Gregory, M., Klein, B., Sahin, O. & Girgis, G. Isolation and characterization of _Campylobacter hepaticus_ from layer chickens with spotty liver disease in the United States.
_Avian. Dis._ 62, 78–85. https://doi.org/10.1637/11752-092017-Reg.1 (2018). Article Google Scholar * Crawshaw, T. R. _et al._ Isolation of _Campylobacter hepaticus_ from free-range
poultry with spotty liver disease in New Zealand. _N. Z. Vet. J._ 69, 58–64. https://doi.org/10.1080/00480169.2020.1801532 (2020). Article PubMed Google Scholar * Grimes, T. & Reece,
R. Spotty liver disease—An emerging disease in free-range egg layers in Australia. in _Proceedings of the Sixtieth Western Poultry Disease Conference_. 53–56 (2011). * Khan, I. U. H., Hill,
S., Nowak, E. & Edge, T. A. Effect of incubation temperature on the detection of thermophilic _Campylobacter_ species from freshwater beaches, nearby wastewater effluents, and bird fecal
droppings. _Appl. Environ. Microbiol._ 79, 7639–7645. https://doi.org/10.1128/AEM.02324-13 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Kim, J. _et al._ An improved
culture method for selective isolation of _Campylobacter jejuni_ from wastewater. _Front. Microbiol._ 7, 1345. https://doi.org/10.3389/fmicb.2016.01345 (2016). Article PubMed PubMed
Central Google Scholar * Ismail, Y., Lee, H., Riordan, S. M., Grimm, M. C. & Zhang, L. The effects of oral and enteric _Campylobacter concisus_ strains on expression of TLR4, MD-2,
TLR2, TLR5 and COX-2 in HT-29 cells. _PLoS ONE_ 8, e56888. https://doi.org/10.1371/journal.pone.0056888 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Reilly, S. S.
& Gilliand, S. E. Improved culturing techniques for _Campylobacter_. _J. Food Sci._ 68, 2752–2757 (2003). Article CAS Google Scholar * Van, T. T. H. _et al._ Induction of spotty liver
disease in layer hens by infection with _Campylobacter hepaticus_. _Vet. Microbiol._ 199, 85–90. https://doi.org/10.1016/j.vetmic.2016.12.033 (2017). Article PubMed Google Scholar * Van,
T. T. H. _et al._ Survival mechanisms of _Campylobacter hepaticus_ identified by genomic analysis and comparative transcriptomic analysis of _in vivo_ and _in vitro_ derived bacteria.
_Front. Microbiol._ 10, 107–107. https://doi.org/10.3389/fmicb.2019.00107 (2019). Article PubMed PubMed Central Google Scholar * Aziz, R. K. _et al._ The RAST server: Rapid annotations
using subsystems technology. _BMC Genomics_ 9, 75. https://doi.org/10.1186/1471-2164-9-75 (2008). Article CAS PubMed PubMed Central Google Scholar * Kanehisa, M. & Goto, S. KEGG:
Kyoto encyclopedia of genes and genomes. _Nucleic Acids Res._ 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000). Article CAS PubMed PubMed Central Google Scholar * Alazzam, B.,
Bonnassie-Rouxin, S., Dufour, V. & Ermel, G. MCLMAN, a new minimal medium for _Campylobacter jejuni_ NCTC 11168. _Res. Microbiol_ 162, 173–179.
https://doi.org/10.1016/j.resmic.2010.09.024 (2011). Article CAS PubMed Google Scholar * Velayudhan, J. & Kelly, D. J. Analysis of gluconeogenic and anaplerotic enzymes in
_Campylobacter jejuni_: an essential role for phosphoenolpyruvate carboxykinase. _Microbiology_ 148, 685–694. https://doi.org/10.1099/00221287-148-3-685 (2002). Article CAS PubMed Google
Scholar * Kawanishi, T. _et al._ New detection systems of bacteria using highly selective media designed by SMART: Selective medium-design algorithm restricted by two constraints. _PLoS
ONE_ 6, e16512. https://doi.org/10.1371/journal.pone.0016512 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Salahudeen, A. K., Clark, E. C. & Nath, K. A. Hydrogen
peroxide-induced renal injury. A protective role for pyruvate _in vitro_ and _in vivo_. _J. Clin. Invest._ 88, 1886–1893. https://doi.org/10.1172/jci115511 (1991). * Kim, J. C., Oh, E., Kim,
J. & Jeon, B. Regulation of oxidative stress resistance in _Campylobacter jejuni_, a microaerophilic foodborne pathogen. _Front. Microbiol._ 6, 751.
https://doi.org/10.3389/fmicb.2015.00751 (2015). Article PubMed PubMed Central Google Scholar * Benoni, R. _et al._ Modulation of _Escherichia coli_ serine acetyltransferase catalytic
activity in the cysteine synthase complex. _FEBS Lett._ 591, 1212–1224. https://doi.org/10.1002/1873-3468.12630 (2017). Article CAS PubMed PubMed Central Google Scholar * Vorwerk, H.
_et al._ Utilization of host-derived cysteine-containing peptides overcomes the restricted sulphur metabolism of _Campylobacter jejuni_. _Mol. Microbiol._ 93, 1224–1245.
https://doi.org/10.1111/mmi.12732 (2014). Article CAS PubMed Google Scholar * Dickgiesser, N. & Czylwik, D. Chemically defined media for auxotyping of _Campylobacter jejuni_.
_Zentralbl. Bakteriol. Mikrobiol. Hyg. A_ 260, 57–64. https://doi.org/10.1016/S0176-6724(85)80098-5 (1985). Article CAS PubMed Google Scholar * Chandrashekhar, K., Kassem, I. I. &
Rajashekara, G. _Campylobacter jejuni_ transducer like proteins: Chemotaxis and beyond. _Gut Microbes_ 8, 323–334. https://doi.org/10.1080/19490976.2017.1279380 (2017). Article CAS PubMed
PubMed Central Google Scholar * Li, Z. _et al._ Methyl-accepting chemotaxis proteins 3 and 4 are responsible for _Campylobacter jejuni_ chemotaxis and jejuna colonization in mice in
response to sodium deoxycholate. _J. Med. Microbiol._ 63, 343–354. https://doi.org/10.1099/jmm.0.068023-0 (2014). Article CAS PubMed Google Scholar * Vegge, C. S., Brøndsted, L., Li,
Y.-P., Bang, D. D. & Ingmer, H. Energy taxis drives _Campylobacter jejuni_ toward the most favorable conditions for growth. _Appl. Environ. Microbiol._ 75, 5308–5314.
https://doi.org/10.1128/aem.00287-09 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Seong, P. N. _et al._ Characterization of chicken by-products by mean of proximate
and nutritional compositions. _Korean. J. Food. Sci. Anim. Resour._ 35, 179–188. https://doi.org/10.5851/kosfa.2015.35.2.179 (2015). Article PubMed PubMed Central Google Scholar *
Visscher, C. _et al._ Influence of a specific amino acid pattern in the diet on the course of an experimental _Campylobacter jejuni_ infection in broilers. _Poult. Sci._ 97, 4020–4030.
https://doi.org/10.3382/ps/pey276 (2018). Article CAS PubMed PubMed Central Google Scholar * Adedokun, S. A., Adeola, O., Parsons, C. M., Lilburn, M. S. & Applegate, T. J. Factors
affecting endogenous amino acid flow in chickens and the need for consistency in methodology. _Poult. Sci._ 90, 1737–1748. https://doi.org/10.3382/ps.2010-01245 (2011). Article CAS PubMed
Google Scholar * Hoffman, P. S., George, H. A., Krieg, N. R. & Smibert, R. M. Studies of the microaerophilic nature of _Campylobacter fetus_ subsp. _jejuni_. II. Role of exogenous
superoxide anions and hydrogen peroxide. _Can. J. Microbiol._ 25, 8–16. https://doi.org/10.1139/m79-002 (1979). * Karmali, M. A. _et al._ Evaluation of a blood-free, charcoal-based,
selective medium for the isolation of _Campylobacter_ organisms from feces. _J. Clin. Microbiol._ 23, 456–459. https://doi.org/10.1128/JCM.23.3.456-459.1986 (1986). Article CAS PubMed
PubMed Central Google Scholar * Mendz, G. L., Ball, G. E. & Meek, D. J. Pyruvate metabolism in _Campylobacter_ spp. _Biochim. Biophys. Acta_ 1334, 291–302.
https://doi.org/10.1016/S0304-4165(96)00107-9 (1997). Article CAS PubMed Google Scholar * Verhoeff-Bakkenes, L., Arends, A. P., Snoep, J. L., Zwietering, M. H. & de Jonge, R.
Pyruvate relieves the necessity of high induction levels of catalase and enables _Campylobacter jejuni_ to grow under fully aerobic conditions. _Lett. Appl. Microbiol._ 46, 377–382.
https://doi.org/10.1111/j.1472-765X.2008.02326.x (2008). Article CAS PubMed Google Scholar * Reilly, S. S. & Gilliland, S. E. Improved culturing techniques for _Campylobacter_. _J.
Food Sci._ 68, 2752–2757. https://doi.org/10.1111/j.1365-2621.2003.tb05800.x (2003). Article CAS Google Scholar * Davis, L. & DiRita, V. Growth and laboratory maintenance of
_Campylobacter jejuni_. _Curr. Protoc. Microbiol._ 8(8A), 1 1–8A 1 7. https://doi.org/10.1002/9780471729259.mc08a01s10 (2008). * Secker, D., Tompkins, D. & Alderson, G. Gas-permeable
lifecell tissue culture flasks give improved growth of _Helicobacter pylori_ in a liquid medium. _J. Clin. Microbiol._ 29, 1060–1061. https://doi.org/10.1128/JCM.29.5.1060-1061.1991 (1991).
Article CAS PubMed PubMed Central Google Scholar * Rollins, D. M., Coolbaugh, J. C., Walker, R. I. & Weiss, E. Biphasic culture system for rapid _Campylobacter_ cultivation. _Appl.
Environ. Microbiol._ 45, 284–289. https://doi.org/10.1128/AEM.45.1.284-289.1983 (1983). Article ADS CAS PubMed PubMed Central Google Scholar * Shadowen, R. D. & Sciortino, C. V.
Improved growth of _Campylobacter pylori_ in a biphasic system. _J. Clin. Microbiol._ 27, 1744–1747. https://doi.org/10.1128/JCM.27.8.1744-1747.1989 (1989). Article CAS PubMed PubMed
Central Google Scholar * Sellars, M. J., Hall, S. J. & Kelly, D. J. Growth of _Campylobacter jejuni_ supported by respiration of fumarate, nitrate, nitrite, trimethylamine-n-oxide, or
dimethyl sulfoxide requires oxygen. _J. Bacteriol._ 184, 4187–4196. https://doi.org/10.1128/jb.184.15.4187-4196.2002 (2002). Article CAS PubMed PubMed Central Google Scholar * Kaakoush,
N. O., Miller, W. G., De Reuse, H. & Mendz, G. L. Oxygen requirement and tolerance of _Campylobacter jejuni_. _Res. Microbiol._ 158, 644–650.
https://doi.org/10.1016/j.resmic.2007.07.009 (2007). Article CAS PubMed Google Scholar * Ghaffar, N., Connerton, P. & Connerton, I. Filamentation of _Campylobacter_ in broth
cultures. _Front. Microbiol._ 6, 657. https://doi.org/10.3389/fmicb.2015.00657 (2015). Article PubMed PubMed Central Google Scholar * Wright, J. _et al._ Metabolite and transcriptome
analysis of _Campylobacter jejuni_ in vitro growth reveals a stationary-phase physiological switch. _Microbiology_ 155, 80–94. https://doi.org/10.1099/mic.0.021790-0 (2009). Article CAS
PubMed Google Scholar * Skirrow, M. B. _Encyclopedia of Food Sciences and Nutrition. _2nd Edn. (ed. Benjamin Caballero) 779–786 (Academic Press, 2003). * King, Y. T. & Chen, T. C.
Chemical and physical characteristics of chicken livers following adrenocorticotropic hormone-induced stress. _J. Food. Sci._ 63, 589–591. https://doi.org/10.1111/j.1365-2621.1998.tb15791.x
(1998). Article CAS Google Scholar * Mabelebele, M., John, A., Ng’ambi, J., Norris, D. & Ginindza, M. Comparison of gastrointestinal tracts and pH values of digestive organs of Ross
308 broiler and indigenous Venda chickens fed the same diet. _Asian. J. Anim. Vet. Adv._ 9, 71–76. https://doi.org/10.3923/ajava.2014.71.76 (2014). Article CAS Google Scholar * Ciurescu,
G., Vasilachi, A., Habeanu, M. & Dragomir, C. Effects of dietary lentil seeds inclusion on performance, carcass characteristics and cecal pH of broiler chickens. _Indian J. Anim. Sci._
87, 1130–1134 (2017). CAS Google Scholar * Zaefarian, F., Abdollahi, M. R., Cowieson, A. & Ravindran, V. Avian liver: The forgotten organ. _Animals (Basel)_ 9, 63.
https://doi.org/10.3390/ani9020063 (2019). Article Google Scholar * Bolzani, R., Ruggeri, F. & Olivo, O. M. Average normal temperature of the chicken in the morning and after 1–2 days
of fasting. _Boll. Soc. Ital. Biol. Sper._ 55, 1618–1622 (1979). CAS PubMed Google Scholar * Aroori, S. V., Cogan, T. A. & Humphrey, T. J. The effect of growth temperature on the
pathogenicity of _Campylobacter_. _Curr. Microbiol._ 67, 333–340. https://doi.org/10.1007/s00284-013-0370-1 (2013). Article CAS PubMed Google Scholar * Kim, S. S. _et al._ The effect of
the repeated subcultures of _Helicobacter pylori_ on adhesion, motility, cytotoxicity, and gastric inflammation. _J. Korean. Med. Sci._ 17, 302–306.
https://doi.org/10.3346/jkms.2002.17.3.302 (2002). Article PubMed PubMed Central Google Scholar * Overbeek, R. _et al._ The SEED and the Rapid Annotation of microbial genomes using
Subsystems Technology (RAST). _Nucleic Acids Res._ 42, 206–214. https://doi.org/10.1093/nar/gkt1226 (2014). Article CAS Google Scholar * Altschul, S. F. _et al._ Gapped BLAST and
PSI-BLAST: A new generation of protein database search programs. _Nucleic Acids Res._ 25, 3389–3402. https://doi.org/10.1093/nar/25.17.3389 (1997). Article CAS PubMed PubMed Central
Google Scholar * Phung, C. _et al._ _Campylobacter hepaticus_, the cause of Spotty Liver Disease in chickens: Transmission and routes of infection. _Front. Vet. Sci._ 6, 505.
https://doi.org/10.3389/fvets.2019.00505 (2019). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS CP was supported by a joint Vietnam International Education Development
and RMIT Scholarship. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Science, RMIT University, Bundoora West Campus, Bundoora, VIC, Australia Canh Phung, Robert J. Moore & Thi
Thu Hao Van * Scolexia Pty. Ltd., Moonee Ponds, VIC, Australia Timothy B. Wilson, José A. Quinteros & Peter C. Scott Authors * Canh Phung View author publications You can also search for
this author inPubMed Google Scholar * Timothy B. Wilson View author publications You can also search for this author inPubMed Google Scholar * José A. Quinteros View author publications You
can also search for this author inPubMed Google Scholar * Peter C. Scott View author publications You can also search for this author inPubMed Google Scholar * Robert J. Moore View author
publications You can also search for this author inPubMed Google Scholar * Thi Thu Hao Van View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
C.P. designed, conducted the study and carried out data analysis. R.J.M. and T.T.H.V. conceived and supervised the work. T.B.W., J.A.Q. and P.C.S. facilitated and conducted the animal trial.
C.P. wrote the first draft of the manuscript and T.T.H.V. and R.J.M. critically reviewed the manuscript. All authors commented on the manuscript and approved the final version of the
manuscript. CORRESPONDING AUTHOR Correspondence to Robert J. Moore. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in
this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Phung, C., Wilson, T.B., Quinteros, J.A. _et
al._ Enhancement of _Campylobacter hepaticus_ culturing to facilitate downstream applications. _Sci Rep_ 11, 20802 (2021). https://doi.org/10.1038/s41598-021-00277-8 Download citation *
Received: 05 July 2021 * Accepted: 30 September 2021 * Published: 21 October 2021 * DOI: https://doi.org/10.1038/s41598-021-00277-8 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
