Characterization of a green stentor with symbiotic algae growing in an extremely oligotrophic environment and storing large amounts of starch granules in its cytoplasm
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The genus _Stentor_ is a relatively well-known ciliate owing to its lucid trumpet shape. _Stentor pyriformis_ represents a green, short, and fat _Stentor_, but it is a little-known
species. We investigated 124 ponds and wetlands in Japan and confirmed the presence of _S. pyriformis_ at 23 locations. All these ponds were noticeably oligotrophic. With the improvement of
oligotrophic culture conditions, we succeeded in long-term cultivation of three strains of _S. pyriformis_. The cytoplasm of _S. piriformis_ contains a large number of 1–3 μm refractive
granules that turn brown by Lugol’s staining. The granules also show a typical Maltese-cross pattern by polarization microscopy, strongly suggesting that the granules are made of
amylopectin-rich starch. By analyzing the algal rDNA, it was found that all _S. pyriformis_ symbionts investigated in this study were _Chlorella variabilis._ This species is known as the
symbiont of _Paramecium bursaria_ and is physiologically specialized for endosymbiosis. Genetic discrepancies between _C. variabilis_ of _S. pyriformis_ and _P. bursaria_ may indicate that
algal sharing was an old incident. Having symbiotic algae and storing carbohydrate granules in the cytoplasm is considered a powerful strategy for this ciliate to withstand oligotrophic and
cold winter environments in highland bogs. SIMILAR CONTENT BEING VIEWED BY OTHERS PREY PREFERENCE IN A KLEPTOPLASTIC DINOFLAGELLATE IS LINKED TO PHOTOSYNTHETIC PERFORMANCE Article Open
access 30 June 2023 SYMBIOTIC MICROALGAL DIVERSITY WITHIN LICHENICOLOUS LICHENS AND CRUSTOSE HOSTS ON IBERIAN PENINSULA GYPSUM BIOCRUSTS Article Open access 20 August 2020 CONSUMING FRESH
MACROALGAE INDUCES SPECIFIC CATABOLIC PATHWAYS, STRESS REACTIONS AND TYPE IX SECRETION IN MARINE FLAVOBACTERIAL PIONEER DEGRADERS Article Open access 19 May 2022 INTRODUCTION Mixotrophic
protists are reported to live in a wide range of environments1, even in highly oligotrophic environments where other photoautotrophic and heterotrophic organisms cannot survive2,3. Possible
reasons why these protists are adapted to such a harsh environment are (1) there are few large predator animals in such ponds3, (2) high UV resistance due to symbiosis shading effect4, and
(3) mixotrophy allows adaptation to harsh environmental conditions by optimizing the combination of heterotrophic and photoautotrophic organisms in the same organism1. Mixotrophic protists
such as _Stentor pyriformis_ (algae-retaining ciliate) and _Mayorella viridis_ (algae-retaining amoeba) are frequently observed and documented as the dominant protist species in highland
wetlands in Tohoku district, Japan, where average winter temperatures remain below freezing for a few months5. Even in such harsh conditions, these protists survive in non-freezing locations
at the bottom of the pond, but it remains unclear how survival strategies of such protists are related to mixotrophy. The genus _Stentor_ (family Stentoridae, order Heterotrichida) is a
relatively well-known ciliate characterized by its lucid trumpet shape. _S. pyriformis_ is a poorly described species, although _S. pyriformis_ is clearly distinguishable from other
_Stentor_ species (Table S1). The species was first described in 18936 and then appeared in a microbiota report in 19087. However, its next appearance was not until 1994, in the study on
revision of the genus8. As described in the original literature, difficulties in the cultivation of this species6 may have hindered the research on this species. In Japan, _S. pyriformis_
can be found only in highland highly oligotrophic moors, suggesting that intracellular symbiotic algae would help this species of _Stentor_ survive in such a harsh environment. In this
study, we introduce some unique cell morphology of _S. pyriformis_ and the characteristics of symbiotic algae in relation to its life strategy. METHODS SAMPLING Water containing dead leaves,
twigs, or the remnants of submerged plants was sampled from ponds in Japan. The water sample was brought back to the laboratory at Tokyo and was crudely cultured in Petri dishes. A few days
later, _Stentor_ cells containing green coccoids within their bodies were often observed. If the green _Stentor_ was visible, it was directly collected using Komagome pipette or cup
attached to the tip of the rod. We measured hydrogen ion concentration (pH) in some pond samples using URCERI Digital PH Meter (Shenzhenshi Huanhui Dianzishangwu, Shenzhen, China) and
electric conductivity (EC) using AquaPro Water Quality Tester AP-2 (HM Digital, CA, USA). CULTURE Strains of _S. pyriformis_ were cultured in 2% KCM medium (160 µg/L KCl, 260 µg/L CaCl2, 500
µg/L MgSO4 · 7H2O; pH 6.9) in Petri dishes (diameter, 9 cm; height, 2 cm) under fluorescent (64 W; height, 20 cm) (12L:12D) or LED light conditions at 25 °C. After multiple trials, _S.
pyriformis_ was successfully cultured only in low EC medium such as 2% KCM. Its EC was identified to be 1.5 µS/cm. The culture medium was changed once a week; half the volume of culture
medium (10–15 mL) was discarded and fresh medium was compensated for the shortfall. _S. pyriformis_ were fed a non-photosynthetic cryptophyte, _Chilomonas paramecium_, cultured on _Euglena_
medium (2 g/L tryptone, 1 g/L proteose peptone, 2 g/L yeast extract, 1 g/L sodium acetate, 0.01 g/L CaCl2) in a 50 mL polypropylene tube until stationary phase, which was centrifuged and
washed with pure water or by 2% KCM before feeding. CYTOLOGICAL OBSERVATIONS For electron microscopy, cells were chemically fixed with glutaraldehyde and osmium tetroxide or by metal contact
quick freezing as described previously9,10. After thin sectioning, samples on the grid mesh were stained with a lead citrate stain11 and threefold diluted EM Stainer (a lanthanoid
salts-based stain, Nisshin EM, Tokyo12). The presence of α-1, 4-linked glucose in the cytoplasm of the host _S. pyriformis_ and in the chloroplast of the symbiont was tested using Lugol’s
iodine solution (3% iodine (wt/v), 2% (wt/v) potassium iodine, and 73.4% (v/v) ethanol). Polarized light microscopy using a light microscope (Nikon Eclipse Ni, Nikon, Tokyo) with a set of
orthogonal polarizing filters (Nikon) on both the condenser lens and the CCD camera was used for imaging. For Lugol’s iodine staining, 1-μm-thick sections of chemically fixed, Spurr’s
resin-embedded samples were stained with Lugol’s iodine solution for 1 min and examined under a light microscope. For comparison, potato starch was stained with Lugol’s iodine solution for 1
min and photographed under the same conditions. For electron microscopy of the iodine reaction, sections were first stained with lead citrate and EM Stainer and then photographed. The
sections were further treated with Lugol’s iodine solution for 30 s, and the same field of view of the same sample was photographed again under an electron microscope. REINFECTION EXPERIMENT
We investigated whether endosymbiotic algal cells isolated from _S. pyriformis_ could also be infected with _Paramecium bursaria_ (strain PbKb1) and coexist in the cytoplasm. The
reinfection experiment was conducted according to Omura et al.13. Aposymbiotic _P. bursaria_ was prepared using the method described by Higuchi et al.14. When endosymbiotic _Chlorella
variabilis_ cells isolated from _P. bursaria_ is mixed with aposymbiotic _P. bursaria_, they re-establish symbiosis within a few days. Therefore, symbiotic algal cells were isolated from _S.
pyriformis_ and fed to aposymbiotic _P. bursaria_. After 30 days, microscopic observation was performed to confirm whether _P. bursaria_ accepted the alga as a symbiont. _P. bursaria_ was
fed with _Chlorogonium capillatum_ (NIES-3374) once every 3 to 4 days as food. DNA EXTRACTION, AMPLIFICATION, AND SEQUENCING _Stentor_ cells in the fresh sample from Toriko-Daira (the day
after the collection) were isolated under a stereoscopic microscope, and each was transferred into a depression slide filled with pure water. Each ciliate was washed through the tip of a
micropipette and transferred into another depression, with this process being repeated twice. Before DNA extraction, we cultured these ‘clean’ (without algae outside) ciliates for 2 days.
The aim of this short-term culture was to prompt the ciliates to digest the algae, which they had taken as food, not as symbionts. Thereafter, the isolated individuals were washed twice, and
then their DNA was extracted. For the cultured _Stentor_, we isolated individuals and washed twice, and then their DNA was extracted. For each strain, about 20 individuals were collected
into one sample. DNA extraction was performed using NucleoSpin Plant II kit (Macherey–Nagel, Düren, Germany) with modified cell fracturing. _Stentor_ cells, each containing many algal cells,
were incubated for 5 min in 400 µL Buffer PL1 with 10 µL RNase A at 65 °C. After adding 400 µL of glass beads (ø 0.1 mm), each sample was homogenized in BeadSmash 12 (WakenBTech, Kyoto,
Japan) at 5,000 rpm for 30 s. The homogenization was repeated five times, and then each sample was again incubated for 10 min at 65 °C. The subsequent procedures were performed according to
the manufacturer’s instructions. PCR was performed to amplify _Stentor_ SSU to internal transcribed spacer (ITS) rDNA region using KOD FX Neo (Toyobo, Osaka, Japan) with the primer pair of
SR-115 (5′ SSU)/Hits5 (5′ LSU; –GGT TCR CTC GCC GTT ACT A–). The PCR conditions were as follows. An initial denaturation step at 94 °C for 2 min was followed by 45 cycles of the following
conditions: 10 s at 98 °C, 30 s at 52 °C, and 90 s at 68 °C. The amplification was completed with a final step of 68 °C for 1 min. The PCR products were verified by agarose gel
electrophoresis, cutting out the shorter band (due to shorter ITSs, a general trend in ciliate, and being intron-less) from the gel and purified using NucleoSpin Gel and PCR Clean-up kit
(Macherey–Nagel). The above primer pair amplifies ciliate DNA well but not algal DNA as it is very thin. Therefore, algae-targeted PCR was separately performed with the primer pairs of
SR-1/INT-5R16 (3′ SSU) and INT-4F16 (3′ SSU)/HLR3R17 (5′ LSU). The PCR conditions were the same as those for _Stentor_. The PCR products were verified by agarose gel electrophoresis and
purified using the NucleoSpin Gel and PCR Clean-up kit. The PCR products for both ciliate and algae were directly sequenced. PHYLOGENETIC ANALYSES OF _S. PYRIFORMIS_ AND THEIR SYMBIOTIC
ALGAE SSU rDNA sequences for the _Stentor_ species were obtained by searching the keywords [stentor + ssu] and [stentor + 18 s] from the NCBI database. After rough alignment using Clustal
X218, the shorter sequences, and sequences including several ‘N’ were removed. Recent phylogenetic analyses including that of _Stentor_ species have indicated stable relationships between
the _Stentor_ species and its sister clades19,20,21,22. Therefore, the _Stentor_ sequences were aligned with a limited number of outgroup taxa. A bootstrap tree was constructed using the
neighbor-joining (NJ) method with default setting in Clustal X2 and examined using 1000 bootstrap replicates. For maximum likelihood (ML) and Bayesian inference (BI) analyses, the best
nucleotide substitution model for the data set was analyzed using the Akaike information criterion (AIC) via MEGA X23, and the GTR + G + I model was selected. ML analyses were performed with
MEGA X using the nearest-neighbor interchange (NNI) branch-swapping algorithm and 1,000 bootstrap replicates were used to estimate node support values. BI analyses were conducted using the
Markov chain Monte Carlo (MCMC) method implemented in MrBayes v3.2.624. MCMC was run for 107 generations with four chains, and trees were sampled every 1000th generation. The fixed number of
samples (25,000) was discarded as burn-in, and convergence was checked by Tracer v1.649. The SSU rDNA sequences of _S. pyriformis_ algae were first checked for group I intron insertions,
following the method described by Hoshina25. The joined exons were then submitted to BLASTN (NCBI), which indicated that the algae are closely related to species of _Chlorella_ clade
(Chlorellaceae). The alignment data of chlorellacean SSU + ITS2 rDNA sequences have been published by Heeg and Wolf26. Based on this, we added several recently described species, symbionts
of some protozoa and sequences obtained here, and then re-aligned them. Tree construction methods (and selected models) were identical to those for the host ciliate, except for MCMC running
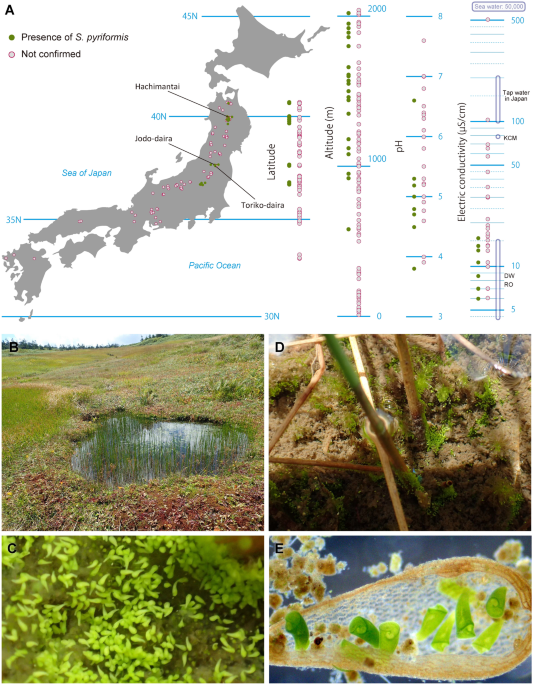
for 108 generations. RESULTS DISTRIBUTION AND ENVIRONMENT We investigated 124 ponds and wetlands in Japan and confirmed the presence of _S. pyriformis_ at 23 locations (Fig. 1A).
Distribution areas were somewhat biased into four areas of the middle to Northern part of Japan, which are located at 550 to 2020 m altitude. Water conditions were slightly acidic with a pH
of 3.8 to 6.6 but showed extremely low values of electric conductivity (EC): 6–16 µS/cm. These EC values overlap with those of distilled water or reverse osmosis water (Fig. 1A). Bogs where
_S. pyriformis_ was detected were usually located near the mountain peak or along the ridge (Fig. 1B). Frequently, we encountered blooming of _S. pyriformis_ on the bottom of the bogs (Fig.
1C). Other times, they were almost all attached to plant stalks or plant debris (Fig. 1D,E). LIGHT MICROSCOPY Cells of _S. pyriformis_ were broadly trumpet-shaped, usually 220–500 × 120–300
µm. This length–width ratio did not change significantly between the cells attached to something and swimming (Figs. 1E, 2A). The cells were colored green due to their endosymbiotic green
algae that were distributed along the whole body (Fig. 2B,C). A large number of transparent vesicles were present along the ciliary rows immediately under the cell surface (Fig. 2D). To see
the contents, the crushed cells were observed. Symbiotic algae appeared to be typical _Chlorella_-like algae, but no dividing alga was observed (Fig. 2E). The algal cells appeared more
vividly green when compared to those in _P. bursaria_, suggesting that they are richer in photosynthetic pigments (Fig. 2E,F, and Table S2). The symbiotic algae in _S. pyriformis_ had the
same size (Table S2, Fig. S1) and morphology as those in _P. bursaria_, but their biological properties were slightly different. As shown in Table S2, _S. pyriformis_’s algae did not grow on
agar plates, but could only be cultured in well-aerated liquid media. Reinfection experiments showed that _S. pyriformis_’s algae failed to re-infect the aposymbiotic strain of _P.
bursaria_, but those isolated from _P. bursaria_ easily re-infected aposymbiotic _P. bursaria_. Macronuclei were, in general, large and spherical (ø 20–35 µm, Fig. 2G). The average number of
macronuclei was 6.1 (range 4–10, n = 9) for freshly obtained samples, whereas four-year cultured cells (Table 1) contained only one or two. Micronuclei could not be identified. CELLULAR
STRUCTURE OF _S. PYRIFORMIS_ The ultrastructural observations were performed on samples collected on Oct. 28, 2019 at a small pond in Toriko-daira, Japan (37°42′17″N 140°14′53″E). First, the
chemically fixed _S. pyriformis_ was observed with an electron microscope. Large vacuoles were found inside the cells, and the symbiotic algal cells were inside the vacuoles. The symbionts
were found uncovered by individual symbiosome membranes (Fig. 3A,B). Many dark gray stained granules were found in the cytoplasm (asterisks in Fig. 3B). Granules were spherical or oval. The
dyeability was not uniform, and the periphery was dyed more intensely. When the same sample was observed by the quick-freezing and freeze substitution method, the appearance in the cytoplasm
was observed quite differently (Fig. 3C). Large intracellular vacuoles as observed under chemical fixation were not seen. In addition, individual symbiotic algae were covered by a single
symbiosome membrane (Fig. 3C,D). The distance between the symbiosome membrane and the cell wall of alga was extremely close (20–50 nm). Fluffy projections were observed on the cell wall of
the symbiotic algae (arrows in Fig. 3D). Pyrenoids were observed in the chloroplasts of the symbiotic algae, through which thylakoid membranes penetrated (arrow in Fig. 3C). Many
multi-vesicular bodies were observed in the cytoplasm (mv in Fig. 3C,E). The multi-vesicular bodies were not observed at all in the samples prepared by chemical fixation, suggesting that
this structure is very fragile and chemical treatment disintegrates it completely. The number of multi-vesicular bodies per cell was not clear, but there were several granules in each cell.
The maximum size of multi-vesicular bodies was about 1 µm, and the size of small vesicles was 100–400 nm in diameter (Fig. S2). CYTOPLASMIC GRANULES Cytoplasmic granules are colored brown by
Lugol staining, indicating that the granules contained glucans composed of α-1, 4-linked glucose (Fig. 4A). As summarized in Table 2, the stored carbohydrate granules with α-1,4-linked
glucose as a backbone are classified into three types based on their physical and chemical properties, amylose-type starch, amylopectin-type starch, and glycogen. The brown color of the
intracellular granules of _S. pyriformis_ suggests that these granules are rich in amylopectin. For comparison, potato starch, which is an amylose-rich starch, was stained with Lugol under
the same condition and turned blue (Fig. 4B). This indicates that this glucan is of the amylopectin-glycogen type. Observation of the isolated granules with a differential interference
contrast (DIC) microscope revealed that the granules had a strong refractive index (Fig. 4C). Figure 4D,E shows DIC (D) and polarization (E) microscopy of the cytoplasmic granules of _S.
pyriformis_. In the crossed polarizer orientation, each cytoplasmic granule showed a Maltese cross pattern characteristic of starch granules. An ultra-thin section of chemically fixed _S.
pyriformis_ showed that the granules were stained with heavy metals including osmium, lead, and lanthanoid ions (Fig. 4F, asterisks). The starch sheath in the pyrenoid of the symbiont was
also well stained, as shown in Fig. 4F (arrow). After taking the micrograph, the section shown in Fig. 4F was treated with Lugol’s iodine solution, as shown in Fig. 4G. The stain of both the
cytoplasmic granules and the starch sheath was removed by iodine treatment, suggesting that the glucan granules and the starch in symbiotic algae share the same affinity to heavy metals.
HOST RDNA SEQUENCE AND PHYLOGENY SSU, ITS1, 5.8S, ITS2, and 5′ LSU rDNA sequences of four _S. pyriformis_ strains were obtained (Table 1). There were 2049 nucleotides, and all four sequences
were completely identical, including one C/T mixture (Y) at the tetraloop of helix E23_1227 in the SSU rRNA structure (data not shown). _Stentor_ SSU rDNA were collected and aligned with
those of _Blepharisma_ and several outgroup taxa. The phylogenetic tree (Fig. 5A) clearly shows the monophyly of the genus _Stentor_ (the only genus of family Stentoridae). The monophyly of
each species was supported by values of Bayesian posterior probabilities (PP) = 0.99–1 and bootstrap values (BV) = 96–100. For the branching pattern of the relationships of the _Stentor_
species, BI, ML, and NJ analyses showed somewhat different topologies. Here, we provide the species relationships reflecting the differences in these three analyses with iconic morphological
characters (Fig. 5B). The monophyletic relationship of _S. roeseli_ and _S. muelleri_ was perfectly supported. _S. polymorphus_, _S. igneus,_ and _S_. cf. _katashimai_ (see Thamm et al.19)
made a clade, which was placed as a sister to _S. coeruleus_. _S. multiformis_ and _S. elegans_ made a clade. _S. pyriformis_ was clustered with _S. amethystinus_ in all analyses, although
supporting values were not high (PP/MLBV/NJBV = 0.85/74/ < 50). Sequence differences between _S. pyriformis_ and _S. amethystinus_ were 32 substitutions and 5 indels (Y is counted as one
substitution). SYMBIOTIC ALGAL RDNA SEQUENCE AND PHYLOGENY Algal sequences covering SSU, ITS1, 5.8S, ITS2, and 5′ LSU rDNA for four _S. pyriformis_ strains were obtained (Table 1). All
sequences contained group I introns at positions S943, S1367, S1512, and L200 (corresponding to the _Escherichia coli_ SSU and LSU rRNA). Because of this, each sequence reached more than
3,900 bases (L200 introns were not completely determined). The algal sequences among Jodo-daira 1436, Toriko-daira 1256, and fresh samples from Toriko-daira were identical, even including
the introns and the fast evolving ITS rDNA. The algal sequence of Hachimantai 1204 had only one different site from the others. It was at the bulge loop of helix P1 of S1512 intron28, where,
Hachimantai 1204 had A/T mixture (W), whereas the others had A. Search for matching sequences using combined SSU rDNA showed they were closely related to the member of _Chlorella_ clade
(sensu Krienitz et al.29), Chlorellaceae (Trebouxiophyceae). Using SSU + ITS2 rDNA of the member of _Chlorella_ clade, phylogenetic analyses were performed. All tree analyses (only ML tree
is shown) indicated the symbiotic algae of _S. pyriformis_ are clustered with _C. variabilis_, with which monophyletic relationships were fully supported (Fig. S3). DISCUSSION DISTRIBUTION
OF _STENTOR PYRIFORMIS_ IN JAPAN AND ITS OPTIMAL CULTURE CONDITIONS _S. pyriformis_ was described by Johnson in 18936. This algae-bearing _Stentor_ has separated spherical macronuclei
without pigmentation, which certainly differentiates it from other _Stentor_ species (see Table S1, Fig. 5B). While the most common algae-bearing _Stentor_, _S. polymorphus_ assumes a
slender trumpet shape (often shortened), _S. pyriformis_ never resembles such a slender trumpet, but assumes a pear or short conical shape, even when it is swimming6. Presence or absence of
colored pigmentation is also a prominent characteristic for separating _Stentor_ species. Among algae-bearing _Stentor_ spp., _S. polymorphus_ and _S. pyriformis_ only are considered
colorless species, whereas colored species are _S. amethystinus_, _S. fuliginosus_, _S. araucanus,_ and _S. tartari_8 (Table S1). Therefore, _S. pyriformis_ is a clearly discernible species;
however, it remains underexplored. Indeed, we could only find one paper on the new habitats of _S. pyriformis_7, with the exception of the paper of species consolidation of this genus8. We
confirmed the presence of _S. pyriformis_ at 23 locations (Fig. 1A). This indicates that _S. pyriformis_ is by no means a rare organism. We assume one of the reasons why _S. pyriformis_ has
been poorly studied is the difficulty of cultivation. In fact, Johnson6 noted that he could not keep them more than a month and never observed any cells in fission. In addition, after five
years of failure, it was finally possible to culture _S. pyriformis_ for more than several months. Because of objectively unfounded data that we could not include in the distribution data
(Fig. 1A), we noticed the wetlands where we found _S. pyriformis_ were limited to small ponds or bogs locating near the mountain peak or along the ridge (Fig. 1B). That is, the ponds
depending on rainfall without inflowing rivers. Because there is no nutrient flowing in, waters in these ponds showed noticeable oligotrophic tendency, i.e., extremely low electric
conductivity (Fig. 1A), which gave us some clues on culture. The most important point of culture for _S. pyriformis_ was keeping the medium lower electric conductivity. We use the KCM medium
diluted by 2% with Milli-Q water, and changed medium once a week. A non-photosynthetic cryptophyte, _Chilomonas paramecium_ was selected for food. We selected the food so that it would not
itself grow in the culture medium. Growing organisms, like photosynthetic algae, seemed to cause damage to _S. pyriformis_. Using this culture method, _S. pyriformis_ can be maintained for
more than four years (see Table 1). For the organisms not easy to grow in culture, Professor Michael Melkonian mentioned no protist is ‘uncultivable’, there is just human failure30. Here, it
just became possible to culture _S. pyriformis_ 120 years after its discovery; however, this method does not always work. _S. pyriformis_ appears to be extremely fragile and disintegrates
when any variables are unintentionally altered, that is, the culture is still unstable. When its condition deteriorates, the cells divide unevenly in such a way that a part of the cell is
broken. When this happens, the cells become spherical, and the drug drops to the bottom of the dish. It retains this shape for more than a month, but eventually disappears. The doubling time
of _S. pyriformis_ remains 3 to 4 weeks, even under favorable conditions (data not shown). We occasionally encountered the blooming of _S. pyriformis_ all over the bottom of the ponds (Fig.
1C). _S. pyriformis_, therefore, does not seem to be a particularly slow growing species, but our culture method appears to be far from the optimal culture conditions for them. Three _S.
pyriformis_ strains used in this study are available from the authors upon request. ULTRASTRUCTURE In this study, we compared the conventional chemical fixation method with the
rapid-freezing fixation method for electron microscopic observation. As a result, large vacuoles were observed in the cytoplasm when chemical fixation was used, but not by rapid freezing.
Instead, many multi-vesicular bodies were observed in the cytoplasm. The quick-freezing and freeze-substitution method is considered superior in that it can prevent deformation of the
intracellular structure compared to chemical fixation31. Therefore, it is possible that the originally existing multi-vesicular bodies were artificially disintegrated by chemical fixation,
and the constituent biological membranes fused together, eventually forming large vacuoles. To the authors' knowledge, no intracellular structure similar to the multi-vesicular body in
_S. pyriformis_ has been reported in protists. As multi-vesicular bodies of _S. pyriformis_ could only be observed using the freeze-substitution method, similar granules may also be found in
other protists if the same technique is used for electron microscopy. In animals, on the other hand, aggregates of secretory vesicles resembling the multi-vesicular bodies of _S.
pyriformis_ are present in cardiac telocytes32. The extracellular vesicles form multi-vesicular structures of about 1 μm in diameter and contain materials for intercellular communication
that are involved in cardiac physiology and regeneration. Because _S. pyriformis_ cells often form aggregates at the bottom of the pond, some chemicals may be released from the
multi-vesicular body, attracting nearby cells and forming aggregates. Observation by the freeze-substitution method revealed that the symbiosome membrane was in close contact with the
symbiotic chlorella. Furthermore, fluffy projections were observed on the cell wall of the symbiotic chlorella. These characteristics were consistent with those of _C. variabilis_, which is
symbiotic in the cells of _P. bursaria_9. The only difference was that in _S. pyriformis_, the symbiotic chlorella cells were scattered in the cytoplasm, whereas the symbiotic _Chlorella_ in
_P. bursaria_ were anchored directly below the cell surface. STORAGE GRANULES The iodine in Lugol’s solution selectively binds to α-1, 4-linked glucose found in polysaccharides, such as
starch33 and glycogen34. The color stained with Lugol’s solution reflects the type of glucose polymer. Starches with high amylose content stain blue-violet (cf. Fig. 4B), high amylopectin
stains red–purple, and glycogen stains reddish brown (Table 2). The granules in the cytoplasm of _S. pyriformis_ stained reddish brown with Lugol’s solution (Fig. 4A), suggesting that these
granules are composed of α-1,4-linked glucans with high number of α-1,6-linked branch points, either amylopectin-rich starch or glycogen. The pyrenoid of _Chlorella_ spp. is surrounded by a
starch sheath of two large plates35. As shown in Fig. 4F,G, the image contrast formed by electron staining of the starch granule in the chloroplast (arrow) was lost by treatment with Lugol’s
solution. Although the detailed mechanism is unknown, this observation suggests that electron-stained heavy metals (osmium, lead, and lanthanoid ions) bound to the granules may have been
eliminated by iodine in Lugol’s solution. The cytoplasmic granules of _S. pyriformis_ showed the same staining properties as the starch granules in the chloroplasts of symbiotic chlorella,
suggesting that both types of granules share chemical characteristics as polysaccharides. Alveolates make up one of the most diverse and largest groups of protists. They include three major
taxa: dinoflagellates, ciliates, and apicomplexan protozoa. All three alveolate lineages store glucose in an α-1,4-linked glucose chain with α-1, 6 branches. Ciliates are known to synthesize
glycogen granules. For example, _Tetrahymena_ has glycogen granules between 35 and 40 nm in diameter, each granule being a collection of small γ-granules of 2–3 nm in size36.
Dinoflagellates and apicomplexans typically produce more complex and larger spherical starch particles, usually greater than 1 μm in size37,38. Amylopectin-rich starch and glycogen are very
similar polysaccharides, but they differ in granule size and birefringence (Table 2). Starch granules are large, birefringent, and have a high refractive index, but glycogen does not exhibit
birefringence, and its granules generally have a size of 300 nm or less. When observed with a polarizing microscope, the starch granules show a Maltese cross pattern. This pattern is
derived from the radial arrangement of amylose and amylopectin molecules in granules and is one of the criteria for starch identification. Since the cytoplasmic granules of _S. pyriformis_
are large in size (1–3 μm) and show a typical Maltese cross pattern as shown in Fig. 4E, these granules are likely to be starch granules rich in amylopectin. PHYLOGENY OF _S. PYRIFORMIS_ AND
ITS MORPHOLOGY Relationships of _Stentor_ species were not clearly resolved. BI and ML analyses indicated basal diverging of the _S. pyriformis_ + _S. amethystinus_ clade from others, but
NJ analysis did not indicate so (Fig. 5). Recent phylogenetic analyses inclusive of _Stentor_ species also indicated basal diverging of _S. amethystinus_ from the others; however, the
monophyly of the others is not highly supported21,22. Therefore, the one thing that can be said is that _S. pyriformis_ is closely related to _S. amethystinus_. For the identification of
_Stentor_ species, the shape of macronucleus, presence or absence of cortical pigmentation, and symbiotic algae are very important and iconic characteristics8,19. _S. pyriformis_ and _S.
amethystinus_ share beaded macronuclei and the presence of symbiotic chlorella (Table S1, Fig. 5B). Pigmentation is present in _S. amethystinus,_ but not in _S. pyriformis_. Pigmentation is
a noticeable characteristic, which tinctures the whole body of _Stentor_ cells. The pigment is thought to function as a defense against predators39. However, the kind of pigment compound
depends on the species40, and the relationship between pigment possession and phylogeny is poor (Fig. 5). Of note, colorless vesicles exist in _S. pyriformis_ (Fig. 2D). The short and fat
shape is also a common characteristic for _S. pyriformis_ and _S. amethystinus_, in this genus with many elongated trumpet shape species6,8. SYMBIOTIC ALGAE IN _S. PYRIFORMIS_ Algae-targeted
PCR products from whole cells of _S. pyriformis_ were sequenced directly, and clear peaks were obtained for each. This shows that all or nearly all of the algal symbionts in each _Stentor_
cell are unified, regardless of samples under long-term culture or nature. In addition, all symbionts were closely related to _C. variabilis_ (Fig. S3), which has been known as a
representative symbiont of _P. bursaria_ (Oligohymenophorea), the model organism of multi-algae retaining protists (MARP41) style symbioses. For the chlorellacean species, the diversity of
ITS2 sequence comparisons has often been adopted. For two organisms to compare, ITS2 sequence differences (gaps are counted as a fifth character) usually fall either less than 2% for single
species or more than 10% for different species42,43. This characteristic simply encourages a species concept. The ITS2 sequences of _S. pyriformis_ algae differ only by one nucleotide site
out of 248 sites from those of _P. bursaria_ algae (Fig. 6A), which strongly suggests the symbiotic chlorella of _S. pyriformis_ are also _C. variabilis._ Several _Stentor_ species retain
coccoid green algae8 (Table S1), but only three algal sequences have been published. Two algal sequences from _S. polymorphus_ belonged to different clades from Chlorellaceae44,45. As for
the other algal sequence of _S. amethystinus_, the symbiont may belong to Chlorellaceae46. This sequence (EF589816) is short (991 bp) and only covers a part of SSU rDNA; therefore, it was
not included in our phylogenetic analyses (Fig. S3). The sequence differs from _C. variabilis_ with 10 base changes and 3 indels, indicating that it is not _C. variabilis._ In the case of
_P. bursaria-C. variabilis_ symbiosis, _C. variabilis_ has been shown to be vastly different from other free-living species. _C. variabilis_ demands organic nitrogen compounds47 and leaks
nearly half of the photosynthate to outside algal cells48,49. Furthermore, they are sensitive to the _C. variabilis virus_ (CvV; so-called ‘NC64A virus’), which is abundant in natural
freshwater50,51,52. Therefore, _C. variabilis_ should be considered an already evolved species that is unable to survive without the protection of the host cell53. Four _C. variabilis_ rDNA
sequences obtained from _S. pyriformis_ were identical, with the exception of a nucleotide position in the S1512 intron. Here, the regions without group I introns, i.e., SSU, ITS1, 5.8S, and
ITS2 rDNA, are compared among _C. variabilis_ sequences of _S. pyriformis_ and of _P. bursaria_. Several published sequences cover the above SSU-ITS region, of which varieties are shown as
_P. bursaria_ symbiont genotype (PbS-gt) 1 to 3 (Fig. 6A). Due to the small number of sequences, it is still unknown whether these genotypes depend on (or are related to) their living
regions. Genotype 1 was from USA and Japan, genotype 2 was from China, and genotype 3 was from Australia. All available sequences for _S. pyriformis_ symbionts were obtained in this study,
and they were all from Japan. As a result, all sequences of _S. pyriformis_ symbionts were aggregated into a single genotype SpS, which was distantly related to all _P. bursaria_ symbionts,
including those from Japan (Fig. 6B). Five variable sites are found in SSU rDNA among _C. variabilis_ genotypes, of which four are concentrated to that of the symbionts of _S. pyriformis_
(SpS) (Fig. 6A). C/T substitution at alignment position 656 will be a hemi-compensatory base change (hemi-CBC) at the E23_2 helix of SSU rRNA structure (Fig. 6C), whereas the other four
sites are at single strand regions (data not shown). Mutations (1821–1828) including comparatively large indels were seen in ITS1 region (Fig. 6A). It was found that all these mutations are
assembled in helix 1 (for chlorellacean ITS1 structure, see Bock et al.54,55). Thermodynamic analysis via Mfold56,57 predicted that PbS sequences form linear helix 1 similar to the other
chlorellacean species, but SpS sequences including the additional nucleotides may form a dichotomous branching of helix 1 (Fig. 6D). The group I introns inserted in SSU rDNA of _S.
pyriformis_ symbionts are identical to those of _P. bursaria_ symbionts28,58 in terms of numbers (three introns) and insertion sites (S943, S1367 and S1512). The sequences of S943 and S1512
introns are matched more than 99%. However, with respect to the S1367 intron, a large length gap was found (168 nucleotides) at the tip of P8 (Fig. S4). This section has been indicated as a
homing endonuclease gene remnant28, and those of _S. pyriformis_ symbionts are presumed to be a more degenerated form than those of _P. bursaria_ symbionts. At any rate, the symbiotic algae
of _S. pyriformis_ were found to be _C. variabilis_. Because _S. pyriformis_ never lost the symbiotic algae in four years of culture, and all four algae had nearly identical genetic
characteristics, the symbiotic relationships between _S. pyriformis_ and _C. variabilis_ can be regarded as stable, or permanent. Although _S. pyriformis_ and _P. bursaria_ share _C.
variabilis_ as their endosymbionts, considering the genetic differences depending on their host species, the sharing event has not happened recently. Symbiont sharing among various host
species has also been known for some ciliates41,59 (_Carolibrandtia ciliaticola_ in Fig. S3), and a script to spread a particular algal symbiont has been suggested41. Given the physiological
characters of _C. variabilis_ (mentioned above), this algal species might be an ideal algal symbiont, and it will be no surprise if the other protists also retained _C. variabilis_ as their
algal partners. Research on the symbiotic algae that other _Stentor_ spp. have and on host and regional dependencies are awaited. ADAPTATION OF _S. PYRIFORMIS_ TO OLIGOTROPHIC ENVIRONMENT
IN HIGHLAND MARSH In Japan, _S. pyriformis_ lives only in alpine ponds (Fig. 1), where the winter is cold, and the surface of the pond is always covered with ice. The water in these ponds
has low electrical conductivity (~ 10 μS/cm), and there are few living organisms except _S. pyriformis_, meaning that only little food is available in wintertime. The reason this ciliate is
rich in stored carbohydrate granules may be due to its need for nutrients to survive such harsh winter environments. Preliminary studies suggest that many protists, especially ciliates, may
make starch. Large amounts of cytoplasmic granules that show a Maltese cross were observed in chlorella-bearing ciliates such as _P. bursaria_, while only a small amount of such granules was
observed in _Euplotes aediculatus, Paramecium caudatum, Blepharisma japonicum, and Tetrahymena pyriformis_. Protists with symbiotic algae seem to produce particularly large amounts of
stored carbohydrate granules in the cytoplasm, but the mechanism of starch synthesis may be widely shared by ciliates. _P. bursaria_ has been shown to be more resistant to starvation
conditions than the aposymbiotic strain of the same species13. Under food-deprived conditions, _P. bursaria_ was interpreted to have survived by digesting symbiotic algae. Resting cyst
formation and cannibalism are known as other strategies for protozoans to survive starvation conditions60. This study suggests that the use of carbohydrate granules stored in cells may be
another possible strategy for ciliates to survive harsh environments such as highland oligotrophic bogs. REFERENCES * Selosse, M.-A., Charpin, M. & Not, F. Mixotrophy everywhere on land
and in water: The _grand écart_ hypothesis. _Ecol. Lett._ 20, 246–263 (2017). Article PubMed Google Scholar * Sonntag, B., Posch, T., Klammer, S., Teubner, K. & Psenner, R.
Phagotrophic ciliates and flagellates in an oligotrophic, deep, alpine lake: Contrasting variability with seasons and depths. _Aquat. Microb. Ecol._ 43, 193–207 (2006). Article Google
Scholar * Woelfl, S., Garcia, P. & Duarte, C. _Chlorella_-bearing ciliates (_Stentor, Ophrydium_) dominate in an oligotrophic, deep North Patagonian lake (Lake Caburgua, Chile).
_Limnologica_ 40, 134–139 (2010). Article CAS Google Scholar * Sonntag, B., Summerer, M. & Sommaruga, R. Are freshwater mixotrophic ciliates less sensitive to solar ultraviolet
radiation than heterotrophic ones?. _J. Eukaryot. Microbiol._ 58, 196–202 (2011). Article PubMed PubMed Central Google Scholar * Tsukii, Y. _Protist Information Server_.
http://protist.i.hosei.ac.jp (2017). * Johnson, H. P. A contribution to the morphology and biology of the stentors. _J. Morph._ 8, 467–563 (1893). Article Google Scholar * Walker, E. R.
Observations on the micro-fauna of an Oregon pond. _Trans. Am. Microsc. Soc._ 28, 75–84 (1908). Article Google Scholar * Foissner, W. & Wölfl, S. Revision of the genus _Stentor_ Oken
(Protozoa, Ciliophora) and description of _S. araucanus__nov_. _spec_. from South American lakes. _J. Plankton Res._ 16, 255–289 (1994). Article Google Scholar * Song, C., Murata, K. &
Suzaki, T. Intracellular symbiosis of algae with possible involvement of mitochondrial dynamics. _Sci. Rep._ 7, 1221. https://doi.org/10.1038/s41598-017-01331-0 (2017). Article ADS CAS
PubMed PubMed Central Google Scholar * Hoshina, R., Hayakawa, M. M., Kobayashi, M., Higuchi, R. & Suzaki, T. _Pediludiella daitoensis_ gen. et sp. nov. (Scenedesmaceae,
Chlorophyceae), a large coccoid green alga isolated from a Loxodes ciliate. _Sci. Rep._ 10, 628. https://doi.org/10.1038/s41598-020-57423-x (2020). Article ADS CAS PubMed PubMed Central
Google Scholar * Reynolds, E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. _J. Cell. Biol._ 17, 208–212 (1963). Article CAS PubMed PubMed
Central Google Scholar * Hosogi, N., Nishioka, H. & Nakakoshi, M. Evaluation of lanthanide salts as alternative stains to uranyl acetate. _Microscopy_ 64, 429–435 (2015). Article CAS
PubMed Google Scholar * Omura, G. _et al._ A bacteria-free monoxenic culture of _Paramecium bursaria_: Its growth characteristics and the re-establishment of symbiosis with chlorella in
bacteria-free conditions. _Jpn. J. Protozool._ 37, 139–150 (2004). Google Scholar * Higuchi, R., Song, C., Hoshina, R. & Suzaki, T. Endosymbiosis-related changes in ultrastructure and
chemical composition of _Chlorella variabilis_ (Archaeplastida, Chlorophyta) cell wall in _Paramecium bursaria_ (Ciliophora, Oligohymenophorea). _Eur. J. Protistol._ 66, 149–155 (2018).
Article PubMed Google Scholar * Nakayama, T., Watanabe, S., Mitsui, K., Uchida, H. & Inouye, I. The phylogenetic relationship between the _Chlamydomonadales_ and _Chlorococcales_
inferred from 18S rDNA sequence data. _Phycol. Res._ 44, 47–55 (1996). Article CAS Google Scholar * Hoshina, R., Kamako, S.-i & Imamura, N. Phylogenetic position of endosymbiotic
green algae in _Paramecium bursaria_ Ehrenberg from Japan. _Plant Biol._ 6, 447–453 (2004). Article CAS PubMed Google Scholar * Hoshina, R., Kato, Y., Kamako, S.-i & Imamura, N.
Genetic evidence of “American” and “European” type symbiotic algae of _Paramecium bursaria_ Ehrenberg. _Plant Biol._ 7, 526–532 (2005). Article CAS PubMed Google Scholar * Larkin, M. A.
_et al._ Clustal W and Clustal X version 2.0. _Bioinformatics_ 23, 2947–2948 (2007). Article CAS PubMed Google Scholar * Thamm, M., Schmidt, S. L. & Bernhard, D. Insights into the
phylogeny of the genus _Stentor_ (Heterotrichea, Ciliophora) with special emphasis on the evolution of the macronucleus based on SSU rDNA data. _Acta Protozool._ 49, 149–157 (2010). Google
Scholar * Shazib, S. U. A., Vd’ačný, P., Kim, J. H., Jang, S. W. & Shin, M. K. Phylogenetic relationships of the ciliate class Heterotrichea (Protista, Ciliophora,
Postciliodesmatophora) inferred from multiple molecular markers and multifaceted analysis strategy. _Mol. Phylogenet. Evol._ 78, 118–135 (2014). Article PubMed Google Scholar * Fernandes,
N. M., da Silba Paiva, T., da Silva-Neto, I. D., Schlegel, M. & Schrago, C. G. Expanded phylogenetic analyses of the class Heterotrichea (Ciliophora, Postciliodesmatophora) using five
molecular markers and morphological data. _Mol. Phylogenet. Evol._ 95, 229–246 (2016). Article PubMed Google Scholar * Chen, X., Shazib, S. U. A., Kim, J. H., Kim, M. S. & Shin, M. K.
New contributions to _Gruberia lanceolata_ (Gruber, 1884) Kahl, 1932 based on analyses of multiple populations and genes (Ciliophora, Heterotrichea, Gruberiidae). _Eur. J. Protistol._ 65,
16–30 (2018). Article PubMed Google Scholar * Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. _Mol.
Biol. Evol._ 35, 1547–1549 (2018). Article CAS PubMed PubMed Central Google Scholar * Ronquist, F. _et al._ MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice
across a large model space. _Syst. Biol._ 61, 539–542 (2012). Article PubMed PubMed Central Google Scholar * Hoshina, R. Analysis of an intron intervening the SSU rDNA of _Chlorella_ sp.
T-24–5, a photobiont of _Paramecium bursaria_. _Jpn. J. Protozool._ 45, 17–27 (2012). Google Scholar * Heeg, J. S. & Wolf, M. ITS2 and 18S rDNA sequence-structure phylogeny of
_Chlorella_ and allies (Chlorophyta, Trebouxiophyceae, Chlorellaceae). _Plant Gene_ 4, 20–28 (2015). Article CAS Google Scholar * Wuyts, J., Van De Peer, Y. & De Wachter, R.
Distribution of substitution rates and location of insertion sites in the tertiary structure of ribosomal RNA. _Nucleic Acids Res._ 29, 5017–5028 (2001). Article CAS PubMed PubMed Central
Google Scholar * Hoshina, R. & Imamura, N. Eu-_Chlorella_ large subunit rDNA sequences and group I introns in ribosomal DNA of the paramecian symbiotic alga NC64A. _Phycol. Res._ 56,
21–32 (2008). Article CAS Google Scholar * Krienitz, L. _et al._ Phylogenetic relationship of _Chlorella_ and _Parachlorella_ gen. nov. (Chlorophyta, Trebouxiophyceae). _Phycologia_ 43,
529–542 (2004). Article Google Scholar * Melconian, M. Editorial. _Protist_ 158, 1 (2007). Article Google Scholar * Song, C. & Suzaki, T. Improved preservation of organelles in
_Paramecium bursaria_ by freeze-substitution with glutaraldehyde and osmium tetroxide. _J. Electr. Microsc. Technol. Med. Biol._ 27, 1–8 (2013). Google Scholar * Fertig, E. T.,
Gherghiceanu, M. & Popescu, L. M. Extracellular vesicles release by cardiac telocytes: Electron microscopy and electron tomography. _J. Cell. Mol. Med._ 18, 1938–1943 (2014). Article
CAS PubMed PubMed Central Google Scholar * Dhar, N. R. The starch-iodine reaction. _J. Phys. Chem._ 28, 125–130 (1924). Article CAS Google Scholar * Quain, D. E. & Tubb, S. A
rapid and simple method for the determination of glycogen in yeast. _J. Inst. Brewing_ 89, 38–40 (1983). Article CAS Google Scholar * Němcová, Y. & Kalina, T. Cell wall development,
microfibril and pyrenoid structure in type strains of _Chlorella vulgaris_, _C. kessleri_, _C. sorokiniana_ compared with _C. luteoviridis_ (Trebouxiophyceae, Chlorophyta). _Algol. Stud._
100, 95–105 (2000). Google Scholar * Barber, A. A., Harris, W. W. & Padilla, G. M. Studies of native glycogen isolated from synchronized _Tetrahymena pyriformis_ (HSM). _J. Cell Biol._
27, 281–292 (1965). Article CAS PubMed PubMed Central Google Scholar * Coppin, A. _et al._ Evolution of plant-like crystalline storage polysaccharide in the protozoan parasite
_Toxoplasma gondii_ argues for a red alga ancestry. _J. Mol. Evol._ 60, 257–267 (2005). Article ADS CAS PubMed Google Scholar * Deschamps, P. _et al._ The heterotrophic dinoflagellate
_Crypthecodinium cohnii_ defines a model genetic system to investigate cytoplasmic starch synthesis. _Eukaryot. Cell_ 7, 872–880 (2008). Article CAS PubMed PubMed Central Google Scholar
* Miyake, A., Iio, H. & Harumoto, T. Defence function of pigment granules in _Stentor coeruleus_. _Eur. J. Protistol._ 37, 77–88 (2001). Article Google Scholar * Höfle, G., Reinecke,
S., Laude, U. & Spitzner, D. Amethystin, the coloring principle of _Stentor amethystinus_. _J. Nat. Prod._ 77, 1383–1389 (2014). Article PubMed CAS Google Scholar * Hoshina, R.
& Kusuoka, Y. DNA analysis of algal endosymbionts of ciliates reveals the state of algal integration and the surprising specificity of the symbiosis. _Protist_ 167, 174–184 (2016).
Article CAS PubMed Google Scholar * Hoshina, R. & Fujiwara, Y. Molecular characterization of _Chlorella_ cultures of the National Institute for Environmental Studies culture
collection with description of _Micractinium inermum_ sp. nov., _Didymogenes sphaerica_ sp. nov., and _Didymogenes soliella_ sp. nov. (Chlorellaceae, Trebouxiophyceae). _Phycol. Res._ 61,
124–132 (2013). Article CAS Google Scholar * Hoshina, R. DNA analyses of a private collection of microbial green algae contribute to a better understanding of microbial diversity. _BMC
Res. Notes_ 7, 592. https://doi.org/10.1186/1756-0500-7-592 (2014). Article PubMed PubMed Central Google Scholar * Hoshina, R. _et al._ Cytological, genetic, and biochemical
characteristics of an unusual non-_Chlorella_ photobiont of _Stentor polymorphus_ collected from an artificial pond close to the shore of Lake Biwa Japan. _Phycol. Res._ 61, 7–14 (2013).
Article Google Scholar * Pröschold, T., Darienko, T., Silva, P. C., Reisser, W. & Krienitz, L. The systematics of _Zoochlorella_ revisited employing an integrative approach. _Environ.
Microbiol._ 13, 350–364 (2011). Article PubMed CAS Google Scholar * Pucciarelli, S. _et al._ Biomonitoring of Lake Garda: Identification of ciliate species and symbiotic algae
responsible for the “black-spot” bloom during the summer of 2004. _Environ Res._ 107, 194–200 (2008). Article CAS PubMed Google Scholar * Kamako, S.-I., Hoshina, R., Ueno, S. &
Imamura, N. Establishment of axenic endosymbiotic strains of Japanese _Paramecium bursaria_ and the utilization of carbohydrate and nitrogen compounds by the isolated algae. _Eur. J.
Protistol._ 41, 193–202 (2005). Article Google Scholar * Reisser, W., Vietze, S. & Widowski, M. Taxonomic studies on endocytobiotic chlorophycean algae isolated from different American
and European strains of _Paramecium bursaria_. _Symbiosis_ 6, 253–270 (1988). Google Scholar * Kamako, S.-I. & Imamura, N. Effect of Japanese _Paramecium bursaria_ extract on
photosynthetic carbon fixation of symbiotic algae. _J. Eukaryot. Microbiol._ 53, 136–141 (2006). Article PubMed Google Scholar * Van Etten, J. L., Lane, L. C. & Meints, R. H. Viruses
and viruslike particles of eukaryotic algae. _Microbiol. Rev._ 55, 586–620 (1991). Article PubMed PubMed Central Google Scholar * Yamada, T., Higashiyama, T. & Fukuda, T. Screening
of natural waters for viruses which infect chlorella cells. _Appl. Environ. Microbiol._ 57, 3433–3437 (1991). Article CAS PubMed PubMed Central Google Scholar * Hoshina, R. _et al._
Isolation and characterization of a virus (CvV-BW1) that infects symbiotic algae of _Paramecium bursaria_ in Lake Biwa, Japan. _Virol. J._ 7, 222. https://doi.org/10.1186/1743-422X-7-222
(2010). Article CAS PubMed PubMed Central Google Scholar * Hoshina, R., Iwataki, M. & Imamura, N. _Chlorella variabilis_ and _Micractinium reisseri_ sp. nov. (Chlorellaceae,
Trebouxiophyceae): Redescription of the endosymbiotic green algae of _Paramecium bursaria_ (Peniculia, Oligohymenophorea) in the 120th year. _Phycol. Res._ 58, 188–201 (2010). Article CAS
Google Scholar * Bock, C., Pröschold, T. & Krienitz, L. Two new _Dictyosphaerium_-morphotype lineages of the Chlorellaceae (Trebouxiophyceae): _Heynigia_ gen. nov. and _Hindakia_ gen.
nov.. _Eur. J. Phycol._ 45, 267–277 (2010). Article CAS Google Scholar * Bock, C., Pažoutová, M. & Krienitz, L. Phylogenetic position of _Coronastrum ellipsoideum_ and description of
_Parachlorella hussii_ sp. Nov.. _Biologia_ 66, 585–594 (2011). Article Google Scholar * Mathews, D. H., Sabina, J., Zuker, M. & Turner, D. H. Expanded sequence dependence of
thermodynamic parameters improves prediction of RNA secondary structure. _J. Mol. Biol._ 288, 911–940 (1999). Article CAS PubMed Google Scholar * Zuker, M. Mfold web server for nucleic
acid folding and hybridization prediction. _Nucleic Acids Res._ 31, 3406–3415 (2003). Article CAS PubMed PubMed Central Google Scholar * Hoshina, R. & Imamura, N. Phylogenetically
close group I introns with different positions among _Paramecium bursaria_ photobionts imply a primitive stage of intron diversification. _Mol. Biol. Evol._ 26, 1309–1319 (2009). Article
CAS PubMed Google Scholar * Summerer, M., Sonntag, B. & Sommaruga, R. Ciliate-symbiont specificity of freshwater endosymbiotic _Chlorella_ (Trebouxiophyceae, Chlorophyta). _J.
Phycol._ 44, 77–84 (2008). Article CAS PubMed Google Scholar * Gutiérrez, J., Callejas, S., Borniquel, S., Benítez, L. & Martín-González, A. Ciliate cryptobiosis: A microbial
strategy against environmental starvation. _Intern. Microbiol._ 4, 151–157 (2001). Article CAS Google Scholar * Kasaai, M. R. A comparative study of molecular structure, solution
properties and food application for three branched polysaccharides: Amylopectin, glycogen, and dextran. _Curr. Trends Polymer Sci._ 16, 49–63 (2012). CAS Google Scholar * Bailey, J. M.
& Whelan, W. J. Physical properties of starch. _J. Biol. Chem._ 236, 969–973 (1961). Article CAS PubMed Google Scholar * Fuentes, C. _et al._ Fractionation and characterization of
starch granules using field-flow fractionation (FFF) and differential scanning calorimetry (DSC). _Anal. Bioanal. Chem._ 411, 3665–3674 (2019). Article CAS PubMed PubMed Central Google
Scholar * Prats, C., Graham, T. E. & Shearer, J. The dynamic life of the glycogen granule. _J. Biol. Chem._ 293, 7089–7098 (2018). Article CAS PubMed PubMed Central Google Scholar
* Baker, F. & Whelan, W. J. Birefringence of amylose and amylopectin in whole structural starches. _Nature_ 166, 34 (1950). Article ADS CAS PubMed Google Scholar * Hall, M. B. A
method for isolating glycogen granules from ruminal protozoa for further characterization. _J. Dairy Sci._ 99, 1956–1958 (2016). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS On 11 February 2018, Professor Dr. Yuuji Tsukii, one of the authors of this article and known as the creator and curator of “Protist Information Server”
(http://protist.i.hosei.ac.jp/), suddenly passed away while preparing for this article. This paper is dedicated to the memory of Dr. Tsukii, who expressed a deep interest in algae-bearing
ciliates in his later years. This work was supported by Japan Society for the Promotion of Science KAKENHI [Grant Number 19K06814]. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Nagahama
Institute of Bio-Science and Technology, Tamura 1266, Nagahama, Shiga, 526-0829, Japan Ryo Hoshina * Laboratory of Biological Science, Hosei University, 2-17-1 Fujimi, Chiyoda-ku, Tokyo,
102-8160, Japan Yuuji Tsukii * Research Group of Biological Sciences, Division of Natural Sciences, Nara Women’s University, Kitauoya-Nishimachi, Nara, 630-8506, Japan Terue Harumoto *
Department of Biology, Graduate School of Science, Kobe University, 1-1 Rokkodai-cho, Nada-ku, Kobe, 657-8501, Japan Toshinobu Suzaki Authors * Ryo Hoshina View author publications You can
also search for this author inPubMed Google Scholar * Yuuji Tsukii View author publications You can also search for this author inPubMed Google Scholar * Terue Harumoto View author
publications You can also search for this author inPubMed Google Scholar * Toshinobu Suzaki View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
R.H.: DNA and RNA analyses. Y.T.: Distribution of _Stentor pyriformis_ and establishment of culture method. H.T.: Culture maintenance. T.S.: Detailed observation and analyses of storage
granules. R.H., T.S.: Drafted the manuscript. All authors contributed to preparation of the manuscript. CORRESPONDING AUTHOR Correspondence to Ryo Hoshina. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hoshina, R., Tsukii, Y., Harumoto, T. _et al._ Characterization of a green
_Stentor_ with symbiotic algae growing in an extremely oligotrophic environment and storing large amounts of starch granules in its cytoplasm. _Sci Rep_ 11, 2865 (2021).
https://doi.org/10.1038/s41598-021-82416-9 Download citation * Received: 19 May 2020 * Accepted: 30 December 2020 * Published: 03 February 2021 * DOI:
https://doi.org/10.1038/s41598-021-82416-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
