Long-term course of phrenic nerve injury after cryoballoon ablation of atrial fibrillation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT While phrenic nerve palsy (PNP) due to cryoballoon pulmonary vein isolation (PVI) of atrial fibrillation (AF) was transient in most cases, no studies have reported the results of
the long-term follow-up of PNP. This study aimed to summarize details and the results of long-term follow-up of PNP after cryoballoon ablation. A total of 511 consecutive AF patients who
underwent cryoballoon ablation was included. During right-side PVI, the diaphragmatic compound motor action potential (CMAP) was reduced in 46 (9.0%) patients and PNP occurred in 29 (5.7%)
patients (during right-superior PVI in 20 patients and right-inferior PVI in 9 patients). PNP occurred despite the absence of CMAP reduction in 0.6%. The PV anatomy, freezing parameters and
the operator’s proficiency were not predictors of PNP. While PNP during RSPVI persisted more than 4 years in 3 (0.6%) patients, all PNP occurred during RIPVI recovered until one year after
the ablation. However, there was no significant difference in the recovery duration from PNP between PNP during RSPVI and RIPVI. PNP occurred during cryoballoon ablation in 5.7%. While most
patients recovered from PNP within one year after the ablation, PNP during RSPVI persisted more than 4 years in 0.6% of patients. SIMILAR CONTENT BEING VIEWED BY OTHERS ELECTROPHYSIOLOGICAL
CHARACTERISTICS OF PULMONARY VEIN CONDUCTION RECOVERY AFTER RADIOFREQUENCY ABLATION OF ATRIAL FIBRILLATION Article Open access 14 November 2024 PHRENIC NERVE BLOCK DURING NONINTUBATED
VIDEO-ASSISTED THORACOSCOPIC SURGERY: A SINGLE-CENTRE, DOUBLE-BLIND, RANDOMIZED CONTROLLED TRIAL Article Open access 22 June 2021 NOVEL STRATEGY OF REMOTE MAGNETIC NAVIGATION-GUIDED ABLATION
FOR VENTRICULAR ARRHYTHMIAS FROM RIGHT VENTRICLE OUTFLOW TRACT Article Open access 20 October 2020 INTRODUCTION Cryoballoons have proven to be effective for pulmonary vein isolation (PVI)
in patients with atrial fibrillation (AF). Several recent randomized trials have shown the noninferiority of cryoballoon ablation to radiofrequency ablation with respect to the treatment
efficacy in patients with drug-refractory paroxysmal AF1, 2. Phrenic nerve palsy (PNP) is the most frequently observed complication during cryoballoon ablation. Since the cryoballoon is not
variable, it is difficult to freeze PV away from the position of the phrenic nerve. Thus, the incidence of PNP was reported higher in comparison to that during radiofrequency catheter
ablation1,2,3,4,5. Several methods for the early detection of PNP have been reported6. Although, PNP is usually transient with complete resumption of right diaphragmatic contraction before
the end of ablations, PNP persisted in some patients. However, the long-term course of cryothermal PNP have not well been determined. The purpose of this study was to investigate the
long-term consequence of PNP after cryoballoon ablation. METHODS STUDY SUBJECTS A total of 511 consecutive patients who underwent cryoballoon ablation for AF were included in the present
study. Paroxysmal AF was defined as AF that spontaneously terminated within seven days. Antiarrhythmic drugs were discontinued for at least five half-lives prior to ablation. A 3D image of
the PV was reconstructed by preprocedural enhanced computed tomography. The size and length of the PV trunk (between the ostium and the first branch) were measured by an electro-anatomical
mapping system7. ETHICS Clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki. All data were compliant with the International
Conference on Harmonization guidelines. All experimental protocols were approved by The Ethical Committee of the Jikei University School of Medicine. All methods were carried out in
accordance with relevant guideline and regulations. The informed consent was obtained from all participants. CRYOBALLOON ABLATION OF AF Details of cryoballoon ablation for AF and early
detection of phrenic nerve injury were reported as previously described8,9,10. A single transseptal puncture was performed using a radiofrequency needle (Baylis Medical, Montreal, QC,
Canada) and an 8.5-Fr long sheath (SL0; Abbott, Chicago, IL). The transseptal sheath was exchanged over a guidewire for a 15-Fr steerable sheath (Flexcath Advance; Medtronic, Minneapolis,
MN). Another SL0 sheath was inserted to the left atrium via the same puncture site and a circumferential 20-pole catheter (Lasso 2515 NAV eco variable catheter; Biosense Webster, Diamond
Bar, CA) was inserted to map all PVs before and after the cryoballoon to confirm electrical isolation. PVI was performed with a single balloon technique using a second-generation (Arctic
Front Advance; Medtronic) or 4th-generation cryoballoon (Arctic Front Advance Pro; Medtronic). A 28-mm cryoballoon catheter was used in all of the patients. A spiral mapping catheter
(Achieve; Medtronic) was used to advance the cryoballoon and map the PV potentials. Complete sealing at the antral aspect of the PV was confirmed by the injection of contrast medium. The
proximal-seal technique was used, if possible. This was followed by a freeze cycle of 180 s. In 100 patients, a 120-s bonus-freeze was applied after the successful application of a 180-s
initial freeze. If electrical isolation was not achieved by cryoballoon, additional touch-up ablation was performed with a conventional radiofrequency or cryothermal (Freezer Max; Medtronic)
catheter. EARLY DETECTION OF PHRENIC NERVE INJURY To avoid phrenic nerve injury, the diaphragmatic compound motor action potentials (CMAPs) were monitored during right phrenic nerve pacing,
as previously described6, 11, 12. A standard decapolar catheter or a circumferential 20-pole catheter was placed in the superior vena cava (SVC) cranial to the RSPV in order to pace the PN,
in recent cases, the circular catheter was generally positioned in the subclavian vein in order to achieve better stability and a more reliable PN capture in comparison to the SVC. For the
early detection of PNP, the pacing threshold was measured, and the pacing output was set slightly above the pacing threshold. If a 30% reduction of CMAP or a loss of capture was observed,
the freeze was immediately aborted using a double-stop technique13 and observed for recovery. Additional cryo-applications were not performed even if PNI recovered during the procedure. CMAP
monitoring between left side PVI was not performed. PATIENT FOLLOW-UP Details of patient follow-up were reported as previously described8,9,10. In patients with paroxysmal AF, no
antiarrhythmic drugs were prescribed after the procedure. The patients underwent continuous, in-hospital ECG monitoring for 2–4 days after the procedure. The patients underwent careful
observation (two weeks after discharge, then every month thereafter) at the cardiology clinic. The outcome of AF ablation was evaluated based on the patient’s symptoms, ECG at periodical
follow-up examinations, and periodic 24-h ambulatory monitoring (at 1, 3, 6, 9, 12 months and yearly after the procedure). The recurrence of AF was defined as AF lasting for more than 30 s
after a blanking period of 90 days. IMPENDING, TRANSIENT AND PERSISTENT PNP The degree of PNP was divided into three grades. A > 30% reduction in CMAP without weakening of diaphragmatic
motility was considered as impending PNP. Definition of transient and persistent PNP was previously described14. Specifically, transient PNP was defined as a progressive weakening of
diaphragmatic motility, as assessed by manual palpation on the abdomen, confirmed by fluoroscopy or the occurrence of a hemidiaphragm paralysis detected by both manual palpation and
fluoroscopy during the procedure, and completely resolving before the end of the procedure. Persistent PNP was defined as an elevated hemidiaphragm noted on post-procedural radiography,
which persisted after the procedure. The diaphragmatic function on a chest X-ray was confirmed by a radiologist based on the shape of an elevated hemidiaphragm in both postero-anterior and
in lateral fluoroscopy projections during a sniff maneuver. The position and shape of the diaphragm were determined by correlating measurements to skeletal structures and the radius of the
curvature, respectively. Once the diagnosis of PNP was established, the patient was closely monitored in the clinic with tests repeated every 3 months. Complete recovery of the phrenic nerve
function was diagnosed in the case of normalization of the diaphragm position in X-ray images both at rest and during a sniff test by comparison with the pre-procedural chest images.
STATISTICAL ANALYSES Categorical variables were analyzed using a chi-squared test, unless the expected values in any cells were < 5, in which case Fisher’s exact test was used. _P_-values
of < 0.05 were considered to indicate statistical significance. Continuous variables were expressed as the mean ± standard deviation. A Mann–Whitney U test or unpaired Student’s _t_-test
was used for the analysis of continuous variables. The Kaplan–Meier method were used for survival curve analysis and comparisons between groups were performed using a log-rank test. All
statistical analyses were performed using the SPSS software program (version 27; SPSS, Chicago, IL, USA). RESULTS PULMONARY VEIN ISOLATION A total of 511 patients who underwent cryoballoon
ablation for AF were included in this study. The baseline characteristics are shown in Table 1. The mean age and left atrial diameter were 59.8 ± 10.1 years and 37.9 ± 16.3 mm, respectively.
Cryoballoon isolation was performed in the LSPV (n = 511), LIPV (n = 511), RSPV (n = 506) and RIPV (n = 495). In right-side PV, the PV was isolated in the order of the RSPV to the RIPV in
369 patients and the RIPV to the RSPV in 142 patients. Touch-up ablations were required 5.5% in LSPV, 6.8% in LIPV, 2.3% in RSPV and 22% in RIPV. Isolation of the SVC was performed in 61
(12%) patients by RF catheter. PHRENIC NERVE INJURY DURING CRYOBALLOON PROCEDURE During right-side PVI, the CMAP was reduced in 46 (9.0%) patients. PNP occurred during a cryoballoon
procedure in 29 (5.7%) patients (4.0% [20/506] in the RSPV and 1.8% [9/495] in the RIPV). Among these cases, CMAP reduction preceded PNP in 26 (5.1%) patients (3.6% [18/506] in the RSPV and
1.6% [8/495] in the RIPV). On the other hand, PNP occurred despite the absence of CMAP reduction during the procedure in 3 (0.6%) patients. None of these 3 patients underwent SVC isolation
and/or RF touch-up ablation (Table 2). The incidence of PNP in men was higher than that in women (84% vs. 69%, _P_ = 0.04, Table 1). The details of freezing application in the RSPV and RIPV
(Table 3) were compared between patients with and without PNP. In both RSPV and RIPV, the number of freezing applications was smaller, and the total freezing time was shorter in the patients
with PNP than in those without. The nadir balloon temperature, time to isolation, time to − 30 °C, time to − 40 °C, circumferential length of the PV ostium and length of the PV trunk were
similar between two groups. Cryoballoon ablation was performed by 8 operators in this study. Incidence of PNP was similar among 8 operators (_P_ = 0.57, range 0–9.1%). Incidence of PNP was
not different between the first 20 cases of the operator and subsequent cases (3.4% vs. 3.8%, _P_ = 0.81 in RSPV and 1.0% vs 2.4%, _P_ = 0.23 in RIPV). FOLLOW UP In three patients, PNP
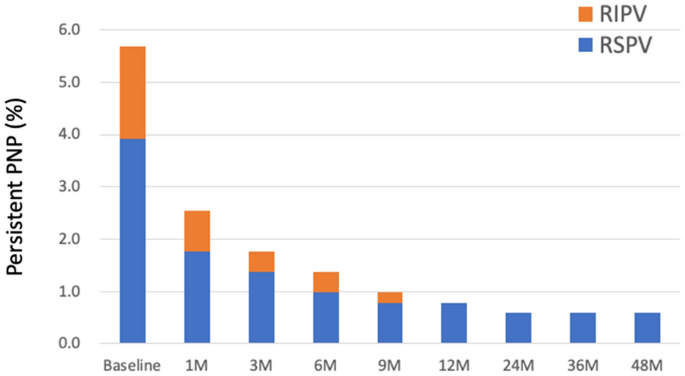
recovered at the end of the procedure. AF free rate was similar between patients with PNP and those without (_P_ = 0.82). No patients complained any symptoms regarding as PNP. At one years
after the procedure, 25 of 29 patients had recovered from PNP. On the other hand, PNP persisted more than 4 years in 3 (0.6%) patients (Fig. 1). In all three patients, PNP occurred during
freezing of the RSPV. The balloon position on the fluoroscopy (Fig. 2) and procedural parameters (Table 4) of these patients were shown. The mean nadir temperature and time to isolation were
− 52 °C and 25 s, respectively. In patient 2 and 3, deep balloon position during RSPV application was shown during freezing of the RSPV. SVC isolation and touch-up RF ablation was not
performed in these 3 patients. All PNPs that occurred during RIPV isolation recovered within one year after ablation. The duration of recovery from PNP was similar between patients who
developed PNP during freezing of the RSPV and those who developed PNP freezing of the RIPV in survival analysis (log rank _P_ = 0.21). The median time to recovery from PNP after ablation was
30 days in the RSPV and 39 days in the RIPV. REPEAT ABLATION PROCEDURE In 46 patients with CMAP reduction during PV application, repeat ablation procedure was performed in 4 patients (RSPV
in 3, RIPV in 1). Of those, PV reconnection was observed in 67% (2/3) of the RSPV and none of the RIPV (Table 5). While freezing time after the elimination of RSPV potential was 51 s and 55
s in the patient with RSPV reconnection, it was 166 s in the patient without RSPV reconnection. Reconnected site was located in anterior wall in one patient and both anterior and posterior
wall in another patient. DISCUSSION PHRENIC NERVE PALSY This study reports the consequences of patients with PNP during cryoballoon PVI with a long-term follow-up period (> 4 years). This
is the first study to use a survival analysis to compare the consequences of PNP occurring during RSPVI to PNP occurring during RIPVI. PVI using cryoballoon was performed for the LSPV (n =
511), LIPV (n = 511), RSPV (n = 506) and RIPV (n = 495), respectively. When PNP occurs in either the RSPV or RIPV, the remaining PVI was not performed by the cryoballoon. Thus, there was a
difference in the number of cases among 4 PVs. PNP occurred in 5.7% of patients. This is consistent with previous reports1, 4, 5, 14, 15. However, this rate was higher than that in recent
studies reporting on data obtained using contemporary techniques11, 16, 17. The incidence of PNP can be decreased by increased operator experience to avoid deep seating of the cryoballoon in
PV, and improved techniques that allow the early detection of PNP. However, incidence of PNP was not related to the operator’s proficiency in this study. The PN runs in front of the distal
right PV; thus, cooling at a deeper position increases the risk of PNP4. In previous studies, the distance from the RSPV ostium to the right peri-cardiophrenic bundles15, the larger RSPV
ostial area5, 11 and the external RSPV-left atrium angle5 were predictors of PNP during cryoballoon PVI. In this study, the time to − 30 °C, time to − 40 °C and the nadir balloon
temperature did not differ to a statistically significant extent and the PV size and length of the PV trunk measured by preoperative CT did not differ between the two groups to a
statistically significant extent. PNP occurred even during RIPVI in 1.6% of patients. In a previous study, the incidence of PNP during cryoballoon RIPVI was 3.5%14. The authors reported that
the velocity of the temperature drop from basal to − 20 °C and the presence of a right common ostium were predictors of PNP. In our institute, cryoballoon was not used in cases involving a
common pulmonary vein trunk. The proximal-seal technique to better define the PV ostium and ensure a proximal ablation lessened the risk for PNP at RSPV12. However, this technique often
difficult to apply during the RIPVI. No patients experienced irreversible PNP during freezing of the RIPV. The right PN descends parallel along the anterolateral aspect of the SVC, and then
courses posteriorly between the RSPV and the SVC. Histological studies have shown that there may be as little as 2.1 mm (± 0.4 mm) and 7.8 mm (± 1.2 mm) of distance between the PN and the
RSPV and RIPV, respectively18. Cryothermal energy can create lesions to depths of 2 to 5 mm19. This close proximity accounts for the higher risk of both transient and persistent PNP during
RSPVI than RIPVI. PNP during RIPVI was more frequent when PN pacing was performed using a circular catheter. It is considered that there were some other intervening factors such as when the
ablation was performed. However, since the number of patients with PNP during RIPVI was small, it is difficult to perform a multivariate analysis. Further larger-scale studies are needed to
reveal the predictors of PNP during RIPVI. PNP occurred in 3 cases without CMAP reduction during freezing. It is unclear when it occurred. In these cases, PNP may have occurred during the
thawing time or the onset may have been late. All 3 patients recovered from the PNP within one month after the procedure. PN pacing and CMAP recording should be continued during thawing
until cryoballoon deflation. TIME-COURSE OF RECOVERY AND IRREVERSIBLE PNP The long-term outcomes of the STOP AF PAS study demonstrated that the rate of PNP was 3.2%. PNP resolved within 36
months in all but 1 of the patients17. However, the rate was not separately evaluated in the RSPV and RIPV. The results of this study showed that a median duration of recovery from PNP was
30–40 days and most patients recovered from PNP within one year after the ablation procedure. On the other hand, none of PNP recovered after the second year. In all three of these patients,
PNP occurred during freezing of the RSPV. However, a survival analysis showed no significant difference in the duration of recovery from PNP between the RSPV and RIPV. No left PNP occurred
in this study. A previous study reported on cases with left PNP and recommended the performance of PN pacing during left PVI20. MECHANISM OF PNP DURING CRYOBALLOON APPLICATION Since the
cryoballoon is not variable, it is difficult to change the site of the freezing application and higher rates of PNP have been reported in comparison to other ablation systems. The incidence
of PNP during RF ablation was reported to be 0.3%3. While the incidence of PNP during cryoballoon PVI was higher than that during RF ablation, PNP was transient in most cases. Since the ion
channel function is temperature-dependent, cryoablation in close proximity to adjacent nerves can result in focal cold-induced conduction block. In a previous study21, large axonal loss was
observed as a histological change during cryothermal PNP. Wallerian degeneration associated with the subsequent regeneration of axons was observed in most cases. Since the extent of damage
from cryoballoon applications was proportional to the temperature changes and the total amount of energy delivered, the early detection of PNP by observing CMAP reduction could prevent the
aggravation of PNP. However, the number of freezing applications and total freezing time were shorter in patients with PNP compared to those without in this study. This was likely a result
of operators urgently terminating the freezing application when CMAP reduction or a loss of the PN capture occurred. In patients with CMAP reduction during RPV application, repeat ablation
procedure was performed in three patients. Of those, RSPV was reconnected in 2(67%) patients. In two reconnected RSPV, freezing time after PV potential elimination was shorter than the PV
without reconnection. The durability of PV isolation was lower for PVs with earlier double stop due to CMAP reduction. A previous study showed 50% of RSPVs were reconnected during the second
procedure11. They also conclude that 1-min freezes seem to be insufficient, however, 2-min freezes might be sufficient to obtain good durability. The results of this study indicated that
when careful observation was performed to detect CMAP reduction, the incidence of irreversible PNP was low. In this study, no patient with PNP reported experiencing symptoms. Several
limitations associated with the present study warrant mention. This study was a non-randomized, observational, single-center study. Since respiratory function tests were not performed in
this study, we could not assess the severity of PNP and influence on the respiratory function and it is unclear whether the respiratory function fully recovered. The relative health and
preserved functional capacity at baseline may relate that all patients with PNP were asymptomatic. Moreover, patients in the current study underwent preprocedural MDCT and those with
non-conventional anatomy were not offered cryoballoon ablation. CONCLUSIONS PNP occurred in 5.7% of patients after cryoballoon ablation for AF. PNP even occurred during RIPVI in 1.8% of
patients. The anatomy of PV, freezing parameters and the operator’s proficiency were not predictors of PNP. While all PNP due to RIPVI recovered within one year after the ablation,
persistent PNP more than 4 years due to RSPVI was observed in 0.6% of patients. However, there was no significant difference in the recovery duration from PNP between PNP during RSPVI and
RIPVI. REFERENCES * Kuck, K. H. _et al._ Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. _N. Engl. J. Med._ 374, 2235–2245. https://doi.org/10.1056/NEJMoa1602014
(2016). Article PubMed Google Scholar * Luik, A. _et al._ Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective,
randomized, controlled, noninferiority freeze AF study. _Circulation_ 132, 1311–1319. https://doi.org/10.1161/CIRCULATIONAHA.115.016871 (2015). Article PubMed PubMed Central Google
Scholar * Schmidt, M. _et al._ German ablation registry: cryoballoon versus radiofrequency ablation in paroxysmal atrial fibrillation: one-year outcome data. _Heart Rhythm_ 13, 836–844.
https://doi.org/10.1016/j.hrthm.2015.12.007 (2016). Article PubMed Google Scholar * Saitoh, Y. _et al._ Fluoroscopic position of the second-generation cryoballoon during ablation in the
right superior pulmonary vein as a predictor of phrenic nerve injury. _Europace_ 18, 1179–1186. https://doi.org/10.1093/europace/euv362 (2016). Article PubMed Google Scholar * Stroker, E.
_et al._ Anatomic predictors of phrenic nerve injury in the setting of pulmonary vein isolation using the 28-mm second-generation cryoballoon. _Heart Rhythm_ 13, 342–351.
https://doi.org/10.1016/j.hrthm.2015.10.017 (2016). Article PubMed Google Scholar * Franceschi, F., Dubuc, M., Guerra, P. G. & Khairy, P. Phrenic nerve monitoring with diaphragmatic
electromyography during cryoballoon ablation for atrial fibrillation: the first human application. _Heart Rhythm_ 8, 1068–1071. https://doi.org/10.1016/j.hrthm.2011.01.047 (2011). Article
PubMed Google Scholar * Narui, R. _et al._ Incidence and factors associated with the occurrence of pulmonary vein narrowing after cryoballoon ablation. _Circ. Arrhythm. Electrophysiol._
https://doi.org/10.1161/CIRCEP.116.004588 (2017). Article PubMed Google Scholar * Tokuda, M. _et al._ Adenosine testing during cryoballoon ablation and radiofrequency ablation of atrial
fibrillation: a propensity score-matched analysis. _Heart Rhythm_ 13, 2128–2134. https://doi.org/10.1016/j.hrthm.2016.08.018 (2016). Article PubMed Google Scholar * Tokuda, M. _et al._
Clinical significance of early recurrence of atrial fibrillation after cryoballoon versus radiofrequency ablation-A propensity score matched analysis. _PLoS ONE_ 14, e0219269.
https://doi.org/10.1371/journal.pone.0219269 (2019). Article CAS PubMed PubMed Central Google Scholar * Tokuda, M. _et al._ Effect of air removal with extracorporeal balloon inflation
on incidence of asymptomatic cerebral embolism during cryoballoon ablation of atrial fibrillation. _Heart Rhythm_ 14, 1291–1296. https://doi.org/10.1016/j.hrthm.2017.05.035 (2017). Article
PubMed Google Scholar * Miyazaki, S. _et al._ Characteristics of phrenic nerve injury during pulmonary vein isolation using a 28-mm second-generation cryoballoon and short freeze strategy.
_J. Am. Heart Assoc._ https://doi.org/10.1161/JAHA.117.008249 (2018). Article PubMed PubMed Central Google Scholar * Su, W. _et al._ Best practice guide for cryoballoon ablation in
atrial fibrillation: the compilation experience of more than 3000 procedures. _Heart Rhythm_ 12, 1658–1666. https://doi.org/10.1016/j.hrthm.2015.03.021 (2015). Article PubMed Google
Scholar * Ghosh, J., Sepahpour, A., Chan, K. H., Singarayar, S. & McGuire, M. A. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein
isolation by balloon cryoablation. _Heart Rhythm_ 10, 646–652. https://doi.org/10.1016/j.hrthm.2013.01.011 (2013). Article PubMed Google Scholar * Abugattas, J. P. _et al._ Phrenic nerve
injury during right inferior pulmonary vein ablation with the second-generation cryoballoon: clinical, procedural, and anatomical characteristics. _Europace_ 20, e156–e163.
https://doi.org/10.1093/europace/eux337 (2018). Article PubMed Google Scholar * Ichihara, N. _et al._ Prevalence and pre-procedural predictors associated with right phrenic nerve injury
in electromyography-guided, second-generation cryoballoon ablation: single large balloon and single 3-minute freeze techniques. _JACC Clin. Electrophysiol._ 2, 508–514.
https://doi.org/10.1016/j.jacep.2016.01.009 (2016). Article PubMed Google Scholar * Molenaar, M. M. D. _et al._ Shorter cryoballoon applications times do effect efficacy but result in
less phrenic nerve injury: results of the randomized 123 study. _Pacing Clin. Electrophysiol._ 42, 508–514. https://doi.org/10.1111/pace.13626 (2019). Article PubMed PubMed Central Google
Scholar * Knight, B. P. _et al._ Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval
study. _JACC Clin. Electrophysiol._ 5, 306–314. https://doi.org/10.1016/j.jacep.2018.11.006 (2019). Article PubMed Google Scholar * Sanchez-Quintana, D. _et al._ How close are the phrenic
nerves to cardiac structures? Implications for cardiac interventionalists. _J. Cardiovasc. Electrophysiol._ 16, 309–313. https://doi.org/10.1046/j.1540-8167.2005.40759.x (2005). Article
PubMed Google Scholar * Coulombe, N., Paulin, J. & Su, W. Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. _J. Cardiovasc. Electrophysiol._
24, 919–925. https://doi.org/10.1111/jce.12157 (2013). Article PubMed Google Scholar * Okishige, K. _et al._ Left phrenic nerve injury during electrical isolation of left-sided pulmonary
veins with the second-generation cryoballoon. _Pacing Clin. Electrophysiol._ 40, 1426–1431. https://doi.org/10.1111/pace.13201 (2017). Article PubMed Google Scholar * Andrade, J. G. _et
al._ Histopathology of cryoballoon ablation-induced phrenic nerve injury. _J. Cardiovasc. Electrophysiol._ 25, 187–194. https://doi.org/10.1111/jce.12296 (2014). Article PubMed Google
Scholar Download references ACKNOWLEDGEMENTS We are grateful to Dr. Brian Quinn (Japan Medical Communication Inc.) for the comments on the language of the manuscript. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department of Cardiology, The Jikei University School of Medicine, 3-25-8, Nishi-shinbashi, Minato-ku, Tokyo, 105-8461, Japan Michifumi Tokuda, Seigo Yamashita,
Hidenori Sato, Hirotsuna Oseto, Hirotsugu Ikewaki, Masaaki Yokoyama, Ryota Isogai, Ken-ichi Tokutake, Ken-ichi Yokoyama, Mika Kato, Ryohsuke Narui, Shin-ichi Tanigawa, Seiichiro Matsuo,
Michihiro Yoshimura & Teiichi Yamane Authors * Michifumi Tokuda View author publications You can also search for this author inPubMed Google Scholar * Seigo Yamashita View author
publications You can also search for this author inPubMed Google Scholar * Hidenori Sato View author publications You can also search for this author inPubMed Google Scholar * Hirotsuna
Oseto View author publications You can also search for this author inPubMed Google Scholar * Hirotsugu Ikewaki View author publications You can also search for this author inPubMed Google
Scholar * Masaaki Yokoyama View author publications You can also search for this author inPubMed Google Scholar * Ryota Isogai View author publications You can also search for this author
inPubMed Google Scholar * Ken-ichi Tokutake View author publications You can also search for this author inPubMed Google Scholar * Ken-ichi Yokoyama View author publications You can also
search for this author inPubMed Google Scholar * Mika Kato View author publications You can also search for this author inPubMed Google Scholar * Ryohsuke Narui View author publications You
can also search for this author inPubMed Google Scholar * Shin-ichi Tanigawa View author publications You can also search for this author inPubMed Google Scholar * Seiichiro Matsuo View
author publications You can also search for this author inPubMed Google Scholar * Michihiro Yoshimura View author publications You can also search for this author inPubMed Google Scholar *
Teiichi Yamane View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.T. and T.Y. wrote the main manuscript text and All authors collect the
study data. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Michifumi Tokuda. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or
other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tokuda,
M., Yamashita, S., Sato, H. _et al._ Long-term course of phrenic nerve injury after cryoballoon ablation of atrial fibrillation. _Sci Rep_ 11, 6226 (2021).
https://doi.org/10.1038/s41598-021-85618-3 Download citation * Received: 09 December 2020 * Accepted: 26 February 2021 * Published: 18 March 2021 * DOI:
https://doi.org/10.1038/s41598-021-85618-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
