A modified clot-based assay to measure negatively charged procoagulant phospholipids
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

Growing evidence supports a role for extracellular vesicles (EVs) in haemostasis and thrombosis due to exposure of negatively charged procoagulant phospholipids (PPL). Current commercial
PPL-dependent clotting assays use chemically phospholipid depleted plasma to measure PPL activity. The purpose of our study was to modify the PPL assay by substituting the chemically
phospholipid depleted plasma with PPL depleted plasma obtained by ultracentrifugation This in order to get readily access to a sensitive and reliable assay to measure PPL activity in human
plasma and cell supernatants. The performance of the assay was tested, including the influence of individual coagulation factors and postprandial lipoproteins and compared to a commercial
PPL assay (STA-Procoag-PPL). The two PPL assays displayed similar sensitivity to exogenously added standardized phospholipids. The PPL activity measured by the modified assay strongly
correlates with the results from the commercial assay. The intraday- and between-days coefficients of variation ranged from 2–4% depending on the PPL activity in the sample. The modified PPL
assay was insensitive to postprandial lipoprotein levels in plasma, as well as to tissue factor (TF) positive EVs from stimulated whole blood. Our findings showed that the modified assay
performed equal to the comparator, and was insensitive to postprandial lipoproteins and TF+ EVs.
Procoagulant phospholipid (PPL) activity has regained interest in recent years, mainly due to the increased understanding of the role of extracellular vesicles (EVs) in thrombosis and
haemostasis1,2. As early as 1946, Chargaff and West observed that the clotting time of plasma was prolonged by applying high-speed centrifugation to remove “thromboplastic substances”3.
Accordingly, Connor and colleagues demonstrated that the amount of annexin A5 positive EVs, measured by flow cytometry, showed a significant and inverse correlation with clotting time4.
These findings suggest that EVs play a significant role in coagulation, apparently due to exposure of phospholipids, and phosphatidylserine (PS) in particular, on their surface. The increase
in surface expression of negatively charged PPL will facilitate the assembly of coagulation factors upon cell activation or apoptosis5. This is crucial for several stages of the coagulation
pathway, namely the formation of the intrinsic and extrinsic tenase complexes, as well as the conversion of prothrombin to thrombin by coagulation factor Xa (FXa)6. The activity of the
extrinsic tenase complex, the tissue factor (TF)—factor VIIa (FVIIa) complex, is increased by several orders of magnitude in the presence of negatively charged membrane phospholipids7.
Several assays have been developed to measure the PPL activity in human plasma. While some are based on the ability of annexin A5 to bind PS in the presence of Ca2+4,8, others are
clot-based, utilizing the ability of PPL to accelerate the conversion of prothrombin to thrombin. Annexin A5-based assays are widely used, often in a flow cytometry setting, a method that is
time consuming, requires expensive equipment and experienced personnel. In addition, annexin A5 is commonly used in chromogenic FXa assays, where the activity measured is based on the EVs
exposing PS that are bound to the microplate. These EVs are then able to accelerate the cleavage of the chromogenic substrate by FXa9.
Compared to FXa chromogenic assays, which measure procoagulant activity of EVs in a purified system, clotting assays involve a more complex reaction and a physiological end-point as they
measure the PPL activity of plasma, and not only captured PS-positive EVs9. To the best of our knowledge, there are currently two clot-based assays commercially available, the
STA-Procoag-PPL assay from Diagnostica Stago (Asnières sur Seine Cedex, France) and the XACT assay from Haematex (Hornsby, NSW, Australia). Both assays use chemical phospholipase treatment
to deplete phospholipids from plasma, but differ with regard to the phospholipase used, plasma origin, and the use of a phospholipid calibrator. The XACT assay uses a snake phospholipase10
and porcine plasma, while an unspecified phospholipase and human plasma is used for the Stago assay.
In this study, we aimed to develop a modified PPL-dependent clotting assay, capable of measuring the PPL activity in human plasma and cell supernatants of in vitro experiments, by removing
PPL from plasma by sequential centrifugation, including final ultracentrifugation. The performance of the modified assay was then validated against the commercially available Stago
STA-Procoag-PPL assay.
The removal of PPL from plasma is fundamental for the clot-based assays. As EVs are the main source of PPL, we first tested whether sequential centrifugation reliably depleted EVs from
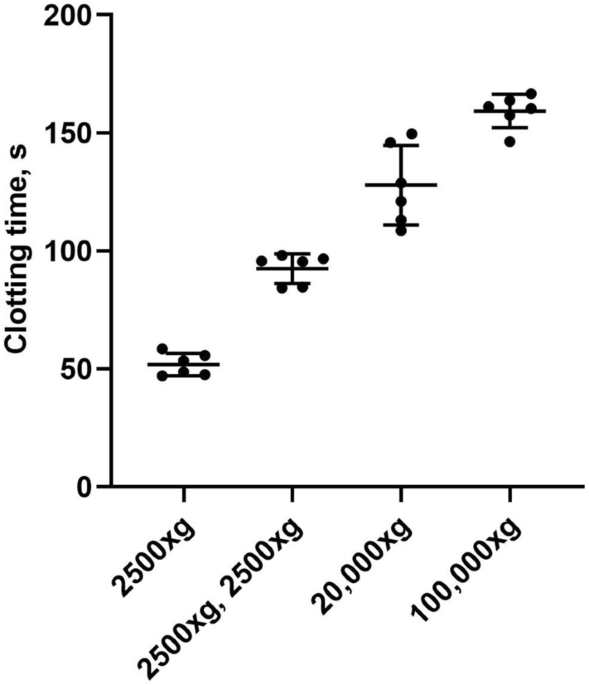
plasma. To achieve this, we compared the PPL clotting times (PPLCT) of plasma samples (n = 6) subjected to sequential centrifugation procedures (Fig. 1). Plasma prepared by centrifugation at
2500×g for 15 min caused clotting times of 51.8 ± 4.7 s (mean ± 1 SD). A second centrifugation step of 2500×g for 15 min resulted in a prolongation of the clotting times to 92.5 ± 6.3 s
(mean ± 1 SD). Pelleting larger EVs (e.g. microvesicles) from platelet free plasma (PFP) by an additional spin of 20,000×g for 30 min at room temperature (RT) further prolonged the clotting
times to 127.8 ± 16.9 s (mean ± 1 SD). The final spin at 100,000×g for 60 min to remove the smallest and lightest EVs (e.g. exosomes) further prolonged the clotting times to 159.3 ± 7.1 s
(mean ± 1 SD).
The effect of sequential centrifugation on plasma PPL clotting times. Citrated blood collected from 6 healthy volunteers was subjected to consecutive centrifugations: 2500×g for 15 min to
obtain platelet poor plasma (PPP), 2500×g for 15 min to obtain platelet free plasma (PFP), 20,000×g for 30 min and 100,000×g for 1 h to deplete for EVs. A sample of plasma after each
centrifugation was analyzed with the modified PPL assay. The PPL activity is presented in seconds (s) of clotting time. Dot plot with mean ± 1 SD (n = 6 for each condition).
Procoagulant phospholipid depleted plasma (PPL depleted plasma) prepared from pooled PFP (2500×g for 15 min twice) by ultracentrifugation (100,000×g for 60 min) resulted in a mean clotting
time of 163.2 ± 7.5 s (mean ± 1 SD) (Supplementary Figure S1).
To test whether the preparation of PPL depleted plasma affected standard coagulation assays, we measured activated partial thromboplastin time (aPTT) and prothrombin time (PT). The aPTT was
30.2 (normal range 25–37 s) and PT 21.3 s, corresponding to a PT-INR of 1.00 (normal range
