Impact of environmental changes on the behavioral diversity of the odonata (insecta) in the amazon
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT The odonates are insects that have a wide range of reproductive, ritualized territorial, and aggressive behaviors. Changes in behavior are the first response of most odonate species
to environmental alterations. In this context, the primary objective of the present study was to assess the effects of environmental alterations resulting from shifts in land use on
different aspects of the behavioral diversity of adult odonates. Fieldwork was conducted at 92 low-order streams in two different regions of the Brazilian Amazon. To address our main
objective, we measured 29 abiotic variables at each stream, together with five morphological and five behavioral traits of the resident odonates. The results indicate a loss of behaviors at
sites impacted by anthropogenic changes, as well as variation in some morphological/behavioral traits under specific environmental conditions. We highlight the importance of considering
behavioral traits in the development of conservation strategies, given that species with a unique behavioral repertoire may suffer specific types of extinction pressure. SIMILAR CONTENT
BEING VIEWED BY OTHERS RAPID FUNCTIONAL TRAITS TURNOVER IN BOREAL DRAGONFLY COMMUNITIES (ODONATA) Article Open access 21 September 2020 INDIVIDUAL VARIABILITY IN HABITAT SELECTION BY AQUATIC
INSECTS IS DRIVEN BY TAXONOMY RATHER THAN SPECIALISATION Article Open access 01 December 2022 CONDITION- AND CONTEXT-DEPENDENT VARIATION OF SEXUAL DIMORPHISM ACROSS LIZARD POPULATIONS AT
DIFFERENT SPATIAL SCALES Article Open access 10 October 2022 INTRODUCTION The enormous variety of behavior exhibited by most animals has inspired human thought, arts, and Science for
centuries, from rupestrian paintings to the Greek philosophers. One prominent group of animals, the insects, present a wide range of complex behaviors1,2, mostly related to reproduction,
such as elaborate courtship rituals, and stereotyped territorial and mating behaviors3,4. Over the years, a large body of research has sought to identify and describe the evolutionary and
ecological processes that have created and maintained the myriad of behavioral patterns found among the different insect groups5. The insects of the order Odonata are good models for the
assessment of ecological questions on animal behavior, in particular because of their diverse reproductive modes and mating strategies6,7. These diverse behaviors include territoriality in
many species, which is usually associated with mating and oviposition sites8. The oviposition behavior of odonates can be classified in three main types: (1) exophytic, when the female lays
eggs directly in the water, usually touching the surface a number of times while hovering; (2) endophytic, when the female lays eggs inside the living tissue of plants, and (3) epiphytic,
when the female oviposits on exposed surfaces, such as roots, debris, moss, phytotelmata or even the ground or rocks6. Odonate territories may vary considerably in size, from a few square
centimeters to many square meters, and may contain a range of valuable resources, which include sunning spots, perches, and oviposition substrates9,10. In many species, dominant males can
often be observed patrolling their territories, and these individuals tend to copulate within the area or relatively close to their territory11. Odonate males may also present agonistic
behavior, settling territorial disputes through physical aggression or non-contact aerial displays, flashing their wings toward intruders or chasing rivals away9,12. Many odonates present
specific mating behaviors, and some species engage in courtship, with the males courting the females prior to mating13. For example, the males of _Calopteryx xanthostoma_ (Charpentier, 1825)
and _Calopteryx haemorrhoidalis_ Vander Linden, 1825, perform elaborate flights, dropping to the water and floating with the current to demonstrate the oviposition site to the potential
female mate14, or extend their legs and iridescent wings toward the female15. After mating, the males of some species may exhibit mate-guarding strategies, which are typically categorized
as: (1) contact guarding, when the male remains in the tandem position (or perched directly on the female) during oviposition; (2) non-contact guarding, when the male perches or hovers near
the female during oviposition, and (3) no mate-guarding, when the female oviposits alone, without the presence of the male16,17. The enormous behavioral diversity of the Odonata, the
conspicuity of the males in the field, and the favorable conditions for the collection of behavioral data combine to make this insect order an excellent model for comparative studies6.
Despite this, little is known about the behavior of most South American species, and data are especially scant for the species from the Amazon region, which are in constant threat from
anthropic actions18. The ongoing increase in the modification of natural landscapes has raised concerns among researchers with regard to the loss of or changes to behavioral traits, in
particular those related to reproduction19,20,21. Environmental alterations may affect both mating behavior and habitat selectivity, which may ultimately alter community structure,
influencing species richness, and their abundance and distribution19,20. In addition to the recent discussion of the need to conserve ethodiversity and behavioral repertoires, a range of
studies have focused on the effects of modifications in the landscape on local animal communities and their associated behavioral patterns, with this ethological focus now being considered a
major ally of biodiversity conservation programs22. Caro & Sherman23 and Harabiš et al.24 demonstrated that many odonate taxa have behavioral traits that are highly sensitive to local
ecological conditions, and that the characteristics of the environment are fundamental to the structuring of odonate communities25. We predicted that odonate behavioral diversity will be
equally vulnerable to environmental change. Given this, the principal objective of the present study was to assess the effects of environmental alterations provoked by shifts in land use on
the behavioral diversity of adult odonates of the suborders Zygoptera and Anisoptera. We assume that the environmental modifications caused by shifts in land use alter the behavioral
diversity of both odonate suborders26. In particular, these changes may lead to the exclusion of species with specific habits that are dependent on a given type of microhabitat27. Given
this, we would predict a greater overall behavioral richness (i.e., a larger number of different types of behavior) in the zygopterans, and greater behavioral evenness in preserved areas,
where the more favorable resource availability may allow for more univariate niche overlap. In altered areas, by contrast, we would expect an increase in niche differentiation, to allow
species to coexist. In these areas, we would predict that the removal of the riparian vegetation and the more open forest canopy of the streams will lead, in particular, to the loss of
behaviors associated with specific types of substrate, such as oviposition sites and perches28,29. In the case of the anisopterans, we would predict the opposite pattern, due to the
ecophysiological differences between the two suborders6,25,30, with greater behavioral richness and evenness in altered areas, and more behavioral divergence in more preserved areas. We
predict specifically that areas with higher deforestation rates and more open canopies will be associated with the establishment of more generalist species, such as those of the genera
_Orthemis_ Hagen, 1861 and _Erythrodiplax_ Brauer, 1868, and an increase in the overall behavioral repertoire of the different species found in these areas, albeit with increasing similarity
in these behaviors. In well-preserved areas, we expect the conditions to favor the presence of more specialized species, with more divergent behaviors31, such as the species of the genus
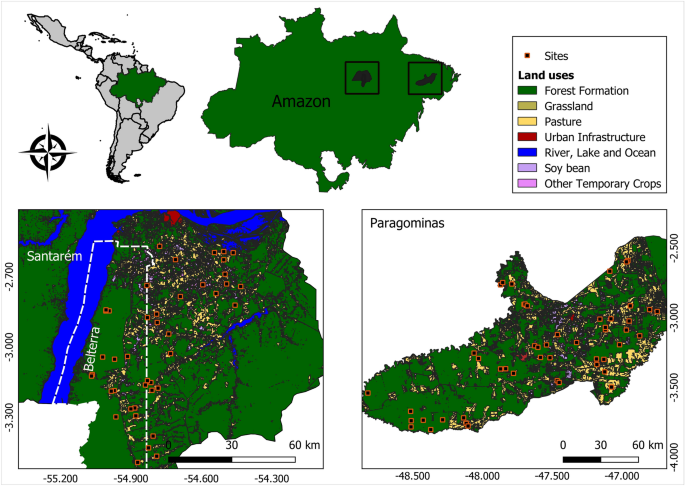
_Microstigma_ Rambur, 1842. MATERIAL AND METHODS STUDY AREA Fieldwork was conducted at 92 streams (first to third order watercourses, in the classification of Strahler32), in two different
regions of the eastern Amazon (Fig. 1). We collected data at 42 streams in Santarém and Belterra, and 50 streams in Paragominas, all in Pará state (Brazil). Both regions have a humid
tropical climate, classified as Af in the Köppen system. The local vegetation is predominantly rainforest, with a few tracts of Amazonian savannah near Santarém. Both regions encompass a
gradient of land use, which varies from highly impacted areas—primarily monocultures and pasture—to well-preserved primary forest. BIOLOGICAL DATA Biological data were collected in both
study regions during the dry season only, for four reasons: (1) the ecophysiological requirements of the odonates6; (2) to standardize the sampling period and minimize sampling noise in the
analyses (see33); (3) because a number of previous studies have shown that odonate diversity may be higher during the dry season in the Amazon region, and (4) the reduced depth of the water
during this season, which forces the odonates to aggregate at smaller bodies of water, facilitating sampling. During the rainy season, by contrast, conditions typically hamper, or even
prohibit altogether the collection of a realistic and representative sample of the odonate communities found on the floodplains of the Amazon25. The species of the suborders Anisoptera and
Zygoptera were collected using the “fixed area transect” method or the “Odonate Sampling Protocol” (OSP)34. We collected specimens within a total of 20 5-m segments at each stream (100 m of
total sampling effort at each site). We captured the specimens using an entomological net while walking along the transect for one hour. Each transect was sampled invariably between 10:00
and 14:00 h, when most of the target species are active, and always on sunny days. We identified all the specimens to the species level, using taxonomic keys and illustrated
guides35,36,37,38,39,40,41, and all the specimens were deposited as vouchers in the collection of the Zoology Museum at the Universidade Federal do Pará (UFPA) in Belém, Brazil.
ENVIRONMENTAL FEATURES We collected environmental data and described the physical habitat, together with the biological data on each stream. We measured a total of 29 environmental variables
(see Supplementary Material S1), which have all been used in previous studies and have been shown to be important predictors for the assessment of the effects of different types of land use
on odonates26. We measured 26 of these variables at each stream following an adapted version of the protocol published by the United States Environmental Protection Agency (US-EPA) and
calculated the environmental metrics following42. This protocol assesses the characteristics of each stream, providing information on the morphology of the channel, hydraulics, substrates,
the availability of shelters for the aquatic biota, the amount and size of woody debris, the cover and structure of the riparian vegetation, and human influences43. Two physical and chemical
descriptors of the water were also measured, using a multiparametric Horiba device in three equidistant sections of the stream segment (downstream, middle, and upstream). We also calculated
the Habitat Integrity Index, HII (see44) to provide a score of physical integrity for each study stream. This index is generated using 12 parameters that evaluate different aspects of the
morphology of the channel and its surroundings. Values of HII closer to 1 indicate more conserved environments, while those closer to 0 are sites with a high level of degradation45. This
protocol has been widely used to assess environmental conditions in the Amazon (for more information, see25,43,46,47). BEHAVIORAL TRAITS We use direct literature classification data and
morphological data as proxies to assess behavioral syndromes. This strategy has been widely used with success in studies of functional diversity48,49. We categorized the behavioral traits in
five classes: (1) territoriality; (2) contest displays; (3) type of oviposition; (4) use of oviposition substrates, and (5) mate-guarding strategies. These categories were defined based on
the literature indexed in the Web of Science and Google Scholar databases, using the name of each study species as the keywords (Supplementary Material S2). Given the lack of data for most
species, we made every possible effort to complete the categories by consulting specialists on odonate behavior, but even then, some species (in particular, the rarest and most
recently-described taxa) lacked some behavioral parameters. In these cases, we obtained information on the behavioral traits of congeneric species, identified the most common behavior in the
genus, and extrapolated it to the species lacking data. This strategy has been used successfully to reduce knowledge gaps in a number of previous studies of the odonates27,29,50. We also
used five morphological traits as a proxy for dispersal behavior and territoriality: (1) abdomen length; (2) thorax volume; (3) wing stroke; (4) wing load51, and (5) the wing–thorax ratio.
These variables were obtained by measuring the total length (TL), thorax width (Thw), abdomen length (AL), and the forewing length (FL) and width (FW). The total length (1) was calculated as
the distance between the head and the tip of the abdomen, (2) the thorax volume (π radius of the thorax2*4/3) provides an index of flight muscle volume, which is a predictor of flight
capacity or dispersal distance52; (3) the wing stroke (πFL2*FW) is proportional to the area of the wing and the amount of air displaced at each stroke of the wings, whose volume is related
to πr2h (where r represents the length of the wing and h, its width)53—this metric also predicts flight performance, and (4) the wing load (thorax volume/wing stroke), for which, we
considered the wing stroke to be a proxy of the wing area, based on the formula: thorax volume/wing area, to provide the wing load index. We also calculated (5) the wing–thorax ratio by
dividing the squared forewing length by the volume of the thorax, to estimate the allometry of the body. Lower values of this ratio indicate stouter bodies and a capacity for faster flight,
whereas higher values indicate slenderer bodies and slower flight54. To obtain these measurements, we analyzed specimens deposited in the collection of the UFPA Ecology and Conservation
Laboratory in Belém. We selected a total of ten male specimens of each species to obtain the measurements necessary to calculate the parameters described above. To be included in the study,
these specimens had to be in good condition, and were selected randomly from the collection, including individuals collected in both degraded and preserved environments. We obtained the
morphological measurements only for male individuals, given the reduced abundance of females in the study area, and the lack of taxonomic keys for females. This standard analysis of the male
specimens (with all length measures being obtained from the right side of the body) also avoids potential intraspecific differences associated with sexual dimorphism. For species
represented by fewer than ten individuals, we measured all the specimens that were in a good condition. All the measurements were obtained in triplicate by three different researchers (to
minimize error) using a digital calliper (precision of 0.01 mm), with the mean of these values being considered for analysis. BEHAVIORAL DIVERSITY We compiled a matrix of ten behavioral
traits for each species and converted it into a similarity matrix using the Gower distance55 (Supplementary Material S2). We then calculated the FRic, FEVE, and FDiv indices proposed by
Villéger56. The behavioral richness thus estimates the set of niches occupied by the species that make up a community, while the evenness evaluates the distribution of the insects among the
behavioral niches occupied by the different species. Lastly, the behavioral divergence indicates the level of niche differentiation in the community, where the greater the divergence, the
more differentiated the community, and thus, the lower the competition for resources. These three indices are important because they quantify relevant aspects of the behavioral diversity of
a community in a complementary fashion, with the species distributed in a multidimensional behavioral space56. DATA ANALYSIS We checked for multicollinearity in our environmental data using
a variance inflation factor (VIF). This analysis was conducted sequentially, until all the variables presented values of VIF below57. Given the differential responses of the two odonate
suborders (Anisoptera and Zygoptera) to environmental gradients25, we ran the analyses separately for each suborder. We conducted an a priori correspondence analysis (CA) of the abundance
matrices, based on the log(x + 1) transformed values, to determine the association of the behavioral traits with environmental features and species. We also ran a weighted mixed multivariate
analysis (Hill–Smith analysis), using the morphological and behavioral trait matrices, with the species as the weight and the CA values as the response variable. We then ran a Principal
Components Analysis (PCA), which included all the standardized environmental variables and species. Finally, we plotted graphs overlaying the scores of the environmental variables and
species with the morphological and behavioral traits. We ran multiple regressions with forward stepwise model selection to test for the effects of environmental changes on the behavioral
diversity of the odonates. For this, the behavioral richness, evenness, and divergence were defined as the response variables. The variables of the physical and limnological structure of the
streams were defined as the predictive variables (the metrics we selected for each model are shown in Tables 1 and 2). All the analyses were run in the R environment, using the dbDF, lm,
decostand, dudi.coa, dudi.pca, dudi.hillsmith and vif functions of the FD58, vegan59, Ade460, and faraway61 packages. RESULTS ENVIRONMENTAL FEATURES The study streams were located along a
gradient of land use. Some of these streams were located within highly impacted environments, with up to 96% of the area of the drainage basin under anthropogenic land use, while others were
inserted within well-preserved remnants of Amazon forest (Supplementary Material S1). The HII values ranged from 0.08 to 0.99, with a mean of 0.64 and standard deviation (SD) of 0.20. The
canopy openness ranged from 0.0 to 1.0 (mean = 0.85 ± SD = 0.24). The variable that varied most was the substrate with a sediment grain size of D50 (mean = 201.32 mm ± SD = 579.23 mm).
BIOLOGICAL DATA We collected 3107 individuals of 101 odonate species, including 49 anisopterans and 52 zygopterans. The most abundant species were _Erythrodiplax basalis_ (Kirby, 1897) (N =
294), _Mnesarete aenea_ (Selys, 1853) (N = 261), and _Erythrodiplax fusca_ (Rambur, 1842) (N = 200), whereas 18 species were represented by only one individual (Supplementary Material S2).
_Micrathyria romani_ Sjöstedt, 1918, _Macrothemis ludia_ Belle, 1987, _Oligoclada walkeri_ Geijskes, 1931, _Phyllogomphoides cepheus_ Belle, 1980, and _Oligoclada abbreviata_ (Rambur, 1842)
were all found in streams with a higher level of conservation, whereas _Oligoclada amphinome_ Ris, 1919, _Dasythemis esmeralda_ Ris, 1910, _Erythrodiplax paraguayensis_ (Förster, 1905), and
_Progomphus intricatus_ Hagen _in_ Selys, 1858 were found in streams with greater environmental disturbance (Supplementary Material S3). We found that the zygopteran species _Argia infumata_
Selys, 1865, _Heteragrion aurantiacum_ Selys, 1862, _Heliocharis amazona_ Selys, 1853 and _Epipleoneura capilliformis_ (Selys, 1886) were characteristic of better preserved streams, while
_Argia reclusa_ Selys, 1865, _Acanthagrion kennedii_ Williamson, 1916, _Neoneura rubriventris_ Selys, 1860, and _Acanthagrion jessei_ Leonard, 1977 were observed at disturbed streams
(Supplementary Material S4). BEHAVIORAL FEATURES The observed variation in the environment had a range of effects on the behavioral traits of the anisopterans. Species with tandem
oviposition (e.g., _Rhodophygia cardinalis_ Erichson in Schomburgk, 1848) covaried positively with fine litter substrates (V23) and electrical conductivity (V2). Non-territorial and non
mate-guarding species covaried positively with the slope of the hydrographic basin (V11) and the amount of litter (V6). By contrast, territoriality was associated positively with substrates
of fine sediments (V21), secondary riparian forest (V28), and the intensity of the non-forest land use at a local scale (V29). Wing load and thorax volume covaried positively with
non-agricultural land use (V14) (Fig. 2a). Considering the relationship between the species and their behavioral traits, the behavioural repertoires of _Rhodophygia cardinalis, Dasythemis
esmeralda, Progomphus intricatus, Micrathyria pseudeximia_ Westfall, 1992_, Erythemis credula_ Hagen, 1861_, Phyllocycla bartica_ Calvert, 1948, and _Cacoides latro_ Erichson, 1848 may have
been the most affected, given that the alterations of environmental variables have a greater influence on the behaviors of these species (Fig. 2b). In the zygopterans, we observed that the
species which oviposit on decayed wood in the streams (e.g., those of the genus _Chalcopteryx_) covaried positively with the HII (V1). Tandem mate guarding and the absence of agonistic
displays covaried positively with conductivity (V2) and substrates with fine sediments (V21). Territoriality was correlated positively with dissolved oxygen (V3), while non-contact mate
guarding was correlated positively with dissolved oxygen (V3), the volume of woody debris in the channel (V19), the mean small tree cover (V9), and the mean slope (V11). In turn, species
with no mate guarding behavior covaried positively with the intensity of the non-forest land use at a local scale (V29) (Fig. 3a). Considering the relationships between the species and the
behavioral traits, the behavioral repertoires of _Acanthagrion adustum_ Williamson, 1916_, Chalcopteryx radians_ Ris, 1914, _Heteragrion icterops_ Selys, 1862_, Acanthagrion ascendens_
Calvert, 1909 and _Argia tupi_ Calvert, 1909 may have been the most affected, given that the alterations in the environmental variables had a greater influence on their behaviors (Fig. 3b).
ENVIRONMENTAL FEATURES AND BEHAVIORAL DIVERSITY When we analyzed the impact of environmental features on behavioral diversity, we found that the environment explained 61% of the behavioral
divergence of the anisopterans. We highlight the positive relationship between behavioral divergence and riparian canopy cover (V8), and the negative relationship with the HII (V1) and large
woody debris in the channel (V15). Environmental variables explained 46% of the behavioral evenness, having a positive relationship with conductivity (V2) and litter (V6), and a negative
relationship with the slope of the catchment (V26), and 56% of the behavioral richness, having a negative relationship with the HII (V1) (Table 1; Fig. 4). In the case of the zygopterans,
environmental features explained 37% of the behavioral divergence, having a positive relationship with small tree canopy cover (V9), and 19% of the behavioral evenness, with high and
positive relationship with the HII (V1) and the large woody debris in the channel (V15), and lastly, 33% of the behavioral richness, also having a positive relationship with conductivity
(V2) (Table 2; Fig. 5). DISCUSSION The results of the present study support our hypothesis that the behavioral diversity of the insects of the order Odonata is affected by environmental
variables, in particular those related to changes in land use. In the case of the suborder Anisoptera, areas with greater riparian canopy cover, reduced environmental integrity, and the
smallest amount of large woody debris in the channel presented a greater divergence of behavioral traits, while areas with higher electrical conductivity and more litter had greater
behavioral evenness. Areas of reduced environmental integrity were the richest in behavioral traits. In the Zygoptera, we found that areas with a greater canopy cover of small trees were
more behaviorally divergent, i.e., they were occupied by communities composed of species with more varied behavior. Areas with high environmental integrity and larger amounts of large woody
debris in the channel had greater behavioral evenness, that is, a greater similarity among individual behavioral strategies, whereas sites with higher electrical conductivity were
behaviorally richer. Recent studies in the Amazon have highlighted the importance of environmental filters for the structuring of odonate communities62. Behavioral diversity may provide an
indicator that has the potential to contribute to the understanding of the effects of variation in the environment on the species composition of local comunities. Changes in the environment,
in particular those provoked by human activities, would thus affect the occurrence of species with given behavioral traits in odonate communities20,23,29. We found that territorial and
reproductive behaviors (i.e., type of oviposition and mate-guarding behavior) were closely related to environmental features (physical and chemical variables, related to conservation status
of the stream and the presence of vegetation cover). Relationships of this type were expected because oviposition strategies are often linked directly to the amount and quality of the
available perches and other resources necessary for oviposition10,29. The removal of the riparian vegetation has a marked effect on most zygopteran species, leading to the local exclusion of
the species that requires woody substrates within or adjacent to the channel for oviposition27,29. This effect is even more noticeable in species with endophytic oviposition, in particular
those that oviposit on specific types of aquatic or semi-aquatic plants, such as macrophytes (e.g., _Eleocharis_ spp. and _Pontederia parviflora_)10. The effect may also be prominent in
species with epiphytic oviposition, which relies on a certain degree of heterogeneity in the oviposition substrates within the stream (e.g., rocky surfaces, decaying wood, roots, leaves, and
debris)63. The absence of any clear relationship in the species with exophytic oviposition was expected, however, because this type of oviposition depends only on the availability of
water6. The same reasoning can be applied to territoriality, given that territorial males defend perches with certain specific environmental characteristics and the availability of the
resources necessary for the females to oviposit, such as the incidence of sunlight, proximity to the stream, and perch density16. Given this, environmental degradation and deforestation will
likely select against territorial behavior, reducing its frequency or even excluding it altogether from impacted streams64. In the Anisoptera, behavioral divergence was greatest at sites
with both greater dense-canopy riparian vegetation cover and lower environmental integrity. However, behavioral richness was higher only at the sites with less intact environments. Evenness
was highest at sites with higher electrical conductivity and larger amounts of litter. Anisopterans are larger than zygopterans, in general, and thus have a lower body surface:volume ratio,
and require direct sunlight on their bodies to ensure activity (heliotherms)6. Most anisopterans have a greater dispersal capacity in comparison with most zygopterans, however, and have a
greater dietary amplitude, and more generalist behavior65. The larger body size and dispersal capacity of the anisopterans may be reflected in the more intense interspecific competition
observed in this suborder, which would mean that, for its species to coexist, they may have to be more divergent, to avoid niche overlap. The greater morphological similarities of the
coexisting species may reflect either a lack of niche specialization66 or simply the fact that these species are more generalist. Many odonate species (most zygopterans) require habitats
with specific characteristics6,67, while others (most anisopterans) occur in varying environments and are able to exploit the different aquatic habitats available along the course of a
stream68. Obviously, however, considerable variation is found within each suborder, or even family or genus, which limits the potential for the reliable extrapolation or generalization of
these patterns. In this case, basic studies of the biology of odonate species should be the principal priority at the present time, with more ample analytical approaches, which aim to
identify general trends among species, independent of their suborder, in a manner similar to the approach of Bastos et al.46. The greatest behavioral richness was found in environments with
reduced habitat integrity, which can be explained by the fact that these environments favor habitat generalist species (e.g., heliophiles) and the local extinction of species specialized for
forested environments, species that are more dependent on the adequate conservation of environments25. We thus expected the observed increase in the number of behavioral traits recorded in
degraded areas—which are usually more open habitats—as a result of the behavioral gap left by the absence of the more susceptible species which inhabited these areas previously. The observed
pattern of behavioral uniformity may be explained by the fact that the sites that have larger amounts of litter and higher electrical conductivity may also have fewer other types of
substrate for oviposition, which may limit the potential for behavioural variation in these environments. We found evidence that some zygopteran behaviors are highly dependent on the
presence of riparian vegetation10,29, and are thus correlated with environmental integrity and heterogeneity3. As mentioned above, endophytic oviposition requires adequate substrates, and
territorial behavior is highly dependent on specific resources for oviposition, and perch density and quality, as well as being influenced by the local density of both males and females11.
We would thus expect the greater behavioral divergence observed in areas with greater small tree canopy cover to be related to the reduced availability of resources in these areas, given
that environmental shifts can modify habitats or conditions in a way that may exclude species with certain behavioral repertoires and favor other taxa with more specific behaviors. As
certain environmental conditions may favor specific behavioral patterns to the detriment of others, any shift in these conditions may increase the behavioral differentiation of the local
species. The sites with higher electrical conductivity had greater behavioral richness. In the Amazon, streams with high electrical conductivity tend to have more resources for predatory
aquatic larvae by favoring the density of algae and, consequently, that of benthic macroinvertebrates69. This increased availability of resources may favor the establishment of more
specialized species and result in an increase in the behavioral richness of the local zygopterans. The higher behavioral evenness found in the more preserved environments, and in particular
in those with more large woody debris in the channel, may be accounted for primarily by the diversity of resources. In impacted environments, resources tend to be less stable and distributed
more unevenly, which may contribute to reduced behavioral evenness and even a lack of equilibrium in the abundance of individuals with diverse behavioral traits. Impacts provoked by shifts
in land use, such as deforestation, may affect the distribution of species and their specific behavioral traits. In fact, the loss of certain types of perches and oviposition resources may
have a marked effect on the expression of certain types of behavior27,29. Impacts caused by dams, such as changes in water flow patterns, may also limit the species with exophytic
oviposition that prefer to oviposit in fast-flowing water6. Our results indicate that behavioral diversity would be a valuable metric for studies of environmental impact. This diversity may
provide important insights into the mechanisms that determine the differential effects of environmental impacts on different odonate species. We would recommend that future studies amplify
the application of metrics that incorporate behavioral parameters. In the specific case of the odonates, the gomphids and aeshnids are of particular interest, due not only to their
crepuscular habits and elusive behavior, which limits data collection6, but also because of the general lack of behavioral data and information on the conservation status of most species. A
number of studies have now focused on species endemic to the Amazon, and the impacts of deforestation and oil palm plantations on their diversity70. However, the general lack of behavioral
data on the species from the Amazon and other tropical regions, represents a knowledge gap that must be overcome to ensure more conclusive analyses. One possible approach here would be the
systematic interpretation of the pressure of environmental filters on the behavioral and functional diversity of these organisms in forest remnants. Although there is an increasing body of
knowledge on the behavioral diversity and conservation of the Odonata29, this field of research is still incipient. In the present study, we aimed to provide a novel contribution to the
understanding of the behavioral ecology and conservation of one of the biologically most diverse regions of our planet, which is currently under threat from a wide range of anthropic
pressures. REFERENCES * Córdoba-Aguilar, A., González-Tokman, D. & González-Santoyo, I. _Insect Behaviour_ (Oxford University Press, 2018). Book Google Scholar * Shuker, D. M. &
Simmons, L. W. _The Evolution of Insect Mating Systems_ (Oxford University Press, 2014). Book Google Scholar * Cade, W. Alternative male reproductive behaviors. _Florida Entomol._ 63,
30–35 (1980). Article Google Scholar * Eberhard, W. G. Copulatory courtship and cryptic female choice in insects. _Biol. Rev._ 66, 1–31. https://doi.org/10.1111/j.1469-185X.1991.tb01133.x
(1991). Article Google Scholar * Matthews, R. V. & Matthews, J. R. _Insect Behavior_ (Springer, 2010). Book Google Scholar * Corbet, P. S. _Dragonflies: Behavior and Ecology of
Odonata_ (Comstock Publishing Associates, 1999). Google Scholar * Córdoba-Aguilar, A. _Dragonflies and Damselflies. Model Organisms for Ecological and Evolutionary Research_ (Oxford
University Press, 2008). Book Google Scholar * Suhonen, J., Rantala, M. J. & Honkavaara, J. Territoriality in odonates. In _Dragonflies and Damselflies: Model Organisms for Ecological
and Evolutionary Research_ (ed. Córdoba-Aguilar, A.) 203–217 (Oxford University Press, 2008). Chapter Google Scholar * Guillermo-Ferreira, R. & Del-Claro, K. Resource defense polygyny
by _Hetaerina rosea_ Selys (Odonata: Calopterygidae): Influence of age and wing pigmentation. _Neotrop. Entomol._ 40, 78–84 (2011). Article Google Scholar * Guillermo-Ferreira, R. &
Del-Claro, K. Oviposition site selection in _Oxyagrion microstigma_ Selys, 1876 (Odonata: Coenagrionidae) is related to aquatic vegetation structure. _Int. J. Odonatol._ 14, 275–279 (2011).
Article Google Scholar * Guillermo-Ferreira, R., Therézio, E. M., Gehlen, M. H., Bispo, P. C. & Marletta, A. The role of wing pigmentation, UV and fluorescence as signals in a
neotropical damselfly. _J. Insect Behav._ 27, 67–80 (2014). Article Google Scholar * Guillermo-Ferreira, R., Gorb, S. N., Appel, E., Kovalev, A. & Bispo, P. C. Variable assessment of
wing colouration in aerial contests of the red-winged damselfly Mnesarete pudica (Zygoptera, Calopterygidae). _Sci. Nat_. 102, 13 (2015). * Guillermo-Ferreira, R. & Bispo, P. C. Male and
female interactions during courtship of the Neotropical damselfly _Mnesarete pudica_ (Odonata: Calopterygidae). _Acta Ethol._ 15, 173–178 (2012). Article Google Scholar * Gibbons, D. W.
& Pain, D. The influence of river flow rate on the breeding behaviour of calopteryx damselflies. _J. Anim. Ecol._ 61, 283–289 (1992). Article Google Scholar * Robertson, H. M. Mating
behaviour and its relationship to territoriality in _Platycypha caligata_ (Selys) (Odonata: Chlorocyphidae). _Behaviour_ 79, 11–26 (1982). Article Google Scholar * Conrad, K. F. &
Pritchard, G. An ecological classification of odonate mating systems: The relative influence of natural, inter-and intra-sexual selection on males. _Biol. J. Linn. Soc._ 45, 255–269 (1992).
Article Google Scholar * Cordero-Rivera, A. & Andrés, J. A. Male coercion and convenience polyandry in a _Calopterygid damselfly_ (Odonata). _J. Insect Sci._
https://doi.org/10.1093/jis/2.1.14 (2002). Article Google Scholar * Miguel, T. B., Calvão, L. B., Vital, M. V. C. & Juen, L. A scientometric study of the order Odonata with special
attention to Brazil. _Int. J. Odonatol._ 20, 27–42 (2017). Article Google Scholar * Berger-Tal, O. _et al._ A systematic survey of the integration of animal behavior into conservation.
_Biol. Conserv._ 30, 744–753 (2016). Article Google Scholar * Cordero-Rivera, A. Behavioral diversity (ethodiversity): A neglected level in the study of biodiversity. _Front. Ecol. Evol._
5, 1–7 (2017). Article Google Scholar * Guillermo-Ferreira, R. & Juen, L. Behavioral syndromes as bioindicators of anthropogenic impact. _Int. J. Odonatol_. (_In press_) (2020). *
Oliveira-Roque, F. _et al._ The Tinbergen shortfall: Developments on aquatic insect behavior that Are critical for freshwater conservation. In _Aquatic Insects_ (eds Del-Claro, K. &
Guillermo, R.) 365–380 (Springer, 2019). Chapter Google Scholar * Caro, T. & Sherman, P. W. Vanishing behaviors. _Conserv. Lett._ 5, 159–166 (2012). Article Google Scholar * Harabiš,
F., Jakubec, P. & Hronková, J. Catch them if you can! Do traits of individual European dragonfly species affect their detectability_?_. _Insect Conserv. Diver._
https://doi.org/10.1111/icad.12378 (2019). Article Google Scholar * Oliveira-Junior, J. M. B. & Juen, L. The Zygoptera/Anisoptera Ratio (Insecta: Odonata): A New Tool for Habitat
Alterations Assessment in Amazonian Streams. _Neotrop. Entomol._ 48, 552–560 (2019). Article Google Scholar * Silva, D. C. & Oliveira-Junior, J. M. B. Efeito da cobertura de dossel
sobre a comunidade de Odonata (insecta) em igarapés na região de Santarém-Belterra (PA). _Rev. Ibero-Am. Ciênc. Ambient._ 9, 88–97 (2018). Google Scholar * Pereira, D. F. G.,
Oliveira-Junior, J. M. B. & Juen, L. Environmental changes promote larger species of Odonata (Insecta) in Amazonian streams. _Ecol. Indic._ 98, 179–192 (2019). Article Google Scholar *
Carvalho, F. G., Silva-Pinto, N., Oliveira-Junior, J. M. B. & Juen, L. Effects of marginal vegetation removal on Odonata communities. _Acta Limnol. Bras._ 25, 10–18 (2013). Article
Google Scholar * Rodrigues, M. E., Roque, F. O., Guillermo-Ferreira, R., Saito, V. S. & Samways, M. J. Egg-laying traits reflect shifts in dragonfly assemblages in response to different
amount of tropical forest cover. _Insect Conserv. Diver._ 12, 231–240 (2018). Article Google Scholar * De Marco, P., Batista, J. D. & Cabette, H. S. R. Community assembly of adult
odonates in tropical streams: An ecophysiological hypothesis. _PLoS ONE_ 10, e0123023 (2015). Article Google Scholar * Seidu, I., Danquah, E., Nsor, C. A., Kwarteng, D. A. & Lancaste,
L. T. Odonata community structure and patterns of land use in the Atewa Range Forest Reserve, Eastern Region (Ghana). _Int. J. Odonatol._ 20, 173–189 (2017). Article Google Scholar *
Strahler, A. N. Quantitative analysis of watershed geomorphology. _Eos Trans. Am. Geophys. Union._ 38, 913–920 (1957). Article Google Scholar * Heino, J. & Peckarsky, B. L. Integrating
behavioral, population and large-scale approaches for understanding stream insect communities. _Curr. Opin. Insect Sci._ 2, 7–13 (2014). Article Google Scholar * Cezário, R. R. _et al._
Sampling methods for dragonflies and damselflies. In _Measuring Arthropod Biodiversity: A Handbook of Sampling Methods_ (eds Santos, J. C. & Fernandes, G. W.) 223–240 (Springer, 2020).
Google Scholar * Lencioni, F. A. A. _The Damselflies of Brazil in An Illustrated Guide—Coenagrionidae_ (All Print Editora, 2006). Google Scholar * Borror, D. J. A key to the New World
genera of Libellulidae (Odonata). _Ann. Entomol. Soc. Am._ 38, 168–194 (1945). Article Google Scholar * Belle, J. Higher classification of the South-American Gomphidae (Odonata). _Zool.
Meded._ 70, 298–324 (1996). Google Scholar * Garrison, R. W. A synopsis of the genus Hetaerina with descriptions of four new species (Odonata: Calopterigidae). _Trans. Am. Entomol. Soc._
116, 175–259 (1990). Google Scholar * Lencioni, F. A. A. _The Damselflies of Brazil: An Illustrated Guide—The Non Coenagrionidae Families_ (All Print Editora, 2005). Google Scholar *
Garrison, R. W., Von Ellenrieder, N. & Louton, J. A. _Dragonfly Genera of the New Word: An Illustrated and Annotated Key to the Anisoptera_ (The Johns Hopkins University Press, 2006).
Book Google Scholar * Garrison, R. W., Von Ellenrieder, N. & Louton, J. A. _Damselfly genera of the New World in Baltimore, An Illustrated and Annotated Key to the Zygoptera_ (The
Johns Hopkins University Press, 2010). Google Scholar * Kaufmann, P. R., Levine, P., Robison, E. G., Seeliger, C. & Peck, D. V. _Quantifying Physical Habitat in Wadeable Streams.
EPA/620/R-99/003_ (US Environmental Protection Agency, 1999). Google Scholar * Juen, L. _et al._ Effects of oil palm plantations on the habitat structure and biota of streams in Eastern
Amazon. _River Res. Appl._ 32, 2081–2094 (2016). Article Google Scholar * Nessimian, J. L. _et al._ Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian
streams. _Hydrobiologia_ 614, 117–131 (2008). Article Google Scholar * Brasil, L. S., Lima, E. L., Spigoloni, Z. A., Ribeiro-Brasil, D. R. G. & Juen, L. The habitat integrity index and
aquatic insect communities in tropical streams: A meta-analysis. _Ecol. Ind._ 116, 106495 (2020). Article Google Scholar * Bastos, R. C. _et al._ Morphological and phylogenetic factors
structure the distribution of damselfly and dragonfly species (Odonata) along an environmental gradient in Amazonian streams. _Ecol. Indic._ 122, 107257 (2021). Article Google Scholar *
Mendoza-Penagos, C. C., Calvão, L. B. & Juen, L. A new biomonitoring method using taxonomic families as substitutes for the suborders of the Odonata (Insecta) in Amazonian streams.
_Ecol. Indic._ 124, 107388 (2021). Article Google Scholar * Violle, C. _et al._ Let the concept of trait be functional!. _Oikos_ 116, 882–892 (2007). Article Google Scholar *
Luiza-Andrade, A., Assis Montag, L. F. & Juen, L. Functional diversity in studies of aquatic macroinvertebrates community. _Scientometrics_ 111, 1643–1656 (2017). Article Google Scholar
* Dalzochio, M. S. _et al._ Effect of tree plantations on the functional composition of Odonata species in the highlands of southern Brazil. _Hydrobiologia_ 808, 283–300 (2018). Article
Google Scholar * McCauley, S. J. Relationship between morphology, dispersal and habitat distribution in three species of Libellula (Odonata: Anisoptera). _Aquat. Insects._ 34, 195–204
(2012). Article Google Scholar * Turlure, C., Schtickzelle, N. & Baguette, M. Resource grain scales mobility and adult morphology in butterflies. _Land. Ecol._ 25, 95–108 (2010).
Article Google Scholar * Reed, S. C., Williams, C. M. & Chadwick, L. E. Frequency of wing-beat as a character for separating species races and geographic varieties of Drosophila.
_Genetics_ 27, 349–361 (1942). Article Google Scholar * Hall, J. P. & Willmott, K. R. Patterns of feeding behaviour in adult male riodinid butterflies and their relationship to
morphology and ecology. _Biol. J. Linn. Soc._ 69, 1–23 (2000). Article Google Scholar * Pavoine, S., Vallet, J., Dufour, A. B., Gachet, S. & Daniel, H. On the challenge of treating
various types of variables: Application for improving the measurement of functional diversity. _Oikos_ 118, 391–402 (2009). Article Google Scholar * Villéger, S., Mason, N. W. H. &
Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. _Ecology_ 89, 2290–2301 (2008). Article Google Scholar * Stine, R. A. The
graphical interpretation of variance inflation factors. _Am. Stat._ 49, 53–56. https://doi.org/10.1080/00031305.1995.10476113 (1995). Article Google Scholar * Laliberté, E. &
Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. _Ecology_ 91, 299–305 (2010). Article Google Scholar * Oksanen, J. _et al._ Vegan:
Community Ecology Package. R package version 2.5-5, 1–298 (2019). https://CRAN.R-project.org/package=vegan. * Chessel, D., Dufour, A. & Thioulouse, J. “The ade4 Package—I: One-Table
Methods.” R News. 4, 5–10. https://cran.r-project.org/doc/Rnews/ (2004). * Faraway, J. faraway: Functions and Datasets for Books by Julian Faraway. R package version 1, 1–117. (2016).
https://CRAN.R-project.org/package=faraway * Brasil, L. S. _et al._ Net primary productivity and seasonality of temperature and precipitation are predictors of the species richness of the
Damselflies in the Amazon. _Basic. Appl. Ecol._ 35, 45–53 (2019). Article Google Scholar * Remsburg, A. J. & Turner, M. G. Aquatic and terrestrial drivers of dragonfly (Odonata)
assemblages within and among north-temperate lakes. _J. N. Am. Benthol. Soc._ 28, 44–56 (2009). Article Google Scholar * Vilela, D. S., Ferreira, R. G. & Del-Claro, K. The Odonata
community of a Brazilian vereda: Seasonal patterns, species diversity and rarity in a palm swamp environment. _Bioscience_ 32, 486–495 (2016). Google Scholar * Sahlén, G. Specialists vs.
generalists among dragonflies—The importance of forest environments to form diverse species pools. In _Forests and Dragonflies_ (ed. Rivera, A. C.) 153–179 (Pensoft Publishers, 2006). Google
Scholar * Winemiller, K. O. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. _Ecol. Monogr._ 61, 343–365 (1991). Article Google Scholar *
Mc Peek M. A. Ecological factors limiting the distribuitions and abundances of Odonata. In Paulson, _Dragonflies_ & _Damselflies: Model Organisms for Ecological and Evolutionary
Research_ (ed. Córdoba-Aguilar, A.) 51–62 (Oxford University Press, 2008). * Balzan, M. V. Associations of dragonflies (Odonata) to habitat variables within the Maltese Islands: A
spatio-temporal approach. _J. Insect Sci._ https://doi.org/10.1673/031.012.8701 (2012). Article Google Scholar * Ribeiro, J. R. I., Nessimian, J. L., Mendonça, E. C. & Carvalho, A. L.
Aspectos da distribuição dos Nepomorpha (Hemípteros: Heterópteros) em corpos d ́água na restinga de Maricá, Estado do Rio de Janeiro. _Oecol. Aust._ 5, 113–128 (1998). Google Scholar *
Juen, L. & De Marco, P. Dragonfly endemism in the Brazilian Amazon: Competing hypotheses for biogeographical patterns. _Biodivers. Conserv._ 21, 3507–3521 (2012). Article Google Scholar
Download references ACKNOWLEDGEMENTS We thank Cikel Ltd., 33 Forest, Instituto Floresta Tropical (IFT), the BRC (Brazilian Research Consortium) Consortium and Hydro Company for financial
and logistical support. We also thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financing the project entitled “Influência dos diferentes tipos de uso do
solo sobre a biodiversidade na Amazônia Oriental” (project number 449315/2014-2) and “Tempo de resiliência das comunidades aquáticas após o corte seletivo de madeira na Amazônia Oriental”
(project number 481015/2011-6). We are also grateful to the BRC and the Hydro company for providing scholarships to BOR, JSB, and RB. BOR and VRSF would also like to thank the Coordenação de
Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for doctoral scholarships (process numbers 88887.493682/2020-00 and 88887.469089/2019). We are also grateful to CNPq for the research
productivity grants awarded to LJ (process 304710/2019-9) and RGF (process 307836/2019-3), ALA (141991/2016-0) and JSB (141113/2020-0) for doctoral scholarships, and LBC for a research
scholarship (process 154761/2018-4). We are also grateful to CAPES for funding the senior internship scholarship for LJ through PROCAD-AMAZONIA/CAPES, for research at the University of
Florida (process 88881.474457/2020-01). RGF thanks FAPESP for continuous support (process 2013/00406-7; 2019/20130-2). ACR was funded by Grants from the Spanish Ministry of Science,
including the European Regional Development Fund (Grants CGL2014-53140-P and PGC2018-096656-B-I00). Finally, we thank the Pró-Reitoria de Pesquisa e Pós-Graduação (PROPESP)/Universidade
Federal do Pará (UFPA) for funding the revision of the manuscript by Dr. Stephen Ferrari. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratório de Ecologia e Conservação, Instituto de
Ciências Biológicas, Universidade Federal do Pará, Belém, Brazil Bethânia O. de Resende, Victor Rennan S. Ferreira, Leandro S. Brasil, Thiago P. Mendes, Fernando G. de Carvalho, Cristian C.
Mendoza-Penagos, Rafael C. Bastos, Joás S. Brito, Ana Luiza-Andrade & Leandro Juen * Graduate Program in Ecology-PPGECO, Universidade Federal do Pará, Belém, Brazil Bethânia O. de
Resende, Victor Rennan S. Ferreira, Lenize B. Calvão, Fernando G. de Carvalho, Rafael C. Bastos, Joás S. Brito, José Max B. Oliveira-Junior, Karina Dias-Silva & Leandro Juen * Instituto
de Ciências e Tecnologia das Águas (ICTA), Universidade Federal do Oeste do Pará (UFOPA), Rua Vera Paz, s/n (Unidade Tapajós) Bairro Salé, Santarém, Pará, 68040-255, Brazil José Max B.
Oliveira-Junior * LESTES Lab, DHb, UFSCar, São Carlos, São Paulo, Brazil Rhainer Guillermo * Universidade de Vigo, ECOEVO Lab, EE Forestal, Campus Universitario A Xunqueira, 36005,
Pontevedra, Spain Adolfo Cordero-Rivera * Graduate Program in Agriculture and the Environment-PPGAA, Universidade Estadual do Maranhão, Balsas, Maranhão, Brazil Thiago P. Mendes * Graduate
Program in Environmental Sciences-PPGCA, Universidade Federal do Amapá, Macapá, Amapá, Brazil Lenize B. Calvão Authors * Bethânia O. de Resende View author publications You can also search
for this author inPubMed Google Scholar * Victor Rennan S. Ferreira View author publications You can also search for this author inPubMed Google Scholar * Leandro S. Brasil View author
publications You can also search for this author inPubMed Google Scholar * Lenize B. Calvão View author publications You can also search for this author inPubMed Google Scholar * Thiago P.
Mendes View author publications You can also search for this author inPubMed Google Scholar * Fernando G. de Carvalho View author publications You can also search for this author inPubMed
Google Scholar * Cristian C. Mendoza-Penagos View author publications You can also search for this author inPubMed Google Scholar * Rafael C. Bastos View author publications You can also
search for this author inPubMed Google Scholar * Joás S. Brito View author publications You can also search for this author inPubMed Google Scholar * José Max B. Oliveira-Junior View author
publications You can also search for this author inPubMed Google Scholar * Karina Dias-Silva View author publications You can also search for this author inPubMed Google Scholar * Ana
Luiza-Andrade View author publications You can also search for this author inPubMed Google Scholar * Rhainer Guillermo View author publications You can also search for this author inPubMed
Google Scholar * Adolfo Cordero-Rivera View author publications You can also search for this author inPubMed Google Scholar * Leandro Juen View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS B.O.R.: Conceptualization, Methods, Writing—Original Draft; V.R.S.F.: Conceptualization, Methods, Formal analysis, Writing—Review and
Editing; L.S.B.: Investigation, Data Curation, Writing—Review and Editing; L.B.C.: Investigation, Data Curation, Writing—Review and Editing; T.P.M.: Writing—Review and Editing; F.C.:
Investigation, Data Curation, Writing—Review and Editing; C.C.M.-P.: Investigation, Data Curation, Writing—Review and Editing; R.B.: Writing—Review and Editing; J.S.B.: Writing—Review and
Editing; J.M.B.O.-J.: Investigation, Data curation, Writing—Review and Editing; K.D.S.: Investigation, Data curation, Writing—Review and Editing; A.L.-A.: Formal analysis, Writing—Review and
Editing; R.G.: Conceptualization, Methods, Writing—Review and Editing; A.C.-R.: Conceptualization, Methods, Writing—Review and Editing; L.J.: Investigation, Data Curation,
Conceptualization, Writing—Review and Editing, Supervision, Acquisition of funding. CORRESPONDING AUTHOR Correspondence to Bethânia O. de Resende. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative
Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE de Resende, B.O., Ferreira, V.R.S., Brasil, L.S. _et al._ Impact of environmental
changes on the behavioral diversity of the Odonata (Insecta) in the Amazon. _Sci Rep_ 11, 9742 (2021). https://doi.org/10.1038/s41598-021-88999-7 Download citation * Received: 14 August 2020
* Accepted: 13 April 2021 * Published: 07 May 2021 * DOI: https://doi.org/10.1038/s41598-021-88999-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
