Microbial sulfate reduction by Desulfovibrio is an important source of hydrogen sulfide from a large swine finishing facility
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

There is still a lack of understanding of H2S formation in agricultural waste, which leads to poor odour prevention and control. Microbial sulfate reduction is a major process contributing
to sulfide formation in natural and technogenic environments with high sulfate and low oxygen concentration. Agricultural waste can be considered a low-sulfate system with no obvious input
of oxidised sulfur compounds. The purpose of this study was to characterise a microbial community participating in H2S production and estimate the microbial sulfate reduction rate (SRR) in
manure slurry from a large-scale swine finishing facility in Western Siberia. In a series of manure slurry microcosms, we identified bacterial consortia by 16S rRNA gene profiling and
metagenomic analysis and revealed that sulfate-reducing Desulfovibrio were key players responsible for H2S production. The SRR measured with radioactive sulfate in manure slurry was high and
comprised 7.25 nmol S cm−3 day−1. Gypsum may be used as a solid-phase electron acceptor for sulfate reduction. Another plausible source of sulfate is a swine diet, which often contains
supplements in the form of sulfates, including lysine sulfate. Low-sulfur diet, manure treatment with iron salts, and avoiding gypsum bedding are possible ways to mitigate H2S emissions from
swine manure.
Swine yield is considered a dominant portion of the market available livestock trade1. Pork global production is forecasted to reach 102,160 thousand tons in 20212, including 3.6 million
tons in Russia, which is among the five top world producers along with China, the USA, EU, and Brasil. The acrid odour of swine manure is a well-known problem associated with livestock
production facilities and animal waste storage systems3. The most noticeable and highly toxic odorous gas in animal wastes is hydrogen sulfide, which is recognised by its typical rotten-eggs
smell. H2S and other volatile sulfur compounds comprise about one-half of the offensive odourants from swine manure4,5. Hydrogen sulfide has a low odour threshold of around 0.5 ppb (mg L−1)
and causes eye irritation at concentrations of 50–100 ppm, while 300–500 ppm (mg L−1) may result in severe poisoning via inhibition of cytochrome oxidase in mammals6. Prolonged exposure to
low concentrations of H2S was associated with persistent neurobehavioral dysfunction7. Odorous compounds also may affect the comfort, health, and production efficiency of animals as well as
the health and comfort of human workers. Fatal asphyxia incidents were reported during manure handling/maintenance due to H2S poisoning8.
There was much uncertainty on the estimation of H2S emissions from livestock waste until recently. Proton-transfer-reaction mass spectrometry (PTR-MS) provided solid evidence that hydrogen
sulfide from agricultural sources was a major source of atmospheric sulfur in regions with intensive animal production9. In contrast to the general perception of the minor importance of H2S
compared to SO2 from industry, the authors demonstrated that emissions from finisher pig production comprised the largest source of atmospheric sulfur in Denmark. Emissions of H2S contribute
to the atmospheric sulfur compounds via its oxidation to aerosol sulfate.
Sulfate-reducing prokaryotes have long been recognized to be major players in biogenic H2S production. Microbial sulfate reduction is ubiquitous in natural environments and mainly associated
with marine biotopes due to their high sulfate level. Agricultural waste is considered as low-sulfate environment rich in organic compounds4. A highly active but cryptic sulfur cycle has
been described for low-sulfate environments, such as peatlands10 and fresh-water lakes11. In low-sulfate biotopes, reduced sulfur species are rapidly reoxidised by oxygen- or iron-respiring
microorganisms that sustain sulfate reduction rates as high as in sulfate-rich marine surface sediments10. Surprisingly, few reports are available on the presence and activity of
sulfate-reducing bacteria (SRB) in swine manure. Desulfovibrio, Desulfobulbus, and Desulfobacterium have been detected by cloning of fragments of dsrA gene encoding a subunit of
dissimilatory sulfite reductase from swine slurry stored in underground pits or lagoons12. Manure treatment with borax (sodium tetraborate decahydrate)13 and tannins14 were proposed to
control SRB population and reduce H2S production. ZnO nanoparticles were applied as an additive to swine manure to reduce biogenic H2S emissions15. The Desulfovibrio was not detected in the
microbial community from pig slurry was small, and that led authors to the conclusion that Firmicutes and Bacteroidetes, comprising the majority of microbial community, played an important
role in the offensive odour compounds production via protein and carbohydrate degradations16.
The present study aimed to understand the diversity and activity of SRB in swine wastes produced by a large-scales swine finishing facility ‘Tomskii’ with a capacity of 176,000 hogs a year
and located in the close vicinity of Tomsk, the capital city of the Tomsk region in Western Siberia, Russia. Strong odour from the facility reaches residential areas of the city in the
summer time and is a matter of serious public concern. We identified bacterial consortia by 16S rRNA gene profiling and metagenomic analysis, measured sulfate reduction rate with radioactive
tracer in manure slurry, isolated and studied SRB responsible for H2S production.
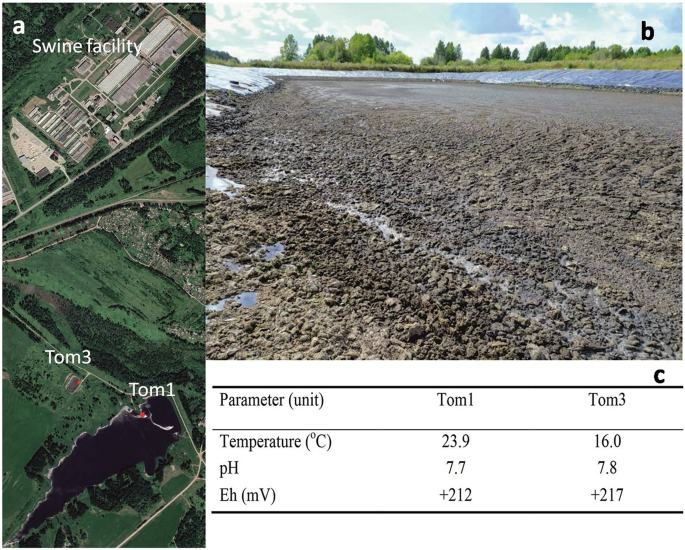
Waste samples were collected from two lagoons at the large-scale swine finishing facility ‘Tomskii’. The swine manure and wastewater from the facility is treated by the lagoons only. The
cumulative manure slurry from the facility pumped to a manure storage lagoon (N56° 58′, E85° 14′; Tom3), which connected by a pipe system with a larger solid–liquid separation lagoon (N56°
57′, E85° 14′; Tom1) of a total volume around 1.5 billion m3 (Fig. 1). The solid–liquid separation lagoon water was used to fertilise agricultural fields via a sprinkler system. However,
this practice was recently abandoned due to the public complaints of malodour from the site. Part of the solid–liquid separation lagoon effluent is released into small stream by an
underdrain.
Google image of swine facility, manure storage lagoon, and solid–liquid separation lagoon with sampling locations (a), manure lagoon (b), and characteristics of the sites at the time of
sampling (c). Maps Data: Google, CNES/Airbus, Landsat/Copernicus, Maxar Technologies, 2021.
The H2S concentration in the ambient air at the solid–liquid separation lagoon (Tom1) was monitored over time (Fig. 2). No measurements were taken during the seasonal snow cover, which
lasted from the beginning of November till the end of March. The H2S concentration in ambient air changed from 0.08 (late October) to 0.69 mg m−3 (mid-September). These concentrations well
exceed the maximum admissible level of 0.008 mg m−3 set up in Russia for residential areas. The average ambient air H2S level tended to correlate with outdoor temperature. The pH values of
both lagoons were 7.7–7.8 and redox potential of + 212–217 mV, indicative of aerobic conditions (Fig. 1). The sulfate concentration in the pore water was 17.4 mg L−1, and chloride was the
major anion with the concentration of 300 mg L−1 (Table 1). Dissolved iron was low and did not exceed 1.36 mg L−1.The mineralogical composition of the sediment and manure slurry revealed the
presence of gypsum (CaSO4·2H2O) (Fig. 3). The manure also contained crystalline sulfur (PDF-24-0733). Calcium carbonates, calcite, CaCO3 (PDF-24-0027), and ankerite (Ca(Fe,Mg,Mn)(CO3)2;
PDF-79-1347) were also present in both samples. Scanning electron microscopy with X-ray microanalysis (SEM–EDS) showed that Ca reached up to 51% of the elemental composition of solids and S
up to 14%. Fe and P comprised up to 20% and 54% of the elemental composition in the solids, respectively.
Time course of hydrogen sulfide levels (1, solid line) and ambient air temperature (2, dashed line) at the solid–liquid separation lagoon (Tom1). H2S concentrations in air were measured via
an OKA-T portable gas analyser with electrochemical sensor (accuracy of ± 25% of reading). Vertical bars show the standard deviation calculated from three readings.
The X-ray diffractograms show the mineralogical composition of the sediment from the solid–liquid separation lagoon, sample Tom1 (a) and manure slurry, sample Tom3 (b). The vertical bar
shows the scale of relative counts. Letter codes: Ae ankerite, Ca(Fe,Mg,Mn)(CO3)2, Cc calcite, CaCO3, Er Erionite, (Na2,K2,Ca)2Al4Si14O36·15H2O, Gy gypsum,CaSO4·2H2O, Qu quartz, SiO2, S
sulfur, S6, Sp Saponite 15ACa0.2Mg3(Si,Al)4O10 (OH)2·4(H2O), St Struvite, NH4MgPO4·6H2O, Td tridymite, SiO2, Wi willhendersonite, KCaAl3Si3O12·5H2O. Note gypsum and sulfur occurrence in both
samples. SEM micrograph (c) and the respective microprobe analysis (d) demonstrates elemental composition of the manure slurry, sample Tom3.
The sulfate reduction rate (SRR) measured directly in manure slurry (sample Tom3) was high and reached 7.25 nmol S cm−3 day−1. The acid volatile sulfide fraction (AVS), which includes H2S
and FeS, was the major product of 35SO42− reduction, and comprised 69% of total reduced sulfur. Sulfate amendment enhanced the rate up to 427 nmol S cm−3 day−1 (Fig. 4). Lactate plus sulfate
had the most pronounced effect on SRR and increased it by nearly 100 times over the control to 674 nmol S cm−3 day−1. The experiments with amendments showed that the higher SRR, the smaller
is the fraction of pyrite sulfur (CRS) in the total pool of reduced sulfur.
Sulfate reduction rate (SRR) and the amount of sulfur in acid volatile sulfides (AVS) and chromium-reducible sulfur (CRS) measured directly in the manure slurry (Tom3) without amendments
(control), manure slurry supplemented with sulfate (Sulfate), and manure slurry supplemented with sulfate plus lactate (sulfate + lactate).
The preliminary investigation of the microbial community by 16S rRNA sequencing did not reveal any taxonomic groups with known capability for dissimilatory sulfate reduction in the sediment
slurry from solid–liquid separation lagoon (Tom1). Considering the low sulfate concentration in the biotope, we set up a microcosm series of the sediment slurry from solid–liquid separation
lagoon (Tom1) amended with sulfate, and sulfate plus lactate with the purpose to reveal low-number SRB limited by electron donor or/and acceptor. The same amendments were applied to the
manure lagoon slurry microcosms (Tom3). All microcosms were supplemented by control without amendments. The H2S content in microcosms was monitored and reached 16 ± 2 mg/L in Tom1 and 19 ± 4
mg/L in Tom3 microcosms. Microbial communities of the sediment slurry from solid–liquid separation lagoon (Tom1), manure slurry from the manure lagoon (Tom3), and corresponding microcosms
were characterised by analysing their 16S rRNA gene sequences. At least 11,000 16S rRNA gene reads (on average 33,316 for Tom1 and 25,760 reads for Tom3 samples) were used to reveal
microbial community composition.
Sulfate and lactate amendments had little effect on the composition of the microbial community of the sediment slurry from solid–liquid separation lagoon (Tom1), and all three samples were
dominated by methanogenic archaea (18.3% to 25.4% of total 16S rRNA gene reads), members of the phyla Bacteroidetes (18.1–21.1%), Campylobacteraeota (17.2–25.3%), Firmicutes (9.4–10.6%),
Patescibacteria (8.4–10.0%), and Synergistetes (7.4–10.2%) (Fig. 5A). Deltaproteobacteria accounted for 1.4–1.8% and were represented by Smithella sp., syntrophic degraders of low molecular
weight organics17. Lineages with known capability for dissimilatory sulfate reduction were not found in any of the Tom1 microcosms. The only detected lineage known to be involved in the
sulfur cycle, bacteria of the genus Sulfurimonas (Campylobacterota), were present below the detection limit in the control microcosm without sulfate and accounted for 0.9% and 5.3% in
microcosms amended with sulfate and sulfate with lactate, respectively. Sulfurimonas species are typically chemolithoautotrophs found in sulfidic environments, such as hydrothermal vents,
marine sediments, sulfidic springs, and groundwater18. They can oxidise reduced sulfur compounds and hydrogen using oxygen, nitrate, or nitrite as electron acceptors.
The relative abundance of taxonomic groups of microorganisms according to 16S rRNA gene profiling in microcosms derived from (A) the sediment slurry (Tom1) and (B) manure slurry (Tom3). S-,
control microcosms with no supplements; S and SL, microcosms amended with sulfate, and sulfate + lactate, respectively. Note that manure slurry samples were analysed in triplicate.
Most of dominant prokaryotic lineages in the sediment slurry samples from the manure lagoon (Tom1) were also abundant in the manure slurry microcosms (Tom3), namely methanogenic
Euryarchaeota (29.6–38.2%), Bacteroidetes (22.0–28.5%), Campylobacterota (3.1–11.9%), and Firmicutes (10.0–13.9%) (Fig. 5B). Spirochaetes (8.0–15.0%) and Proteobacteria (3.0–5.6%) were also
abundant in Tom3, while Patescibacteria and Synergistetes accounted for less than 0.5% of the 16S rRNA gene reads in all Tom3 samples. Several presumably sulfate-reducing bacterial lineages
were identified in the manure slurry microcosms (Tom3): Deltaprotreobacteria of the families Desulfovibrionaceae, Desulfomicrobiaceae, Desulfobacteraceae, and Desulfobulbaceae. All these
groups except Desulfovibrionaceae were present in minor amounts (
