Laser isotope separation of 176lu through off-the-shelf lasers
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We propose a novel and simple method for the laser isotope separation of 176Lu a precursor for the production of 177Lu medical isotope. The physics of the laser-atom interaction has
been studied through the dynamics of the atomic level populations using the density matrix formalism. It has been shown that a combination of cw excitation lasers and pulsed ionization
laser can be used for the laser isotope separation of 176Lu. The optimum conditions for the efficient and selective separation of 176Lu have been derived by studying the time evolution of
level population under laser excitation. It has also been shown that, it might be possible to produce ~ 100% enriched 176Lu isotope at a rate of 5 mg/h, which is higher than all previously
reported methods so far. The isotope separation process proposed can be easily adopted using off-the-shelf lasers, for similar atomic systems. SIMILAR CONTENT BEING VIEWED BY OTHERS LASER
ISOTOPE ENRICHMENT OF 168ER Article Open access 27 March 2025 ISOTOPE SEPARATION OF 176LU A PRECURSOR TO 177LU MEDICAL ISOTOPE USING BROADBAND LASERS Article Open access 17 March 2021 LASER
ISOTOPE SEPARATION OF 223RA Article Open access 28 April 2023 INTRODUCTION Atomic Vapor Laser Isotope Separation (AVLIS) of actinides (particularly Uranium) has applications in nuclear
industry and defence1; therefore, a lot of research has been carried out to achieve the above objective. The technology thus developed is largely limited to a handful of technologically
advanced nations and strongly controlled due to proliferation risks. Most of the AVLIS activity is centred on utilization of high repetition rate Copper Vapor Laser (CVL) or Solid-State
Laser (SSL) pumped dye laser systems for the excitation and ionization of the desired isotope(s). The high pulse repetition frequency (PRF) (10 kHz or higher) of these lasers ensures high
interaction efficiency with the atomic vapor enabling the production of the desired isotopes in large scales. It has been realised that AVLIS technology has the ability to play a pivotal
role in nuclear medicine2. 177Lu isotope has gained importance in the targeted radionuclide therapy of small tumors and metastatic lesions3,4. 177Lu can be produced in a nuclear reactor by
irradiation of its precursor (or parent) 176Lu isotope. The cross-section 4 of this nuclear reaction is 2090 b. 177Lu having a half-life of 6.65 d decays to 177Hf emitting β-particles with
energies 497 keV (76%), 384 keV (9.7%) and 176 keV (12%). The 177Hf which is formed in nuclear excited states decays to the ground state emitting low energy γ-radiation with energies 208 keV
(11%) and 113 keV (6.6%). With mean penetration depths of β-particles in sub-mm, the radionuclide 177Lu is capable of delivering energy in small volumes, thus having localized cytotoxic
effect on the disease cells. The γ-radiation is useful for imaging and studies of bio-distribution and excretion kinetics of the infused radionuclide. Due to these advantages, 177Lu has
evolved as the most effective theranostic (therapeutic + diagnostic) isotope in nuclear medicine. However, natural Lu cannot be used for the production of 177Lu due to the low natural
abundance of its precursor isotope 176Lu (2.59%). It is required to be enriched to ≥ 50% level5. The typical administered quantity4 of 177Lu per course for a patient is ~ 7.4 GBq (1.8 µg).
Therefore, after taking into account the conversion efficiency, 1 mg of enriched 176Lu can be used for the treatment of > 500 patients after irradiation. Despite the requirements in small
quantities, currently only a very few laboratories in the world have the ability to produce enriched 176Lu isotope leading to the worldwide shortage of this isotope. This is primarily due
to the non-availability of the suitable commercial tunable narrow band high repetition rate lasers. AVLIS exploits the small resonance frequency shifts between the constituent isotopes of an
element (called isotope shifts). The lasers in the step-wise ionization are resonantly tuned to the desired isotope causing preferential ionization enabling the separation from the rest of
the isotopes. However, laser isotope separation of Lutetium is extremely complex. The nuclear volume changes6 in adjacent stable Lu isotopes are small (0.041 fm2) resulting in small field
shift. Since the mass shift is also small for the heavy element like Lu, the resultant isotope shifts are small. Fortunately, in case of Lutetium, both stable isotopes of Lu (175Lu and
176Lu) have widely varying nuclear spin, magnetic moment and quadrupole moment values. Consequently, 176Lu has a widely spread hyperfine spectrum than the interfering 175Lu isotope, which
can be exploited for the enrichment of 176Lu. Kurchatov Institute7, Moscow has reported successful enrichment of 176Lu to > 68% with production rate of ~ 4 mg/h. They have used the
following photoionization scheme for the enrichment of 176Lu. $$5d6{s}^{2}\,\,^{2}{D}_{3/2} (0.00 {\mathrm{cm}}^{-1}) \stackrel{540.4068 \mathrm{nm}}{\longrightarrow}\,5d6s6p\,\,
^{4}{F}_{5/2}^{o}\left(18504.58 {\mathrm{cm}}^{-1}\right)\stackrel{535.0626 nm}{\longrightarrow }$$ $$5d6s7s \,\,^{4}{D}_{3/2}\left({37,193.98}
{\mathrm{cm}}^{-1}\right)\stackrel{618.0061\,\mathrm{nm}}{\longrightarrow}\,{53,375} {\mathrm{cm}}^{-1}Autoionization State \stackrel{}{\longrightarrow }{Lu}^{+}$$ We have recently studied8
the isotope selective photoionization of 176Lu and optimization of the process conditions for the enrichment of 176Lu through the above photoionization scheme using density matrix formalism.
The obtained results were in good agreement with the reported experimental data7. Further, we have recently reported a new possible photoionization pathway for the enrichment of 176Lu using
broad band dye lasers9. However, most of these schemes are accessible by high repetition rate (10–30 kHz) CVL or SSL pumped dye laser systems. Since these laser systems have been
categorised as potential dual use technology equipment, hence they have export restrictions to several countries. Therefore, isotope separation using the above scheme(s) cannot be easily
adopted by all the producers of nuclear medicine. This is severely restricting the availability of enriched 176Lu isotope resulting in global shortage of the 177Lu medical isotope. In order
to enhance the production of enriched 176Lu isotope, it is required to look into the photoionization schemes which are easily accessible by off-the-shelf lasers. In the present work, we have
theoretically studied the possibility of using off-the-shelf lasers for the enrichment of176Lu isotope. PHOTOIONIZATION OF LU Lutetium has two stable isotopes. They are 175Lu (97.41%) and
176Lu (2.59%). It has a ground state configuration of 5d6s2 2D3/2 (0.00 cm−1) and has an ionization potential of 43,762.60 cm−1. From the known energy levels10 of Lu, it is possible to
formulate innumerable multi-step photoionization schemes. Apart from the well-known first excitation transitions 540.4068 nm and 573.8130 nm accessible by dye lasers, several strong first
excitation transitions11 exist between the wavelength range 272–495 nm. Among them, the transitions between the wavelength range of 350–495 nm are accessible by continuous wave (cw) diode
laser or second harmonic of the Ti:Sapphire lasers. The hyperfine structure13 of Lu isotopes of several photoionization schemes originating from these transitions have been studied for the
relative separation of the hyperfine components of the constituent isotopes. A list of possible photoionization schemes which showed good separation of the most intense hyperfine component
of 176Lu from the hyperfine structure of 175Lu have been tabulated in Table 1. The requisite wavelengths for these schemes are accessible by commercially available diode lasers or
Ti:Sapphire lasers (fundamental frequency or through second harmonic generation). Among them, the following excitation transition has been considered for further discussion. 465 NM–1179 NM
SCHEME $$5d6{s}^{2}\,^{2}{D}_\frac{3}{2}\left(0.00 \,\,{\mathrm{cm}}^{-1}\right)\stackrel{465.9316\,\,\mathrm{nm}}{\longrightarrow} 5d6s6p \,\,^{2}{D}_\frac{5}{2}^{o}\left(21462.38
{\mathrm{cm}}^{-1}\right)\stackrel{1179.87 \mathrm{nm}}{\longrightarrow}$$ $${5d}^{2}6s\,^{2}{P}_\frac{3}{2}\left({29,937.9}
{\mathrm{cm}}^{-1}\right)\stackrel{}{\longrightarrow}\,\,Autoionization\,\,State\stackrel{}{\longrightarrow}{Lu}^{+}$$ Photoionization of Lu through pulsed blue dye lasers14 for the first
step excitation transition was reported way back in 1981. Later, photoionization schemes originating from UV-blue wavelength region15,16 have been studied for the photoionization of Lu using
second and third harmonics of the pulsed Ti:Sapphire lasers to access the requisite wavelengths. However, such lasers have linewidths in several GHz, hence, cannot be easily adopted for the
isotope separation of Lu. Utilization of diode lasers for isotope separation was first reported by Olivares et al.17, wherein two-step photoionization method was used for the isotope
separation of Li isotopes. Recently Matsuoka et al.18 have studied isotope separation of 48Ca using diode lasers. In both cases ionization has been carried out by a non-resonance ionization
process using pulsed lasers. Further, applicability of such a method for large scale separations has not been stated. The isotope shifts for the first and second excitation transitions of Lu
are typically in the range of 400 MHz and 100 MHz respectively. From the hyperfine structure constant data12,13 (Table 2), the frequency positions of the two-step hyperfine excitation
pathways have been calculated and tabulated in Table 3. As it can be seen from the Table 3, the most intense hyperfine excitation pathway 17/2–19/2–17/2 at the frequency position (15,150
MHz, -25,160 MHz) of 176Lu is well separated from the nearest and the most intense hyperfine excitation pathway 5–6-5 which is at the frequency position (11,100 MHz, −17,720 MHz)of 175Lu.
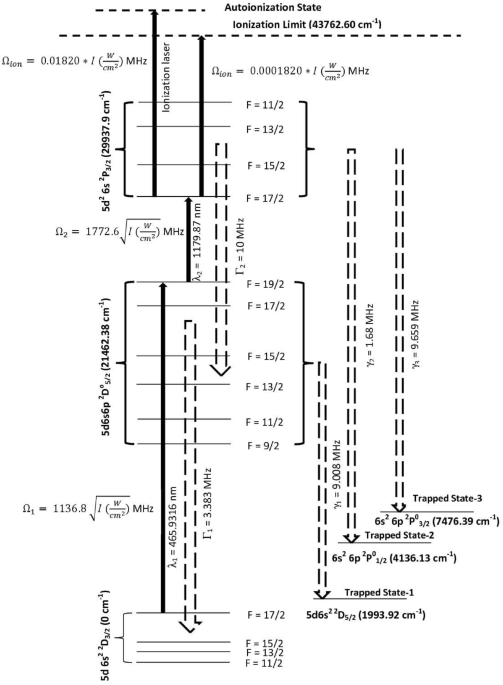
The schematic of the photoionization scheme is shown in Fig. 1. The atoms in the ground state 5d6s2 2D3/2 (0.00 cm−1) are excited to the 5d6s6p 2D05/2 (21,462.38 cm−1) level using the
465.9316 nm laser and atoms from this level are further excited to the 5d26s 2P3/2 (29,937.9 cm−1) level using the 1179.87 nm laser, which are ionized using an ionization laser through
either auto-ionization level or non-resonant ionization. The ionization process is considered as an incoherent process. During the excitation process, due to the finite life-time of the
resonant states, the atoms decay either back to their original states or into the trapped states and lost from ionization process. The excitation rates, decay11 rates of atoms and the
ionization rates are also given in Fig. 1. The radiative decay rate of the transition 1179.87 nm has not been reported so far. Several transitions originating from the lower levels ranging
between 20,000 cm1 to 25,000 cm−1 have shown11 decay rates of about 1 × 107 Hz, therefore; the decay rate is taken as 1 × 107 Hz for the 1179.87 nm transition. Several articles have been
published on the even parity autoionization states19,20,21,22,23 wherein two step laser excitation method has been used. However, very little work has been on the odd parity autoionization
states. D’yachkov7 et al. have found several odd-parity autoionization states between the energy range 53,067 cm−1–53,861 cm−1 among which, autoionization transition to 53,375 cm−1 was the
strongest. Another work24 recently reported nineteen new odd parity autoionization levels in the energy range 50,650–51,650 cm−1. Using Ti:Sapphire laser for the third excitation step, it is
possible to access Rydberg or Auto-ionization state in the range between 39,938 cm−1 and 49,560 cm−1 from the second excitation level 29,937.9 cm−1. Since this is a large energy range, it
is very likely to find a suitable autoionization state, nonetheless, can be confirmed only by experiments. In a laser isotope separation process, the atomic parameters such as isotope
shifts, hyperfine structures of the constituent isotopes, radiative lifetimes of levels, branching ratios and ionization cross-section; laser parameters such as power, spectral bandwidth,
frequency, pulse width (or interaction time) and delay between the pulses; effusive atom source parameters such as atomic velocity distribution and angular divergence (collimation ratio),
all have a complex interplay on the ionization efficiency and selectivity of the photoionization process. The coherent laser–atom interactions can be accurately described by the density
matrix theory25. The density matrix equations relevant for the pulsed two-step excitation process have been published in our recent article8. When, the atoms traverse perpendicular to the
spatially overlapped co-propagating laser beams, for all CW lasers case, the laser-atom interaction time is determined by the atomic velocity and the laser beam diameter. While in case of CW
laser excitation followed by pulsed laser ionization, the laser-atom interaction time is determined by the temporal width of the pulsed laser. The coupled density matrix elements have been
integrated for the entire laser-atom interaction time determined as described above. The effect of linewidth of excitation lasers has been incorporated according to the phase diffusion model
as discussed in our recent article8. In an AVLIS process, when the first and second excitation lasers are tuned to the frequencies ν1 and ν2 respectively, isotope selectivity is defined as
$${\text{Isotope Selectivity S }}({\upnu }_{1},{\upnu }_{2})=\left(\frac{{\upeta }_{{176}_{\mathrm{Lu}}}}{{\upeta }_{{175}_{\mathrm{Lu}}}}\right)$$ (1) where, η is the ionization efficiency
of the isotope. When the abundance of the isotopes is not equal, one needs to normalize the selectivity with the abundance of the constituent isotopes; thus, isotope ratio enhancement factor
is defined as $${\text{Isotope Ratio Enhancement Factor IRE }}({\upnu }_{1},{\upnu }_{2})=\left(\frac{{\upeta }_{{176}_{\mathrm{Lu}}}}{{\upeta }_{{175}_{\mathrm{Lu}}}}\right)\times
\left(\frac{{\mathrm{f}}_{{176}_{\mathrm{Lu}}}}{{\mathrm{f}}_{{175}_{\mathrm{Lu}}}}\right)$$ (2) where, f is the fractional abundance of the isotope. Degree of enrichment is calculated using
the expression $${\text{Degree of enrichment }}(\mathrm{\%}) =\left\{\frac{{\upeta }_{{176}_{\mathrm{Lu}}}{\times \mathrm{f}}_{{176}_{\mathrm{Lu}}}}{\left({\upeta
}_{{175}_{\mathrm{Lu}}}{\times \mathrm{f}}_{{175}_{\mathrm{Lu}}}+{\upeta }_{{176}_{\mathrm{Lu}}}{\times \mathrm{f}}_{{176}_{\mathrm{Lu}}}\right)}\right\}\times 100$$ (3) RESULTS AND
DISCUSSION For a source temperature of 1600 °C, the most probable velocity of 176Lu is 421.8 m/s. At this temperature the Doppler broadening of the 465.9316 nm transition is ~ 1500 MHz. Let
us consider that the atom source to have a length-to-diameter (L/D) ratio of 5 corresponding to the full-angle divergence (θ) of 22.6°. For the orthogonal laser-atom interaction, Doppler
broadening along the laser propagation axis reduces to ~ 565 MHz. Let us also consider that the laser atom interaction region26 to have dimensions of 10 mm diameter and 270 mm long. First,
we look at the possibility of using cw lasers for excitation and ionization of 176Lu isotope. EXCITATION AND IONIZATION USING CW LASERS As mentioned earlier, the primary aim of the present
work is to investigate the enrichment of 176Lu through off-the-shelf lasers. A detailed survey has been carried out on the availability of laser systems in the wavelength ranges indicated in
Table 1. Blue diode laser systems within the wavelength range of the first excitation transition are available with a typical cw power of 30 mW; while diode lasers with accessible
wavelengths of the second excitation transition have a typical power of 120 mW. Therefore, we set the limit to the powers of first and second excitation lasers as 30 mW and 120 mW
corresponding to the power densities of 38.2 mW/cm2 and 153 mW/cm2respectively. The saturation intensities of the transitions 465.9316 nm and 1179.87 nm are 13 mW/cm2 and 3 mW/cm2
respectively which can be easily achieved using the available cw lasers. The frequency of the excitation lasers can be controlled to ~ 1 MHz using the commercially available wavelength
meters. Non-resonance ionization can be carried out using the high power 10 W cw lasers which are available in wavelengths of 532 nm (Nd:YAG laser) or 527 nm laser (Nd:YVO4 laser).
Therefore, we set the limit to the power of ionization laser to 10 W and the corresponding power density is 12.73 W/cm2. Calculation of ionization efficiencies of Lu isotopes has been
carried out varying the frequencies of the first and second excitation lasers around the resonance of Lu isotopes and the results are plotted as two-dimensional contour plots in Fig. 2. The
contours showed resonances in agreement with expected positions (Table 3). Diagonal ridges corresponding to the coherent two photoionization have been observed even at longer detunings due
to the strong first and second excitation transitions. The resonance frequency position of the most intense hyperfine excitation pathway 17/2–19/2–17/2 (15,150 MHz, -25,160 MHz) of 176Lu
lies far away from the most intense hyperfine excitation pathway 5–6–5 (11,100 MHz, −17,720 MHz) of 175Lu. Since the most probable velocity of Lu atoms at 1600 °C is 421.8 m/s, for the cw
laser beam diameter of 1 cm, the interaction time of atoms with spatially overlapped laser beams is 23.7 µs. The cw lasers typically operate in the fundamental mode TEM00 having a Gaussian
intensity distribution across the diameter; nevertheless it is possible to convert them into a flat-top intensity distribution using beam shaping optics. For the present work, we have
considered lasers to have flat-top intensity distribution across its diameter. First, evolution of level populations with interaction time (Fig. 3A,B) have been calculated setting the
excitation lasers to the frequencies corresponding to the hyperfine excitation pathway of 17/2–19/2–17/2 (15,150 MHz, -25,160 MHz) of 176Lu and setting the power of the ionization laser to
zero. As expected in the case of non-resonant 175Lu isotope (Fig. 3A), a very small fraction of atoms which are excited into the upper levels decay into the trapped states. At the end of the
interaction, about 0.56% atoms can be found in the 1993.92 cm−1 trapped level and rest of the atoms in the ground state 0.00 cm−1. On the other hand, for the case of resonant 176Lu isotope
(Fig. 3B), the atoms from the ground F = 17/2 hyperfine level are excited through the 17/2–19/2–17/2 hyperfine excitation pathway. Since the ionization laser power is set to zero, the atoms
excited into the upper hyperfine levels decay to the 1993.92 cm−1, 4136.13 cm−1 and 7476.39 cm−1 trapped levels. Due to the repetitive excitation and decay processes, at the end of the
interaction, the entire population of F = 17/2 (30%) is transferred to the trapped levels mentioned above. It is therefore expected that the introduction of cw ionization laser transfers
atoms from the excited levels into the ionization continuum. The dynamics of the photoionization process with the introduction of a cw ionization laser is discussed below. When a cw
ionization laser with a power density of 12.73 W/cm2 along with the cw excitation lasers interacts with the atomic beam; the ionization efficiency of the non-resonant 175Lu isotope (Fig. 3C)
reaches to the value of 2.3 × 10–10 and the ionization efficiency of resonant176Lu isotope (Fig. 3D) reaches to 2.5 × 10–5 implying that most of the population excited into the higher
levels decays to the trapped states. The low ionization efficiency of the resonant 176Lu isotope can be attributed to multiple factors such as low power density of the cw ionization laser
and low ionization cross-section (1 × 10–16 cm2) of the non-resonant ionization process. From the Eqs. (1)–(3), one can calculate the degree of enrichment, which is ~ 100%. Considering the
Lu number density of 1 × 1013 atoms/cm3 and the dimensions7,26 of the laser-atom interaction region of 10 mm diameter and 270 mm length, the production rate of enriched 176Lu is calculated
to be 4.9 µg/h. In comparison to the value of 3.7 mg/h reported by D’yachkov et al.7, the production rate in the present case is three orders lower. Therefore, enrichment of 176Lu through
“_all_ cw” laser process is very inefficient. Hence, we have adopted a combination of cw lasers for the excitation process and pulsed laser for the ionization process which is discussed in
the next section. CW LASER EXCITATION AND PULSED LASER IONIZATION As discussed in the previous section, the low ionization efficiency of 176Lu under cw laser excitation and ionization is due
to the low non-resonant ionization cross-section and low peak power of the cw ionization laser. This can be overcome by employing the tunable high repetition rate pulsed laser for the
ionization. Theoretical modelling of cw laser excitation followed by pulsed laser ionization for the isotope selective photoionization of Sr has been studied previously27. Currently high
repetition rate (up to 10 kHz) Ti:Sapphire lasers are available with the average power of 1 W. The pulse duration of these lasers is 50 ns. These lasers are also having a wavelength
tunability between 700 and 1000 nm. The lasers can produce peak power density of 2500 W/cm2. Wavelength tunability of these lasers allows atoms to be excited either into high lying Rydberg
states followed by electric field ionization or directly into the autoionization levels. Cross-section28 for such a process can be in the range of 1 × 10–14 cm2 which is two-orders higher
than the cross-section for the non-resonant ionization process. As a result of high peak power density and the high resonance ionization cross-section, ionization efficiency is expected to
be enhanced significantly. If one carefully observes the time evolution of level populations of the first (21,462.38 cm−1) and the second (29,937.9 cm−1) excited states of 176Lu under cw
laser excitation (Fig. 3A,B); one can find that the atomic population from these states starts decaying into the trapped states after ~ 20 ns of its interaction with the excitation lasers.
Therefore, for efficient ionization, it is important for the pulsed ionization laser to interact with the _“nascent”_ atomic beam within 50 ns of its interaction with the cw lasers.
Experimentally, this can be achieved by passing the diode lasers through mechanical choppers and synchronising the pulsed ionization laser with the sync signal of the mechanical chopper
(Supplementary material Fig. S1). Alternatively, it is also possible to use high-frequency intensity modulation of narrow-linewidth laser light using fast-switching electro-optical
modulators29. Evolution of level populations have been calculated for the Lu isotopes by setting the pulsed ionization laser peak power density to 2500 W/cm2 with a pulse width of 50 ns. The
results are plotted in Fig. 4. It can be seen from the Fig. 4B that, in this case the ionization efficiency of 176Lu isotope reaches a value of 4.7 × 10–2, as compared to the 175Lu
ionization efficiency of 5.7 × 10–9 (Fig. 4A) corresponding to the degree of enrichment of ~ 100% of the 176Lu isotope. Considering the atom number density to be 1 × 1013 atoms/cm3 and the
laser-atom interaction region having dimensions of 10 mm diameter and 270 mm length, the production rate of enriched 176Lu is calculated to be 2.7 mg/h. This is comparable to the value of
3.7 mg/h reported by D’yachokov et al.7. However, in our case the degree of enrichment is significantly higher (100%) than that has been previously reported (68.4%) value. In the _“nascent”_
atomic beam, the population of the ground state 0.00 cm−1 is distributed among the four hyperfine components F = 11/2 to F = 17/2 based on the 2F + 1 rule. Therefore, the F = 17/2 hyperfine
level possesses 0.30 (30%) of the population of which 5 × 10–2 is ionized. This implies ionization efficiency of 17% of the targeted F = 17/2 hyperfine level population. The ionization
efficiency can be further increased by increasing the power of the ionization laser; however, it is not practically viable as tunable high repetition rate lasers with average power > 1 W
are not commercially available. Another reason for the low ionization efficiency is due to the large Doppler broadening of the atomic ensemble. As mentioned earlier, for self-collimating
atomic beam effusing out of the atom source having L/D ratio of 5 (full angle divergence of 22.6°), the Doppler broadening along the laser propagation axis will be 565 MHz for the 465.9316
nm transition. The diode laser which can be considered as the single frequency system during short interaction times (50 ns), causes a _“hole burning”_ in the velocity groups of the Doppler
broadened atomic ensemble. The group of atoms having velocities nearly perpendicular to the laser propagation axis induce a very small proportion of velocity induced Doppler shifts. When the
excitation laser is tuned to the resonance of a transition, this group of atoms of the resonant isotope are excited and ionized. The remaining atoms experiencing the velocity induced
Doppler shifts are less likely to get excited and ionized. This causes low ionization efficiency. One of the ways to overcome such a problem is by increasing the linewidth of the excitation
lasers; however, this is associated with the loss in selectivity. Therefore, we have adopted an alternative approach. Angular divergence of the atomic beam can be reduced by increasing the
L/D ratio of the atom source which causes reduction in the angular divergence and hence velocity induced Doppler shifts. Using this approach, most of the atoms in the atomic beam can be
brought into the resonance by adjusting the L/D ratio of the atom source. Calculation of ionization efficiency and degree of enrichment has been carried out varying the L/D ratio of the atom
source. It has been found that for a L/D ratio of 10 corresponding to the full angular divergence of 11.5° enhances the ionization efficiency to 8.8 × 10–2 while the degree of enrichment
remains at ~ 100%. Such source geometry allows production of 176Lu at a rate of 5 mg/h. EFFECT OF DELAY OF IONIZATION LASER Effect of delay and delay jitter of the ionization laser pulse
with reference to the interaction time has been studied (Supplementary material Fig. S2). Ionization efficiency and the degree of enrichment have been calculated varying delay of the
ionization laser with reference to the start time of interaction of cw excitation lasers with _“nascent”_ atomic beam. It has been observed that a delay of 50 ns results in highest
ionization efficiency. Therefore, it is necessary to set the delay of the ionization laser to 50 ± 10 ns to achieve the ionization efficiency within 90% of its value. This requirement can
be easily met with the currently available technological advances in the synchronization electronics. CONCLUSION We have proposed a novel and simple method for the enrichment of 176Lu
isotope a precursor to 177Lu medical isotope. The physics of the laser-atom interaction has been studied through the dynamics of the atomic level populations using density matrix formalism.
It has been shown that a combination of cw excitation lasers and pulsed ionization laser can be used for the laser isotope separation of 176Lu. The optimum conditions for the efficient and
selective separation of 176Lu have been derived by studying the time evolution of level populations during laser excitation. It has been shown that, it might be possible to produce ~ 100%
enriched 176Lu isotope at a rate of 5 mg/h, which is higher than all previously reported methods so far. The enrichment process proposed can be easily adopted using off-the-shelf lasers for
similar atomic systems. METHOD The isotope shift and hyperfine structure data of natural Lu isotopes have been taken from the literature and the frequency positions of all hyperfine pathways
have been derived with reference to centre of gravity of 176Lu. A density matrix code for three-step photoionization has been developed. Separate codes have been used for _all cw lasers
case;_ cw laser excitation followed by pulsed laser ionization case. Ionization efficiencies of natural Lu isotopes have been calculated for various conditions. From the ionization
efficiencies, degree of enrichment of 176Lu has been derived. DATA AVAILABILITY All the relevant data is provided with this paper. Any additional data relevant to the work in this paper are
available upon reasonable request to the corresponding author. CODE AVAILABILITY The code developed is not available for other users. REFERENCES * Cohen, K. & Murphy, G. M. (eds) _The
Theory of Isotope Separation as Applied to the Large Scale Production of U235_ (Mcgraw-Hill Book Company Inc., 1951). Google Scholar * Eerkens, J.W., Kunze, J.F., & Bond, L.J.. Laser
isotope enrichment for medical and industrial applications. in _Proceedings of ICONE14 International Conference on Nuclear Engineering, July 17–20, 2006, Miami, USA_. * de Jong, M. _et al._
Somatostatin receptor-targeted radionuclide therapy of tumors: Preclinical and clinical findings. _Semin. Nuclear Med._ 32, 133–140 (2002). Article Google Scholar * Dash, A., Raghavan, M.,
Pillai, A. & Knapp Jr, F. F. Production of 177Lu for targeted radionuclide therapy: Available options. _Nucl. Med. Mol. Imaging_ 49, 85–107 (2015). Article CAS Google Scholar *
Dvorakova, Z. Production and chemical processing of 177Lu for nuclear medicine at the Munich research reactor FRM-II. Doctoral Thesis, Technische Universiat Munchen (2007). * Georg, U. _et
al._ Laser spectroscopy investigation of the nuclear moments and radii of lutetium isotopes. _Eur. Phys. J. A_ 3, 225–235 (1998). Article ADS CAS Google Scholar * D’yachkov, A. B. _et
al._ Selective photoionisation of lutetium isotopes. _Quantum Electron._ 42, 953–956 (2012). Article Google Scholar * Suryanarayana, M. V. Isotope selective three-step photoionization of
176Lu. _J. Opt. Soc. Am._ 38B, 353–370 (2021). Article ADS Google Scholar * Suryanarayana, M. V. Isotope separation of 176Lu a precursor to 177Lu medical isotope using broadband lasers.
_Sci. Rep._ 11, 6118 (2021). Article ADS CAS Google Scholar * Kramida, A., Ralchenko, Yu., Reader, J. &NIST ASD Team (2020). _NIST Atomic Spectra Database (version 5.8) (online)._
https://physics.nist.gov/asd. https://doi.org/10.18434/T4W30F (National Institute of Standards and Technology, 2021). Accessed 12 Jul 2021. * Atomic spectral line database from CD-ROM 23 of
R. L. Kurucz. https://www.cfa.harvard.edu/amp/ampdata/kurucz23/sekur.html. * Brenner, T., Buttgenbach, S., Rupprecht, W. & Traber, F. Nuclear moments of the low abundant natural isotope
176Lu and hyperfine anomalies in the lutetium isotopes. _Nucl. Phys. A_ 440, 407–423 (1985). Article ADS CAS Google Scholar * Vergès, J. & Wyart, J.-F. Infrared emission spectrum of
lutecium and extended analysis of Lu I. _Phys. Scr._ 17, 495 (1978). Article ADS Google Scholar * Bekov, G. I. & Vidolova-Angelova, E. P. Optimal scheme for multistage photoionization
of lutetium atoms by laser radiation. _Sov. J. Quantum Electron._ 11(1), 137–139 (1981). Article ADS Google Scholar * Gadelshin, V. _et al._ Laser resonance ionization spectroscopy on
lutetium for the MEDICIS project. _Hyperfine Interact_ 238, 28 (2017). Article ADS Google Scholar * Gadelshin, V. M. _et al._ Measurement of the laser resonance ionization efficiency for
lutetium. _Radiochim. Acta_ 107(7), 653–661 (2019). Article CAS Google Scholar * Olivares, I. E., Duarte, A. E., Saravia, E. A. & Duarte, F. J. Lithium isotope separation with tunable
diode lasers. _Appl. Opt._ 41, 2973–2977 (2002). Article ADS CAS Google Scholar * Matsuoka, K. _et al._ The laser Isotope separation (LIS) methods for the enrichment of 48Ca. _J. Phys.
Conf. Ser._ 1468, 012199 (2020). Article CAS Google Scholar * Bekov, G. I. & Vidolova-Angelova, E. P. Optimal scheme for multistage photoionization of lutetium atoms by laser
radiation. _Sov. J. Quantum Electron._ 11, 137 (1981). Article ADS Google Scholar * Xu, C. B. _et al._ The study of autoionizing states of lutetium atoms by resonance ionization
spectroscopy. _J. Phys. B At. Mol. Opt. Phys._ 26, 2821–2835 (1993). Article ADS Google Scholar * Kujirai, O. & Ogawa, Y. Observation of even-parity autoionization states of lutetium
atom by optogalvanic spectroscopy. _J. Phys. Soc. Jpn._ 67, 1056–1057 (1998). Article ADS CAS Google Scholar * Ogawa, Y. & Kujirai, O. Study of even-parity autoionization states of
lutetium atom by atomic beam laser resonance ionization spectroscopy. _J. Phys. Soc. Japan._ 68, 428–433 (1999). Article ADS CAS Google Scholar * Li, R. _et al._ Even-parity Rydberg and
autoionizing states of lutetium by laser resonance-ionization spectroscopy. _Phys. Rev. A_ 95, 052501 (2017). Article ADS Google Scholar * Rath, A. D., Biswal, D. & Kundu, S. Odd
parity autoionization levels of Lu I by three-step photoionization. _J. Quant. Spectrosc. Radiat. Transfer_ 270, 107696 (2021). Article CAS Google Scholar * Shore, B. W. _The Theory of
Coherent Atomic Excitation: Simple Atoms and Fields_ Vol. 1 (Wiley, 1990). Google Scholar * Babichev, A. P. _et al._ Development of the laser isotope separation method (AVLIS) for obtaining
weight amounts of highly enriched 150Nd isotope. _Quantum Electron._ 35, 879–890 (2005). Article ADS CAS Google Scholar * Jones, O. R., Perks, R. M., Telle, H. H. & Morgan, T. G.
Isotope-specific resonance ionization mass spectrometry using continuous-wave diode and pulsed dye lasers for trace detection and enrichment of elements of environmental and medical
importance, exemplified for strontium (Sr)-Part I: Theoretical modelling. _Rapid Commun. Mass Spectrom._ 10, 1725–1738 (1996). Article ADS CAS Google Scholar * D’yachkov, A. B. _et al._
A study of the kinetic parameters of Lu laser photoionization scheme. _Opt. Spectrosc._ 128, 289–296 (2020). Article ADS Google Scholar * Voss, A. _et al._ First use of high-frequency
intensity modulation of narrow-linewidth laser light and its application in determination of 206,205,204Fr ground-state properties. _Phys. Rev. Lett._ 111, 122501 (2013). Article ADS
Google Scholar Download references ACKNOWLEDGEMENTS Authors acknowledge the support of Computational Analysis Division, Bhabha Atomic Research Centre, Visakhapatnam for providing the Super
Computer Facility for this work. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Bhabha Atomic Research Centre, Visakhapatnam, Andhra Pradesh, 530 011, India M. V. Suryanarayana & M.
Sankari Authors * M. V. Suryanarayana View author publications You can also search for this author inPubMed Google Scholar * M. Sankari View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS M.V.S. conceived the idea and developed the requisite codes. Both the authors contributed towards computations, data interpretation and
manuscript preparation. CORRESPONDING AUTHOR Correspondence to M. V. Suryanarayana. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES.
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Suryanarayana, M.V., Sankari, M. Laser isotope separation of 176Lu through off-the-shelf lasers. _Sci Rep_ 11, 18292 (2021). https://doi.org/10.1038/s41598-021-97773-8 Download
citation * Received: 11 March 2021 * Accepted: 31 August 2021 * Published: 14 September 2021 * DOI: https://doi.org/10.1038/s41598-021-97773-8 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
