Structural, optical, and cytotoxicity studies of laser irradiated zno doped borate bioactive glasses
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Borate glasses (BG) doped with different amounts of ZnO (0–0.6 mol%) were formed by the traditional melt quenching technique. The different glasses so made were characterized using
different characterization techniques such as X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), and UV–Vis absorption optical
properties. The XRD patterns showed an amorphous structure with one broad peak at 2_θ_ = 29°, while the phonons bands were studied in terms of the FTIR bands. Optical properties of the
glasses were studied using UV–Vis absorption spectra in the range 190–1100 nm, in which the prominent band lies at about 261.5 nm of peak position, from which the bandgab (Eg) was calculated
from its edge using Tauc’s plot, with Eg ~ 3.5 eV. The laser irradiation showed no significant changes in the absorption bands, despite a significant change observed in the amorphous
behavior in the XRD pattern. The cell viability was performed for two samples of the BG and 0.6 mol% ZnO doped using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)
assay method. The result showed better cell viability and low toxicity. So, ZnO doped BG can be used in various biomedical applications. SIMILAR CONTENT BEING VIEWED BY OTHERS THE
MANUFACTURE, OPTICAL PROPERTIES, AND MECHANICAL ASPECTS OF EUROPIUM-DOPED BORATE GLASSES Article Open access 02 October 2024 FABRICATION, STRUCTURAL, PHYSICAL, OPTICAL CHARACTERISTICS AND
Γ-RAY ATTENUATION CAPACITY OF BOROSILICATE GLASSES DOPED WITH GD2O3 Article Open access 23 December 2024 INVESTIGATION OF PHYSICAL, STRUCTURAL, OPTICAL, AND LUMINESCENCE PROPERTIES OF NICKEL
OXIDE DOPED ALKALI ZINCO-BORATE GLASSES Article Open access 28 February 2025 INTRODUCTION In the last decades, the field of biomaterials has grown at an incredible rate, leading to the
development of bioactive materials, which may elicit specific and predictable responses from cells and tissues1. The discovery of Hench glass in 1969 constituted for the first time a
revolution in the history of biomaterials2,3. The mechanical, bioactive, and structural properties of the bioactive glasses are largely based on synthesis procedures, composition, particle
size, crystallization, etc. Bioactive glasses are made using several approaches. The most common method for making bioactive glasses is the traditional melt-quenching method, in which all
the components are well mixed in a ball mill before being melted at an elevated temperature4. In the melt-quenching process, a volatile part such as B2O3 gets evaporated out5,6,7. In
constructing a bioactive glass, understanding how the physicochemical structures of these materials influence their characteristics is critical, as it allows the material to be adapted for
particular applications. Each component influences the bioactive glass's performance. Calcium, for example, promotes osteoblast development and apatite layer precipitation8,9. Na2O.
K2O. MgO. CaO. P2O5-based glasses found to constitute a promising material for bioactive applications such as bone repair, tissue regeneration in the human body, etc.10,11,12,13. Borate
glasses quickly release significant amounts of boron, leading to a high concentration of local boron near the glass. As a result, compared to silicate glasses, the degradation and sintering
behavior of borate/borosilicate glass is more controllable14,15,16. In borate glasses, the boron oxide appears in BO3 and BO4 in a network structure that forms 'super structural'
units (pentaborate, boroxol ring, diborate, or tetraborate groups), depending on the composition and the kind of added glass modifiers17,18,19,20. The glass exhibits nonlinear change in its
physical properties when one alkali ion is replaced with another alkali content at a constant amount, resulting in a mixed alkali effect (MAE)21. Incorporation of zinc oxide in glass
structures is expected to acts as a intermediate oxide either as network former or as network modifier22,23. Glass composition is modified by introducing the ‘dopants’ to the glass network
to form the desired glass where it can be bioactive, bioresorbable, and/or biodegradable. Dopants like Cu, Zn, In, Ba, La, Y, Fe, Cr, and Sr as ions lead to trigger the properties. ZnO/MgO
additives have been shown to stimulate osteoblast proliferation, differentiation, and bone mineralization24,25,26,27. Zn is an essential trace element that is used by various metalloenzymes
for structure, catalysis, or regulatory functions. Zinc is involved in bone metabolism, enhancing osteoblastic bone formation and preventing osteoclastic bone resorption, raising bone
mass28. Nutritional zinc supplementation has been demonstrated to have preventive and therapeutic effects on bone loss induced by bone disorders29,30. Investigations have also demonstrated
that small quantities of Zn induced early cell proliferation and enhanced differentiation of in vitro biocompatibility studies31,32,33. Methodologies like MTT and MTS are used to assess the
viability and cytotoxicity of the glass samples because these methods generally measure cytotoxicity for bulk constructions indirectly by extracting the materials. According to Saranti et
al., boron oxide has a catalytic action that promotes bioactivity34. Neáková et al. developed mesoporous bioactive glass nanoparticles (MBGNs) based on the SiO2–CaO system using a
micro-emulsion supported sol–gel method. Zn2+ ions were doped into MBGNs with 8 Mol% ZnO concentration (Zn-MBGNs). The findings investigated that the addition of zinc precursors had no
effect on particle morphology, but enhanced their specific surface area when compared to MBGNs35. Lee et al. reported that bone implant and osteointegration utilizing a B2O3-based glass
technology represents no toxicity. Bone implant and osteointegration using B2O3 based glass system are reported by Lee et al. without any toxicity36. Kolavekar et al. studied the optical
properties of Pr2O3-doped multi-component borate glasses37, TeO2-doped lead borate glasses38, Li+ Ions doped zinc borate glass39 and Er3+ and Er3+/Yb3+ co-doped heavy metal borate glasses40
and showed the effect of the dopants on the photophysical properties of the borate glass. Bioactive glasses are effective biomaterials for promoting angiogenesis in both hard and soft tissue
engineering applications. Metallic ions like Cu2+, Ag2+, Mg2+, Zn2+, Fe3+, Sr2+, and Co2+ have been utilized as dopants in oxide glasses41,42,43. The presence of these ions in the glass
network causes antibacterial agents, osteogenesis motivation factors, and angiogenesis enhancers44,45. The effect of laser irradiation was studied for borosilicate glasses attracted much of
scientisits attention because it can stimulate various microspheres that can be controlled within transparent materials due to non-linear optical absorption46,47. The novelty in the work is
to study the effect of laser irradiation on the structure and optical properties of ZnO doped borate bioactive glass. This study is aimed to investigate the effect of ZnO on the structure of
bioactive borate glass using X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). The effects of laser irradiation on the selected glasses are studied in terms of
their UV–Vis absorption spectra and XRD pattern. The cytotoxicity of ZnO-doped bioactive borate glass on cell viability is assessed in a contest to bioapplications. EXPERIMENTAL WORK
MATERIALS (GLASS PREPARATION) The glasses of compositions 6Na2O + 12K2O. + 5MgO + 20CaO + 4P2O5 + (53-x) B2O3 + xZnO (0 ≤ x ≤ 0.6 mol%), were prepared via traditional melt quenching method.
Highly pure chemicals of orthoboric acid (a source for B2O3) and ammonium dihydrogen phosphate (a source for P2O5) supplied by Sigma Aldrich Co were used. Na2O, K2O, MgO, and CaO were added
as carbonates provided by El Nasr Pharmaceuticals. All the chemicals were used as received. The chosen glass batches were mixed and melted in an electrical furnace at 1100–1150 °C and
swirled to assure homogeneity, and then the melts were quenched and pressed between two steel plates at room temperature to obtain the glasses. The so-obtained glasses were characterized
using XRD patterns, FTIR, and UV–visible absorption spectra. Images of the formed glasses are listed in the Table 1. CHARACTERIZATION AND ANALYSIS TECHNIQUES The various methods of analysis
and typical settings that describe ZnO doped borate glass are recorded by utilizing the following instruments: (a) the X-ray diffraction pattern (XRD) was recorded in the range of 4° ≤ 2θ ≤
70° using a Rigaku X-ray diffractometer ultima IV with CuKα radiation of wavelength λ = 0.154600 nm and steps of 0.02°. (b) Microstructure of the samples was examined using a scanning
electron microscope (JEOL JSM-6510LV, USA), which used a focused electron beam (operating at 20 kV accelerating voltage) with a magnification up to 40,000 X, where the samples were coated
with gold so the surface becomes conducting to measure the images. (c) A FTIR spectrometer (type Nicolet i10, Thermo Fisher Co.) was used to record FTIR spectra of the glasses over a
4000–400 cm−1 range, with a 2 cm−1 step resolution. Measurements were performed on powders dispersed in KBr in a 1:100 ratio in the form of thin pellets. (d) UV/Visible absorption spectra
were recorded (in a range of 190 to 1100 nm) for the polished `samples, using a double beam (JASCO V570 UV/Vis./NIR) spectrophotometer, with air as a reference sample. All the glass samples
were irradiated for 30 min by a laser beam (λ = 375 nm) with a 150 mV power. The technique of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay technique was used
to assess cytotoxicity and proliferation was purchased from (Serva, Germany). Cells were seeded in well plates and cultured for 48 h to determine the IC50 for the different glasses and
compared with the control sample (normal cell without glass). The glass was solubilized in dimethyl sulfoxide (DMSO) stoke before treatment. The reduction in cell growth was measured at (570
nm) (BioTek, Elx800, US) and the results were calculated as a percentage of control. Prism software was used to calculate the IC50 of glass concentrations as well as cell viability. RESULTS
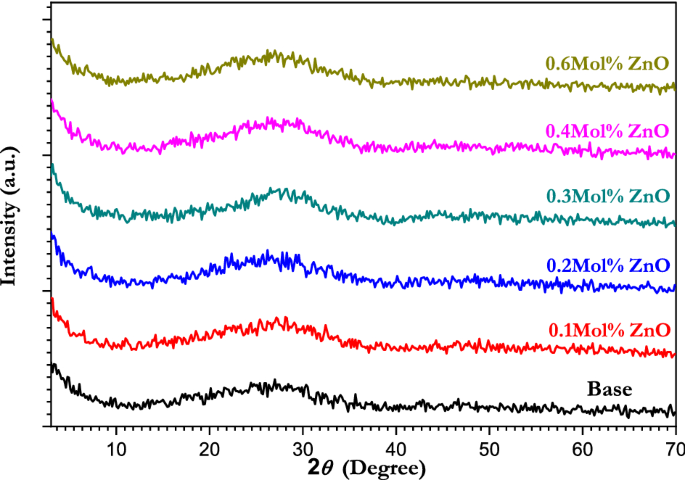
AND DISCUSSION XRD PATTERNS AND MICROSTRUCTURE Figure 1 presents XRD patterns of the ZnO doped borate glass, which reveal an amorphous structure for all the samples with a broad diffraction
peak at 2_θ_ = 29° of diffraction angle ‘2_θ’,_ with no sharp peak in absence of any crystallites. The absence of clearly defined diffraction peaks confirms the samples' glassy nature
and rules out the possibility of long-range atomic organization48,49. The absence of Bragg's peak and the amorphous glassy nature of all the glass samples were confirmed by X-ray
diffraction patterns due to the presence of potassium and magnesium, in particular, which enhanced glass processability, making it easier to make glass without crystallization and use it in
coatings, fibers, scaffolding and other applications50. Figure 2a, b represent SEM of borate glass doped with 0.6 mol% ZnO of lower and higher magnification which showed dispersed particles
or grain-like structure appeared in the morphology of 0.6Mol% ZnO doped borate glass. SEM images indicate the domination of the amorphous structure of the borate matrix as a continuous
phase. A good agreement with the suggested amorphous structure from the XRD pattern was demonstrated from the SEM images. Figure 3 shows FTIR spectra of the ZnO doped borate glass where
there were broad absorption bands that indicate the groups inside the network system. The interpretation of IR of borate glass is summarized as follows: The first broad band around 1396 cm−1
indicated for B–O asymmetric stretching vibration band of trigonal BO3. The second band at 1008 cm−1 pointed to the B–O bond stretching of the tetrahedral BO4. The third band positioned
nearly at 714 cm−1 refers to the bending of B–O–B in trigonal BO3 units and the last band at 563 cm−1 points to the vibration of metal cations like ZnO. The bands face no changes when ZnO
nanoparticles are added to the composition. The strong appearance of vibrational broad bands of triangular and tetrahedral for borate glass owing to the presence of the two alkali metals
Na2O and K2O. Also, many little changes were investigated after the minor addition of ZnO nanoparticles which indicated the effect of the concentration of ZnO nanoparticles on the
transformation process. The band appears in the far infrared region 425 cm−1 due to the stretching vibration of transition metal ions such as (Zn2+, and Mn2+). The other main vibrational
modes mentioned before are due to the borate glass matrix51. PeakFit4.12 computer program was used for the mathematical deconvoluted analysis technique (DAT) that was used to examine and
analyze the collected FTIR spectral data of ZnO-doped borate glasses to obtain quantitative information about the internal changes inside the glass matrix. In the range of 1750–500 cm−1, the
smeared overlapping bands of triangular and tetrahedral borate groups were resolved, while the region of 3200–3600 cm−1 reveals the vibrational modes of the OH group. The deconvoluted
analysis was established using a small number of bands to resolve the spectra and then weaker bands were inserted to improve the fit. Figure 4a–e represents the deconvoluted analysis of
ZnO-doped borate glasses and their residuals. The deconvolution process is based on the previous knowledge of the wavenumber of suggested vibrational groups and the second derivative of the
spectrum that identifies the accurate position of peak maxima as previously described by different authors52,53,54,55,56. In such cases, the differences between the deconvoluted and measured
spectrum can be minimized and plotted as shown in the residual curves. Obtained N4 data of coordinated boron (N4 = BO4/(BO4 + BO3)) for ZnO doped borate glasses is represented in Fig. 5,
which indicates boron atoms transformations occur inside the glass matrix after ZnO nanoparticles addition. It can be figured that the value of N4 increased by increasing the concentration
of ZnO nanoparticles to 0.2 mol% and then faces two stages of decreasing and increasing. The creation of non-bridging oxygen NBOs can be correlated with the decrease in the N4 ratio with
increasing in ZnO nanoparticles content in addition to other parameters. The negative charge on NBOs makes it easier for electrons to be excited at higher wavelengths57. Both ZnO and CaO act
to reduce the BO4/(BO4:BO3) ratio and enhanced the glass network by increasing the number of non-bridging oxygen atoms58. In the glass structure, ZnO acts as a intermediate oxide either as
network former or as network modifier59, where ZnO nanoparticles is a promising candidate as a modifier with a large band gap, allowing it to be used as a potential optical material48,60. As
there was an unnoticeable change ZnO nanoparticles act as a modifier not as a former. ELECTRONIC ABSORPTION SPECTRA Ultraviolet–visible absorption spectroscopy represents the optical
properties that give information about the electronic structure of the material61,62. UV–Vis. absorption spectra of ZnO doped borate glasses were explained in Fig. 6 in the wavelength range
of 190–1100 nm. It can be figured that the absorption of UV–Vis arises from the higher wavelength to the lower wavelength of the glass materials. A high absorption band at the wavelength of
261.75 nm in the UV region of the spectrum was figured out for all doped glasses, which results from the unavoidable trace iron impurities in the raw materials during the glass formation63.
While the broad absorption band between 200 and 340 nm is due to high valence or tetrahedral coordination of the transition metal ions in the alkali borate glass which agreed with the data
reported in64,65. The nonlinear behavior of the position of the edge at around 340 nm was found to be compatible with the N4 values represented in Fig. 5. The UV absorption of glasses is
considered to be influenced by both internal and external factors such as the electronic transitions, which are primarily caused by the addition of dopants and are influenced by the glass
structure and chemical bonding66. Also, the change in the position of the absorption edge is due to the variation of oxygen bonding in the glass network67. The addition of metal cations such
as Pb, Zn, Cd, and others affects the network formation of B2O3 and SiO4. These additives also act as a network modifier and a nucleating agent for glass crystallization. As a result, the
optical properties of borate glasses have changed significantly68. Using a diode laser with a wavelength of 375 nm and 150 mW power the ZnO-doped borate glasses were irradiated for 30 min at
room temperature. Figure 7a–f represents the absorption spectra for ZnO-doped borate glasses before and after the irradiation process. It can be figured that there was an absorption band in
the UV region and no visible bands were observed for ZnO-doped borate glasses before and after the irradiation process as can be seen in Fig. 7a–f, only a change in the absorption intensity
for the absorption band in the UV region and a small shift to the edge wavelength λedge were observed. The values of the λedge and direct optical energy gaps were calculated using Tauc
plots and the Mott-Davis model \({\left(\alpha h\upsilon \right)}^{2}=B\left(h\upsilon -{E}_{g}\right)\)69,70 are listed in Table 2. XRD was performed for o.1mol% ZnO doped sample and it’s
obvious that clearly defined diffraction peaks were absent confirming the samples' glassy nature and ruling out the possibility of long-range atomic organization48. Figure 8 showed that
before and after laser irradiation as there were two broad humps after laser irradiation around 29° and 46° were detected such humps distinguish non-crystalline solids (amorphous solids).
Therefore, it can be stated that all studied samples are short-range order solids (glass solids). CYTOTOXICITY ASSAY It is necessary to evaluate the cytotoxicity of the material used in
bio-applications. The culture of normal human skin fibroblasts (HSF) was used and investigated the influence of the glass with different concentrations on these cells. Glass efficiency and
potency in cells exposed to a drug (glass) are commonly evaluated using drug (glass) dose–response assays (e.g. MTT assay), and then the IC50 (half maximum inhibitory concentration) is
estimated. With cell-based cytotoxicity studies, the 50 percent inhibitory concentration (IC50) is commonly employed to measure drug potency71. Zinc ions have also been linked to a variety
of physiological activities, such as cell proliferation72. The result of cell viability was determined for the undoped and 0.6 mol% ZnO doped borate glasses Table 3. From the data in the
table starting from 50 to 1.5625 μM of the glass material where a low concentration of each glass (1.5625 μM) gives a high percentage of viable cells, and also, they are non-toxic. After
increasing the concentration of the glass material, the values of cell viability are still good compared to cell viability values in73,74. Figure 9a,b represent the dose–response curves
cytotoxicity assay of the undoped and 0.6 mol% ZnO doped borate glasses with different concentrations incubated in cell culture and showed the half-maximal inhibitory concentration (IC50).
The standard error of the mean is represented by the error bars. Prasad S75, investigated in vitro cell proliferation using the MTT test on the base glass (BG0B) selected from the
SiO2–Na2O–CaO–P2O5 system (S53P4 glass), as well as various modified glass compositions generated by replacing SiO2 in the base glass composition by B2O3 (BG1B, BG2B, and BG3B). It
demonstrates that the cell proliferation was better on the B2O3-modified glasses (BG1B, BG2B, and BG3B) compared to the base glass (BG0B). It’s founded that cell growth was better on the
B2O3-modified glasses (BG1B, BG2B, and BG3B) when compared to the base glass (BG0B). According to Balasubramanian et al.76 adding boron to bioactive glasses in various quantities has
substantial effects on glass structure, glass processing characteristics, biodegradability, biocompatibility, bioactivity, and cytotoxicity. For bone and soft tissue engineering, various
compositions of boron-doped, borosilicate, and borate glasses, are being studied. After cytotoxicity tests, the optical microscope image of the cells was taken (Fig. 10a–c). The morphology
of cells that extend over the dish surface was not changed in the samples that showed low cytotoxicity as compared to the control sample. The morphology of the cells was approximately
similar compared to the control sample, where the pulp cells indicated the survival cells and the rounded or shrunk cells indicated dead cells. It’s concluded that non-toxic behavior is
exhibited by the prepared glasses compared to the control sample cells. Also, it is appropriate to be used for human tissue with no harmful effects. CONCLUSIONS In this study, ZnO doped
borate glasses with a composition of 6Na2O + 12K2O. + 5MgO + 20CaO + 4P2O5 + (53-x) B2O3 + xZnO, (0 ≤ x ≤ 0.6 mol%) were synthesized by traditional melt quenching technique. XRD study showed
a high degree of amorphous structure for all samples. The formation of borate glass and the interaction with ZnO nanoparticles were indicated successfully by FTIR spectroscopy.
Deconvolution analyses were applied to analyze the collected FTIR spectral data and showed a slight change in N4 coordinated boron but wasn’t noticeable due to the minor addition of zinc
oxide. The incorporation of TMI was found to produce BO3 and BO4 structural units by shattering the boroxol (B3O6) ring, according to Fourier transform infrared (FTIR) spectra. UV–Vis
optical properties were applied, and the optical energy gap was found to be around 3.4 eV. A highly intense band in the UV region in the range between 200 and 270 nm was noticed and found to
be due to unavoidable trace elements introduced by raw materials. After the laser irradiation process, the optical energy gap was nearly similar for all samples but there was a change in
the absorption intensity. The XRD pattern showed a change in the structure after the irradiation process, which indicate the short-range order of the investigated glass. Good cell viability
was found for 0.6 mol% ZnO doped borate glass compared to the undoped glass after using the MTT assay method, the value of IC50 was decreased from 411.9 μM for borate glass to 126.4 μM for
0.6 mol% ZnO doped borate glass. As a result of that, and after future study on biodegradation and activity, ZnO-doped borate glasses with nominal composition is recommended for in vitro and
in vivo bio applications. DATA AVAILABILITY No data was used for the research described in the article. The data presented in this study are available in the article. REFERENCES * Marchi,
J. _Biocompatible Glasses: From Bone Regeneration to Cancer Treatment_ (Springer, 2016). Book Google Scholar * Hench, L. L. The story of bioglass®. _J. Mater. Sci. Mater. Med._ 17, 967–978
(2006). Article CAS PubMed Google Scholar * Lung, C. Y. _et al._ A multi-element-doped porous bioactive glass coating for implant applications. _Materials_ 14, 961 (2021). Article ADS
CAS PubMed PubMed Central Google Scholar * Ram, S., Chakravorty, D. & Bahadur, D. Effect of nucleating agents on the crystallisation behaviour of barium hexaferrite in a borate
glass. _J. Magn. Magn. Mater._ 62, 221–232 (1986). Article ADS CAS Google Scholar * Kaur, G. _et al._ Synthesis, cytotoxicity and hydroxyapatite formation in 27-Tris-SBF for sol-gel
based CaO-P2O5-SiO2-B2O3-ZnO bioactive glasses. _Sci. Rep._ 4, 1–14 (2014). Article CAS Google Scholar * Mehrabi, T. & Mesgar, A. S. In vitro biomineralization potential in simulated
wound fluid and antibacterial efficacy of biologically-active glass nanoparticles containing B2O3/ZnO. _Colloids Surf., B_ 212, 112338 (2022). Article CAS Google Scholar * Elmowafy, B.
M., Abdelghany, A., Ramadan, R. M., Ghazy, R. & Meaz, T. Synthesis, structural characterization, and antibacterial studies of new borate 13–93B3 bioglasses with low copper dopant.
_Egypt. J. Chem._ 65, 1–2 (2022). Google Scholar * Maeno, S. _et al._ The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D
culture. _Biomaterials_ 26, 4847–4855 (2005). Article CAS PubMed Google Scholar * Wang, C., Meng, C., Zhang, Z. & Zhu, Q. 3D printing of polycaprolactone/bioactive glass composite
scaffolds for in situ bone repair. _Ceram. Int._ 48, 7491–7499 (2022). Article CAS Google Scholar * Kokubo, T. & Takadama, H. How useful is SBF in predicting in vivo bone
bioactivity?. _Biomaterials_ 27, 2907–2915 (2006). Article CAS PubMed Google Scholar * Zhu, H. _et al._ 3D bioprinting of multifunctional dynamic nanocomposite bioinks incorporating
Cu-doped mesoporous bioactive glass nanoparticles for bone tissue engineering. _Small_ 18, 2104996 (2022). Article CAS Google Scholar * Al-Harbi, N. _et al._ Silica-based bioactive
glasses and their applications in hard tissue regeneration: A review. _Pharmaceuticals_ 14, 75 (2021). Article CAS PubMed PubMed Central Google Scholar * Kiran, P., Ramakrishna, V.,
Trebbin, M., Udayashankar, N. & Shashikala, H. Effective role of CaO/P2O5 ratio on SiO2-CaO-P2O5 glass system. _J. Adv. Res._ 8, 279–288 (2017). Article CAS PubMed PubMed Central
Google Scholar * Zhang, X. _et al._ Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. _Biomaterials_ 31,
5865–5874 (2010). Article CAS PubMed Google Scholar * Gupta, S., Majumdar, S. & Krishnamurthy, S. Bioactive glass: A multifunctional delivery system. _J. Control. Release_ 335,
481–497 (2021). Article CAS PubMed Google Scholar * Henaish, A. _et al._ A comparative study of optical vanadium antimony borate glass doped with spinel ferrite using structural,
spectral, and electrical measurements. _Appl. Phys. A_ 128, 1–19 (2022). Article Google Scholar * Yano, T., Kunimine, N., Shibata, S. & Yamane, M. Structural investigation of sodium
borate glasses and melts by Raman spectroscopy.: I. Quantitative evaluation of structural units. _J. Non-Cryst. Solids_ 321, 137–146 (2003). Article ADS CAS Google Scholar * Zakaly, H.
M. _et al._ An experimental evaluation of CdO/PbO-B2O3 glasses containing neodymium oxide: Structure, electrical conductivity, and gamma-ray resistance. _Mater. Res. Bull._ 151, 111828
(2022). Article CAS Google Scholar * Doweidar, H. & Saddeek, Y. B. FTIR and ultrasonic investigations on modified bismuth borate glasses. _J. Non-Cryst. Solids_ 355, 348–354 (2009).
Article ADS CAS Google Scholar * Ram, S. & Haldar, S. Medium-range structural ordering and macroscopic interactions in 1 to 2 mm thin two-dimensional platelets of borate glasses.
_Phys. Status solidi (b)_ 195, 343–351 (1996). Article ADS CAS Google Scholar * Shan, Z. _et al._ Mixed alkaline earth effects on crystallization behavior of basalt glasses and liquids.
_J. Alloy. Compd._ 874, 159986 (2021). Article CAS Google Scholar * Subhashini, Bhattacharya, S., Shashikala, H. & Udayashankar, N. Synthesis and studies on microhardness of alkali
zinc borate glasses. _AIP Conf. Proc._ 1591, 749–750 (2014). Article ADS CAS Google Scholar * Afrizal, N. H., Yahya, N., Yusoff, N. M., Kasim, A. & Hashim, A. Physical, mechanical
and structural properties of yttrium oxide doped zinc borate glasses. _Solid State Phenom._ 307, 327–335 (2020). Article Google Scholar * Kaur, G. _Clinical Applications of Biomaterials:
State-of-the-Art Progress, Trends, and Novel Approaches_ (Springer, 2017). Book Google Scholar * Ram, S. & Narayan, K. A. Controlled crystallization of lead oxide-chromium oxide-boron
oxide (PbO-Cr2O3-B2O3) glasses and a catalytic effect of alumina for the growth of lead chromate (Pb2CrO5) microcrystals. _Ind. Eng. Chem. Res._ 26, 1051–1055 (1987). Article CAS Google
Scholar * Ram, S., Bahadur, D. & Chakravorty, D. Magnetic and microstructural studies of Ca-hexaferrite based glass-ceramics. _J. Non-Cryst. Solids_ 101, 227–242 (1988). Article ADS
CAS Google Scholar * Kumari, K., Ram, S. & Kotnala, R. Self-controlled growth of Fe3BO6 crystallites in shape of nanorods from iron-borate glass of small templates. _Mater. Chem.
Phys._ 129, 1020–1026 (2011). Article CAS Google Scholar * Ghazy, A. R., Al-Hossainy, A. F., El-Sheekh, M. M. & Makhlof, M. E. M. Investigating the differences in structure,
morphology, optical properties, laser photoluminescence and dielectric properties for chitosan-doped commercial and Polycladia myrica mediated ZnO nanoparticles films combined with TD-DFT
simulations. _Algal Res._ 71, 103076 (2023). Article Google Scholar * Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. _Mol. Cell. Biochem._ 338, 241–254 (2010).
Article CAS PubMed Google Scholar * Li, H. _et al._ The role of zinc in bone mesenchymal stem cell differentiation. _Cell. Reprogram._ 24, 80–94 (2022). Article CAS PubMed Google
Scholar * Balamurugan, A. _et al._ Development and in vitro characterization of sol–gel derived CaO–P2O5–SiO2–ZnO bioglass. _Acta Biomater._ 3, 255–262 (2007). Article CAS PubMed Google
Scholar * Naresh, P. _et al._ Preparation and Characterization of melt derived CaO-Sb2O3-Li2O containing borate glass for multiple application. _J. Non-Cryst. Solids_ 589, 121642 (2022).
Article CAS Google Scholar * Oki, A., Parveen, B., Hossain, S., Adeniji, S. & Donahue, H. Preparation and in vitro bioactivity of zinc containing sol-gel–derived bioglass materials.
_J. Biomed. Mater. Res. Part A_ 69, 216–221 (2004). Article Google Scholar * Saranti, A., Koutselas, I. & Karakassides, M. Bioactive glasses in the system CaO–B2O3–P2O5: Preparation,
structural study and in vitro evaluation. _J. Non-Cryst. Solids_ 352, 390–398 (2006). Article ADS CAS Google Scholar * Neščáková, Z. _et al._ Multifunctional zinc ion doped sol–gel
derived mesoporous bioactive glass nanoparticles for biomedical applications. _Bioact. Mater._ 4, 312–321 (2019). Article PubMed PubMed Central Google Scholar * Lee, J. H. _et al._ In
vivo study of novel biodegradable and osteoconductive CaO-SiO2-B2O3 glass-ceramics. _J. Biomed. Mater. Res. Part A_ 77, 362–369 (2006). Article Google Scholar * Kolavekar, S. B. &
Ayachit, N. Impact of Pr2O3 on the physical and optical properties of multi-component borate glasses. _Mater. Chem. Phys._ 257, 123796 (2021). Article CAS Google Scholar * Kolavekar, S.
B. & Ayachit, N. Impact of variation of TeO2 on the thermal properties of lead borate glasses doped with Pr2O3. _Eur. Phys. J. Plus_ 137, 1–8 (2022). Article Google Scholar *
Kolavekar, S. B. & Ayachit, N. Ionic conductivity and dielectric relaxations in Li+ ions doped zinc borate glass system. _ECS J. Solid State Sci. Technol._ 11, 103009 (2022). Article
ADS Google Scholar * Hegde, V. _et al._ Analysis of optical and near-infrared luminescence of Er3+ and Er3+/Yb3+ Co-doped heavy metal borate glasses for optical amplifier applications.
_Photonics_ 9, 355 (2022). Article CAS Google Scholar * Ram, S. & Ram, K. IR and Raman studies and effect of γ radiation on crystallization of some lead borate glasses containing
Al2O3. _J. Mater. Sci._ 23, 4541–4546 (1988). Article ADS CAS Google Scholar * Ram, S. Infrared study of the dynamics of boroxol rings in the crystallization of BaFe12O19 microcrystals
in borate glasses. _Phys. Rev. B_ 51, 6280 (1995). Article ADS CAS Google Scholar * Ram, S. & Ram, K. Infrared reflectance spectra and formalism of precipitation of acicular magnetic
particles in network glasses. _Infrared Phys. Technol._ 37, 457–469 (1996). Article ADS CAS Google Scholar * Kargozar, S., Baino, F., Hamzehlou, S., Hill, R. G. & Mozafari, M.
Bioactive glasses: SPROUTING angiogenesis in tissue engineering. _Trends Biotechnol._ 36, 430–444 (2018). Article CAS PubMed Google Scholar * Abouelnaga, A. M., Meaz, T. M., Othman, A.
M., Ghazy, R. A. & El Nahrawy, A. M. Probing the structural and antimicrobial study on a sol–gel derived velosef-loaded bioactive calcium magneso-silicate xerogel. _SILICON_ 13, 623–631
(2021). Article CAS Google Scholar * Tostanoski, N. J., Youngman, R. E. & Sundaram, S. Effect of femtosecond laser irradiation on structure-terahertz property relationship in sodium
borosilicate glasses. _Int. J. Appl. Glass Sci._ https://doi.org/10.1111/ijag.16634 (2023). Article Google Scholar * Tomita, K., Kishi, T., Matsumura, D. & Yano, T. Laser heating
induced spatial homogenization of phase separated Na2O-B2O3-SiO2 glass plate with bearing NiO for heat center and structural probe. _J. Non-Cryst. Solids_ 597, 121891 (2022). Article CAS
Google Scholar * Samir, A., Hassan, M. A., Abokhadra, A., Soliman, L. & Elokr, M. Characterization of borate glasses doped with copper oxide for optical application. _Opt. Quant.
Electron._ 51, 1–13 (2019). Article CAS Google Scholar * Issever, U., Kilic, G. & Ilik, E. The Impact of CuO on physical, structural, optical and thermal properties of dark VPB
semiconducting glasses. _Opt. Mater._ 116, 111084 (2021). Article CAS Google Scholar * Okumura, M. _et al._ Osteoblastic phenotype expression on the surface of hydroxyapatite ceramics.
_J. Biomed. Mater. Res._ 37, 122–129 (1997). Article CAS PubMed Google Scholar * Pal, M., Roy, B. & Pal, M. Structural characterization of borate glasses containing zinc and
manganese oxides. _J. Modern Phys._ https://doi.org/10.4236/jmp.2011.29129 (2011). Article Google Scholar * Abdelghany, A. The elusory role of low level doping transition metals in lead
silicate glasses. _SILICON_ 2, 179–184 (2010). Article CAS Google Scholar * Abdelghany, A. Novel method for early investigation of bioactivity in different borate bio-glasses.
_Spectrochim. Acta Part A Mol. Biomol. Spectrosc._ 100, 120–126 (2013). Article ADS CAS Google Scholar * Abdelghany, A., Diab, H., Madbouly, A. & Ezz-ElDin, F. Inspection of
radiation shielding proficiency and effect of gamma-ray on ESR and thermal characteristics of copper oxide modified borate bioglasses. _J. Inorg. Organomet. Polym Mater._ 32, 3204–3219
(2022). Article CAS Google Scholar * Kamitsos, E. Infrared studies of borate glasses. _Phys. Chem. Glasses_ 44, 79–87 (2003). CAS Google Scholar * Lin, Y.-T. _et al._ Photothermal
atomic force microscopy coupled with infrared spectroscopy (AFM-IR) analysis of high extinction coefficient materials: A case study with silica and silicate glasses. _Anal. Chem._ 94,
5231–5239 (2022). Article CAS PubMed Google Scholar * Chanshetti, U. B., Sudarsan, V., Jogad, M. S. & Chondhekar, T. K. Effect of CuO addition on the optical and electrical
properties of sodium zinc borophosphate glasses. _Physica B_ 406, 2904–2907 (2011). Article ADS CAS Google Scholar * Boda, R., Srinivas, G., Komaraiah, D., Shareefuddin, M. &
Sayanna, R. Optical properties of bismuth borate glasses doped with Eu3+ ions. _Int. Conf. Condens. Matter Appl. Phys._ 1728, 020377 (2016). Google Scholar * Ahmad, F., Aly, E. H., Atef, M.
& ElOkr, M. Study the influence of zinc oxide addition on cobalt doped alkaline earth borate glasses. _J. Alloy. Compd._ 593, 250–255 (2014). Article CAS Google Scholar * Nazrin, S.
N. _et al._ Dielectric constant, metallization criterion and optical properties of CuO doped TeO2–B2O3 glasses. _J. Inorg. Organomet. Polym. Mater._ 32(7), 2513–2526 (2022). Article CAS
Google Scholar * Ghazy, A. R. _et al._ Synthesis, structural and optical properties of Fungal biosynthesized Cu2O nanoparticles doped Poly methyl methacrylate-co-Acrylonitrile copolymer
nanocomposite films using experimental data and TD-DFT/DMOl3 computations. _J. Mol. Struct._ 1269, 133776 (2022). Article CAS Google Scholar * Ghazy, A. R., Al-Hossainy, A. F., Rizk, H.
F. & Shendy, S. Synthesis, characterization, TD-DFT method, and optical properties of novel nanofiber conjugated polymer. _Synth. Met._ 291, 117206 (2022). Article Google Scholar *
ElBatal, F., Hamdy, Y. & Marzouk, S. UV–visible and infrared absorption spectra of transition metals-doped lead phosphate glasses and the effect of gamma irradiation. _J. Non-Cryst.
Solids_ 355, 2439–2447 (2009). Article ADS CAS Google Scholar * Mercier, C., Palavit, G., Montagne, L. & Follet-Houttemane, C. A survey of transition-metal-containing phosphate
glasses. _C. R. Chim._ 5, 693–703 (2002). Article CAS Google Scholar * Haddon, J., Rogers, E. & Williams, D. Absorption spectra of first row transition metal ions in phosphate
glasses. _J. Am. Ceram. Soc._ 52, 52–52 (1969). Article CAS Google Scholar * ElBatal, F., Marzouk, M. & Abdelghany, A. UV–visible and infrared absorption spectra of gamma irradiated
V2O5-doped in sodium phosphate, lead phosphate, zinc phosphate glasses: A comparative study. _J. Non-Cryst. Solids_ 357, 1027–1036 (2011). Article ADS CAS Google Scholar * El Agammy, E.,
Doweidar, H., El-Egili, K. & Ramadan, R. Physical and optical properties of NaF–TeO2 glasses and glass–ceramics. _Appl. Phys. A_ 127, 1–9 (2021). Google Scholar * Gautam, S., Bundela,
P., Pandey, A., Awasthi, M. & Sarsaiya, S. Diversity of cellulolytic microbes and the biodegradation of municipal solid waste by a potential strain. _Int. J. Microbiol._
https://doi.org/10.1155/2012/325907 (2012). Article PubMed PubMed Central Google Scholar * Tauc, J., Grigorovici, R. & Vancu, A. Optical properties and electronic structure of
amorphous germanium. _Phys. Status solidi (b)_ 15, 627–637 (1966). Article ADS CAS Google Scholar * Ghazy, A., Hemeda, O., Al-Hossainy, A., Ghazy, R. & Henaish, A. Docking of
COVID-19 main protease and TD-DFT/DMOl3 simulated method, synthesis, and characterization with hybrid nanocomposite thin films and its applications. _Surf. Interfaces_ 37, 102722 (2023).
Article CAS Google Scholar * Larsson, P. _et al._ Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. _Sci. Rep._ 10,
1–12 (2020). Article Google Scholar * Chen, S. _et al._ In vitro stimulation of vascular endothelial growth factor by borate-based glass fibers under dynamic flow conditions. _Mater. Sci.
Eng., C_ 73, 447–455 (2017). Article CAS Google Scholar * Palakurthy, S., Reddy, K. V., Patel, S. & Azeem, P. A. A cost effective SiO2–CaO–Na2O bio-glass derived from bio-waste
resources for biomedical applications. _Prog. Biomater._ 9, 239–248 (2020). Article CAS PubMed PubMed Central Google Scholar * Moghanian, A. _et al._ Structural and in vitro biological
evaluation of sol-gel derived multifunctional Ti+4/Sr+2 co-doped bioactive glass with enhanced properties for bone healing. _Ceram. Int._ 47, 29451–29462 (2021). Article CAS Google Scholar
* Datta, S. _et al._ Effect of boron oxide addition on structural, thermal, in vitro bioactivity and antibacterial properties of bioactive glasses in the base S53P4 composition. _J.
Non-Cryst. Solids_ 498, 204–215 (2018). Article ADS Google Scholar * Balasubramanian, P., Buettner, T., Pacheco, V. M. & Boccaccini, A. R. Boron-containing bioactive glasses in bone
and soft tissue engineering. _J. Eur. Ceram. Soc._ 38, 855–869 (2018). Article CAS Google Scholar Download references FUNDING Open access funding provided by The Science, Technology &
Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Physics Department, Faculty of Science, Tanta
University, Tanta, 31527, Egypt Ahmed R. Ghazy, B. M. Elmowafy, T. M. Meaz & R. Ghazy * Spectroscopy Department, Physics Research Institute, National Research Centre, 33 Elbehouth St.,
Dokki, Giza, 12311, Egypt A. M. Abdelghany * Microwave Physics and Dielectrics, Physics Research Institute, National Research Centre, 33 Elbehouth St., Dokki, Giza, 12311, Egypt R. M.
Ramadan Authors * Ahmed R. Ghazy View author publications You can also search for this author inPubMed Google Scholar * B. M. Elmowafy View author publications You can also search for this
author inPubMed Google Scholar * A. M. Abdelghany View author publications You can also search for this author inPubMed Google Scholar * T. M. Meaz View author publications You can also
search for this author inPubMed Google Scholar * R. Ghazy View author publications You can also search for this author inPubMed Google Scholar * R. M. Ramadan View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.R.G.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Review & Editing; B.M.E.:
Conceptualization, Formal analysis, Investigation, Writing; A. M.A.: Conceptualization, Formal analysis, Investigation, Writing, reviewing final version; T.M.M.: Conceptualization,
Methodology, Writing—Original Draft, Writing—Review & Editing; R.G.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Review & Editing; R.M.R.: Supervision,
Conceptualization, Methodology, Writing—Original Draft, Writing—Review & Editing. CORRESPONDING AUTHOR Correspondence to Ahmed R. Ghazy. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if
changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the
material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Ghazy, A.R., Elmowafy, B.M., Abdelghany, A.M. _et al._ Structural, optical, and cytotoxicity studies of laser irradiated ZnO doped borate bioactive glasses. _Sci Rep_ 13,
7292 (2023). https://doi.org/10.1038/s41598-023-34458-4 Download citation * Received: 15 March 2023 * Accepted: 30 April 2023 * Published: 05 May 2023 * DOI:
https://doi.org/10.1038/s41598-023-34458-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
