Genome-wide discovery of di-nucleotide ssr markers based on whole genome re-sequencing data of cicer arietinum l. And cicer reticulatum ladiz
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Simple sequence repeats (SSRs) are valuable genetic markers due to their co-dominant inheritance, multi-allelic and reproducible nature. They have been largely used for exploiting
genetic architecture of plant germplasms, phylogenetic analysis, and mapping studies. Among the SSRs, di-nucleotide repeats are the most frequent of the simple repeats distributed throughout
the plant genomes. In present study, we aimed to discover and develop di-nucleotide SSR markers by using the whole genome re-sequencing (WGRS) data from _Cicer arietinum_ L. and _C.
reticulatum_ Ladiz. A total of 35,329 InDels were obtained in _C. arietinum,_ whereas 44,331 InDels in _C. reticulatum_. 3387 InDels with 2 bp length were detected in _C. arietinum_, there
were 4704 in _C. reticulatum_. Among 8091 InDels, 58 di-nucleotide regions that were polymorphic between two species were selected and used for validation. We tested primers for evaluation
of genetic diversity in 30 chickpea genotypes including _C. arietinum_, _C. reticulatum, C. echinospermum_ P.H. Davis, _C. anatolicum_ Alef., _C. canariense_ A. Santos & G.P. Lewis, _C.
microphyllum_ Benth., _C. multijugum_ Maesen, _C. oxyodon_ Boiss. & Hohen. and _C. songaricum_ Steph ex DC. A total of 244 alleles were obtained for 58 SSR markers giving an average of
2.36 alleles per locus. The observed heterozygosity was 0.08 while the expected heterozygosity was 0.345. Polymorphism information content was found to be 0.73 across all loci. Phylogenetic
tree and principal coordinate analysis clearly divided the accessions into four groups. The SSR markers were also evaluated in 30 genotypes of a RIL population obtained from an interspecific
cross between _C. arietinum_ and _C. reticulatum._ Chi-square (χ2) test revealed an expected 1:1 segregation ratio in the population. These results demonstrated the success of SSR
identification and marker development for chickpea with the use of WGRS data. The newly developed 58 SSR markers are expected to be useful for chickpea breeders. SIMILAR CONTENT BEING VIEWED
BY OTHERS MINING AND VALIDATION OF NOVEL GENOTYPING-BY-SEQUENCING (GBS)-BASED SIMPLE SEQUENCE REPEATS (SSRS) AND THEIR APPLICATION FOR THE ESTIMATION OF THE GENETIC DIVERSITY AND POPULATION
STRUCTURE OF COCONUTS (_COCOS NUCIFERA_ L.) IN THAILAND Article Open access 01 October 2020 GENOME-WIDE SIMPLE SEQUENCE REPEATS (SSR) MARKERS DISCOVERED FROM WHOLE-GENOME SEQUENCE
COMPARISONS OF MULTIPLE SPINACH ACCESSIONS Article Open access 11 May 2021 TRANSCRIPTOME WIDE SSR DISCOVERY CROSS-TAXA TRANSFERABILITY AND DEVELOPMENT OF MARKER DATABASE FOR STUDYING GENETIC
DIVERSITY POPULATION STRUCTURE OF _LILIUM_ SPECIES Article Open access 29 October 2020 INTRODUCTION Chickpea (_Cicer arietinum_ L.) is one of the valuable cool-season grain legume crops in
the world. It is a self-pollinated and diploid plant (2n = 2x = 16) with a genome size of ~ 740 Mb1 which is considerably less than other important legume crops like pea, lentil, alfalfa,
soybean and peanut2. The genus _Cicer_ L. belongs to the family Fabaceae, subfamily Faboideae and contains a total of 49 taxa with 9 annuals and 40 perennials3,4,5,6. Toker et al.7 has been
recently introduced a new annual wild _Cicer_ species, thereby increasing the count to 10 annual species. _C. arietinum_ is solely cultivated species of the genus. _C. reticulatum_ is
considered to be the wild progenitor of the cultivated chickpea8. It is crossable with the cultivated chickpea and possesses 2n = 2x = 16 chromosommes with a smaller genome size of 416 Mb
than that of the cultivated chickpea9. Chickpea plays valuable roles in human diet as a rich source of dietary proteins, complex carbohydrates and micronutrients such as iron, potassium and
zinc as well as vitamins A and B in addition to folate and thiamine10. Because of its capacity of biological fixation of atmospheric nitrogen through nodulation with _Rhizobium_ species, it
is an advantageous crop in crop rotation11. Also, chickpea is the most important cool season food legume in the arid and semi-arid areas under rainfed conditions12. Globally, harvested area
was approximately 14.8 million ha and total production was almost 15.1 million tons of chickpeas in 202013. It is widely grown and consumed in India, Pakistan, Iran and Turkey13. Various
biotic and abiotic factors have been affecting the chickpea production in the worldwide14,15. Due to limited genetic diversity in cultivated chickpea, it has been restricted achievement in
respect to efforts for increasing the productivity16. Conventional methods have been used in crop breeding and tolerance to the environmental stresses while molecular breeding approaches
have potential to accelerate the process of developing new cultivars. Also, the effective usage of plant genetic resources in breeding might be possible with the awareness and information of
genetic variation present within individuals or populations. Molecular markers explore the genetic diversity at the DNA level and have the capability to reflect the precise genetic
diversity between genotypes17. In chickpea, random amplified polymorphic DNA (RAPD)18,19,20, amplified fragment length polymorphism (AFLP)21,22, simple sequence repeat (SSR)23, inter simple
sequence repeat (ISSR)24,25,26 and internal transcribed spacer (ITS)27 have been used for genetic diversity analysis in different germplasm. Recently, an extensive development has been made
regarding the improvement of several genomic or transcript-based SSR markers and SNP markers and their deployment in the large-scale genomics and breeding programs in
chickpea28,29,30,31,32,33,34,35. In contrast to SNP markers, SSRs are very convenient and easy to use. SSRs can be found in both coding and noncoding regions of all higher organisms. The
genome wide occurrence, co-dominant inheritance, highly polymorphic and multi-allelic nature promote wide utilization of SSRs36,37,38. Earlier, the usual protocol for isolation
microsatellite sequences was utilization of microsatellite-enriched libraries by cloning and Sanger sequencing method, which was costly, difficult, and time consuming39. Recently,
development of next-generation sequencing (NGS) technologies has prompted the fast and cost-effective SSR discovery in many crops. There are now numerous methods that apply NGS for
genotyping, reduced representation libraries (RRLs), restriction-site-associated DNA sequencing (RADseq), genotyping-by-sequencing (GBS), whole-genome resequencing (WGRS)40,41,42. WGRS is
more appropriate for pre-breeding activities where less number of elite parents, landraces and wild species require to be examined delicately for genome variation (SNPs, CNV, structural
variation) and association studies43. Efficiency of WGRS have been shown in many such crops such as rice44,45, sorghum46, cotton47, soybean48, tomato49, and chickpea50,51,52,53. In view of
above prospects, genome-wide SSR markers were developed in chickpea in the present study. The utility of these developed markers in F6 population derived from an interspecific cross between
_C. arietinum_ and _C. reticulatum_ was accessed. The cross-transferability of these markers was also examined across 30 chickpea genotypes including cultivated and wild types. RESULTS
GENOTYPING A total of 2.01 GB and 2.16 GB raw sequence reads of _C. arietinum_ and _C. reticulatum_ were generated from 150 bp paired-end sequencing. _C. arietinum_ had 34.77 M reads and 33%
guanine-cytosine (GC) content while _C. reticulatum_ had 33.60 M reads and 34% GC content. The means of reads mapped to the _C. arietinum_ reference genome were 97.56% and 96.62% in _C.
arietinum_ and _C. reticulatum_, respectively. VARIANT DETECTION Using variant calling pipeline, 3.9 M and 4.7 M variants were initially detected in _C. arietinum_ and _C. reticulatum_
genome, respectively. Out of all variants, a total of 3.26 M SNPs were identified in _C. arietinum_, by contrast 3.93 M in _C. reticulatum_ compared to the reference genome. In total, 35,329
and 44,331 InDels were identified in the species of _C. arietinum_ and _C. reticulatum_, respectively. A total of 3387 InDels with 2 bp length was detected in _C. arietinum_, there was 4704
in _C. reticulatum._ Among 8091 InDels, 58 di-nucleotide regions that were polymorphic between two species were selected and used for primer design (Table 1). SSR VALIDATION IN RIL
POPULATION Designed primer pairs were used for validation in 30 chickpea genotypes of F6 population obtained from an interspecific cross between _C. arietinum_ and _C. reticulatum._ Out of
SSR31 and SSR32, all primers were successfully amplified. The obtained PCR products were loaded on a polyacrylamide gel, and allele sizes were determined by comparing with _C. arietinum_ and
_C. reticulatum_. The difference of allele sizes was also confirmed in the gel. It was seen that all 30 genotypes carried one of the alleles which the parents had. While SSR5 and SSR10
produced suitable alleles in 30 RIL genotypes for 2-nucleotide polymorphism between female and male parents, SSR14 primer produced suitable alleles for 8-nucleotide polymorphism and SSR18
primer for 6-nucleotide polymorphism between _C. arietinum_ and _C. reticulatum_ (Table 1). Chi-square (χ2) values were calculated for each marker to test the fit of the markers in 30
genotypes representing the RIL population to the expected 1:1 expression ratio. Markers deviating from expected Mendelian ratios were determined by chi-square analysis (Table 2). According
to the results, it was determined that the markers were suitable for 1:1 expansion ratio, since the calculated p values for all markers except SSR20 were greater than 0.05. SSR DIVERSITY IN
CULTIVATED AND WILD POPULATIONS For genetic diversity analysis, 30 genotypes obtained from cultivated and wild species were tested in polyacrylamide gel, bands were scored according to
allele sizes. As a result of the analysis, a total of 244 alleles belonging to 41 different SSR loci were determined in 30 chickpea genotypes (Table 3). At the population level, allelic
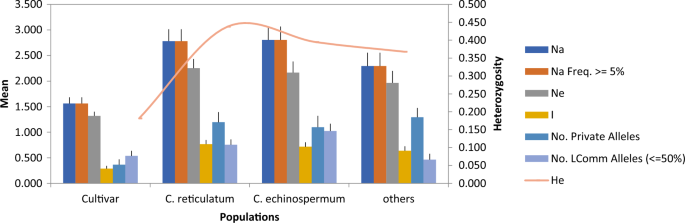
diversity in cultivated and wild populations was shown in Fig. 1. Total allele distribution was 63 in cultivars and 311 in wild genotypes. While a total of 110 alleles were determined in the
genotypes of the _C. reticulatum_, 112 alleles were observed in the genotypes of the _C. echinospermum_. 89 alleles were determined in the population from distantly related wild species.
The mean number of alleles (Na) for 30 genotypes was 2.36 (Table 3). The highest number of alleles was obtained from the primers SSR3, SSR58 and SSR39 (Table 3). The number of effective
alleles (Ne) varied between 0.75 and 3.74. Nei's54 observed (Ho) and expected (He) heterozygosity values were calculated as 0.08 and 0.34, respectively. The mean of polymorphism
information content (PIC) was measured as 0.73 (Table 3). The highest PIC value was observed at the SSR21 (0.90) loci, followed by the SSR56 (0.88), SSR54 (0.86), SSR4 (0.85), SSR7 (0.83)
and SSR34 (0.83) loci. The lowest PIC value was found in the SSR9 (0.51) locus (Table 3). Phylogenetic tree consisting of 30 chickpea genotypes was constructed based on the UPGMA clustering
method with newly developed SSRs (Fig. 2). The chickpea genotypes were divided into four clusters, indicating clear separation between wild and cultivated species. Cluster I contained
cultivated chickpeas including four kabuli and four desi chickpeas. Cluster II, III and IV consist of wild chickpea species, each representing _C. echinospermum, C. reticulatum_ and other
wild chickpea species, respectively. The PCoA analysis confirmed the clusters of the phylogenetic tree (Fig. 3). Cultivated and wild genotypes did not cluster together. The two informative
components explained 92.36% of the cumulative variance, PC1 and PC2 shared 53.72% and 38.64% variation, respectively. DISCUSSION USING NGS TECHNOLOGY IS AN EFFECTIVE TOOL FOR THE
IDENTIFICATION OF SSR MARKERS SSRs are valuable genetic markers due to their co-dominant inheritance, multi-allelic and reproducible nature55. In chickpea, large numbers of SSR markers have
been identified and widely used for genetic diversity analysis, gene/QTL mapping, construction of linkage map, marker assisted selection (MAS)33,56,57,58,59. However, validation and
selection of informative markers from such huge numbers of markers that show polymorphism in chickpea, is an excessive effort. In addition, the narrow genetic base in chickpea may can
restrict use of the identified markers in genotyping studies because of their low intra-specific polymorphism among chickpea genotypes23,30. The NGS technologies have caused impressive
advances in sequencing which creates high-throughput sequences to transform genotyping and plant breeding. It provides opportunities to perform high-throughput SSR identification. In present
study, we developed genome-wide SSR markers from cultivated and wild chickpea genotypes. SSR marker development from genomic data has been reported for various crops such as sesame60, red
clover61, peanut62, sweet potato63, faba bean64, lentil65. DISTRIBUTION OF VARIANTS IN _C. ARIETINUM_ AND _C. RETICULATUM_ GENOME As a result of alignment to the reference genome of
chickpea, a total of 3.26 M SNPs were identified in _C. arietinum_, by contrast 3.93 M in _C. reticulatum_. Previously, 51,632 SNPs were reported by 454 transcriptome sequencing of _C.
arietinum_ and _C. reticulatum_ genotypes35. In addition, couple hundreds of SNPs were also studied using Solexa ⁄ Illumina sequencing, targeted amplicon sequencing, mining of expressed
sequence tag libraries and sequencing of candidate genes30,66,67. VALIDATION AND POLYMORPHIC POTENTIAL OF SSRS The utilization of genetic diversity in chickpea genetic resources is very
important in order to utilize collections and improve breeding studies. Genetic diversity analysis in chickpea was previously performed using RAPD18, AFLP68, STMS69, SSRs70,71. In this
study, the effectiveness of the developed markers was evaluated in 30 chickpea genotypes obtained from cultivated and wild species as well as 30 chickpea genotypes of F6 population obtained
from an interspecific cross between _C. arietinum_ and _C. reticulatum._ The markers were effective for detection of a total of 244 alleles (Na). The mean of number of alleles (2.36)
observed in this study are within the ranges revealed by various previous studies. For instance, the use of 33 SSR markers identifed a total of 111 alleles with an average of 3.7 alleles per
locus in 155 chickpea genotypes72. Similarly, 27 SSRs were used to study genetic diversity in 50 chickpea accessions which reported a total of 81 alleles with an average of 3.0
alleles/locus73. In the present study, heterozygosity was detected in genotypes that ranged from 0.03 to 0.66 with mean of 0.34, which is similar to previous studies reported previously by
Upadhyaya et al.74 and Hajibarat et al.75. Genetic diversity analysis showed that the average PIC value of SSR markers was 0.73, higher than PIC value of the SNPs76, STMS77,78, AFLP20 and
SilicoDArT79 markers used to identify genetic variation in chickpea. Botstein et al.80 reported the PIC values of markers as highly informative (≥ 0.5), reasonably informative (0.50–0.25),
or least informative (≤ 0.25). Our average PIC value (0.73) thus shows that the developed markers identified here are highly informative and greatly sufficient for showing relationships
among genotypes, according to Meszaros et al.81. The principal coordinate analysis clearly separated the whole population into four clusters, and wild and cultivated types in seperate
clusters. Results from the present study are consistant with the previous studies71,82 the grouping followed a clear pattern between cultivated chickpea and the wild species. It is also
clear as the wild progenitor, _Cicer reticulatum_ showed close proximity with the cultivated chickpea. The other close connection was seen between _C. reticultum_ and _C. echinospermum_. It
can be supposed from this study that cluster analysis shows the effectiveness of the designed markers. The results of the present study revealed the success of SSR identification and marker
development in chickpea using NGS genome data. The developed SSR markers were applied successfully for illuminating genetic diversity among cultivated and wild chickpea populations as well
as validation in F6 population obtained from an interspecific cross between _C. arietinum_ and _C. reticulatum._ Therefore, newly developed 58 SSR markers are potentially useful for genetic
studies of chickpea. In conclusion, NGS strategy led to the discovery of a large number of microsatellites markers, providing thousands of SSRs for validation in chickpea. These new SSRs
will become significant molecular tools for chickpea genetic breeding programs. Later, these markers could be integrated in genetic maps to be utilized in MAS. MATERIALS AND METHODS PLANT
MATERIAL _C. arietinum_ L._,_ CA 2969 and _C. reticulatum_ Ladiz._,_ AWC 602 were used as a genetic material for WGRS analysis. CA 2969 and AWC 602 chickpea genotypes were registered by
USDA-ARS and Akdeniz University, Department of Field Crops, respectively. The important traits for these genotypes were given in Table 4. Developed SSRs were validated in 30 chickpea lines
from a RIL population earlier developed by Sari et al.83 and derived from an interspecific cross between CA 2969 and AWC 602. The markers were also used to assess the genetic diversity of
cultivated and wild chickpea accessions including eight accessions of _C. arietinum_ (four kabuli and four desi chickpeas), eight accessions of _C. reticulatum_, eight accessions of _C.
echinospermum_ P.H. Davis and six accessions of _C. anatolicum_ Alef., _C. canariense_ A. Santos & G.P. Lewis_, C. microphyllum_ Benth., _C. multijugum_ Maesen, _C. oxyodon_ Boiss. &
Hohen. and _C. songaricum_ Steph ex DC. (Table 5). Seed samples of ICARDA and USDA are available directly from ICARDA (https://www.icarda.org/) and USDA (https://www.usda.gov/). The
procurement of seeds of all cultivated and wild genotypes used in the present study complies with relevant institutional, national, and international guidelines and legislation. EXPERIMENTAL
AREA Plants belonging the parents (CA 2969 and AWC 602) and 30 cultivated and wild chickpea accessions were grown in separate pods in a greenhouse at the Faculty of Agriculture, Akdeniz
University, Antalya, Turkey (30°38′E, 36°53′N, 33 m above sea level) for genomic DNA extraction. DNA EXTRACTION DNA extraction process was carried out at Plant Molecular Biology Laboratuary,
the Faculty of Agriculture, Akdeniz University, Antalya, Turkey. Genomic DNA was extracted from 3 week-old young leaves of plants individually using the CTAB method as described by Doyle
and Doyle84 with minor adjustments such as extra chloroform-isoamyl alcohol and 70% ethanol cleaning steps. DNA quality and quantity of each sample were estimated by electrophoresis on 1%
agarose gels, and the amount was fixed to 100 ng/μL using lambda DNA as a reference. LIBRARY PREPARATION AND SEQUENCING The genomic data from _C. arietinum_ and _C. reticulatum_ was used for
construction of a HiSeq sequencing library using TruSeq DNA sample Prep kit LT, (set A) FC-121-2001 (Illumina, San Diego, CA, USA) according to manufacturer’s protocol. A reduced
representative genomic library with a target insert size of about 350 bp were sequenced on Illumina Hiseq X to generate 150-bp paired-end reads at Macrogen Inc., (Macrogen, Seoul, Korea).
WGRS data of two available genotypes were deposited into the National Center for Biotechnology Information (NCBI) Sequence-Read Archive (SRA) database. The raw data were demultiplexed using
Je V1.285, a quality control was performed for FASTQ Sanger files using fastp86, and reads with a Phred quality score below 15 were trimmed87. The cleaned data were aligned with kabuli
reference genome 1.01 using Bowtie 2 with default parameters88 in the Galaxy software (www.usegalaxy.org). The created BAM files (*.bam) were analyzed using Freebayes (Galaxy Version
1.1.0.46-0)89, with simple diploid calling and filtering, and a minimum of 20 × coverage for variant detection. The obtained variant files were filtered using VCFfilter (Galaxy Version
1.0.0) and SNPs were chosen. Insertions and deletions from individual (*.vcf) files were later merged into a single VCF file using VCF genotypes (Galaxy Version 1.0.0). The combined variant
file was processed using Microsoft Excel to eliminate duplicated regions and organize the SSRs according to their sizes. SSR regions which have 2 bp long and polymorphic between parents were
checked using the Integrated Genome Browser V9.1.4. PRIMER DESIGN For designing the primer pairs from the flanking sequences of identified SSRs, Primer3 software90,91 was used with the
parameters as follows: primer length of 18–27 nucleotides, melting temperatures of 55–65 °C, GC content of 30–70%, and predicted PCR products of 100–300 bp in length. The primer pairs were
later controlled for possible duplication of sequences in the genome using IGB software. The PCR reactions were performed using the M13 tailing PCR procedure92. The forward primers were
tailed by adding an M13 sequence labeled with IRDye to the 5′ end. The following PCR protocol was applied: 95 °C initial denaturation for 5 min, 30 cycles at 95 °C for 30 s, annealing
temperature 60 °C for 30 s, 72 °C for 1 min, followed by 9 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and then a final extension of 10 min at 72 °C. PCR products were loaded
onto 8% denatured polyacrylamide gel and separated by 4300 DNA analyzer (LI-COR, Inc., Lincoln, Nebraska, USA). 1 kb size marker was used to score markers as 1 or 0 for the presence and
absence of alleles. STATISTICAL ANALYSES RIL data was analyzed using MINITAB 19 software. A Chi square (χ2) test was used to assess goodness of fit to the observed segregation ratios
followed 3:1 ratio in the RIL population. GENETIC DIVERSITY AND PHYLOGENY ANALYSIS Genetic diversity parameters such as number of alleles (Na), number of effective alleles (Ne), Shannon
diversity index (I), expected heterozygosity (He), unexpected heterozygosity (uHe), observed heterozygosity (Ho) and Wright’s fixation index (F) were shown using GenAlEx 6.593. The
phylogenetic tree was constructed in DARwin ver 5.0 software94 using the unweighted pair group method with arithmetic mean (UPGMA)95 clustering method and modified in FigTree v1.4.4
(http://tree.bio.ed.ac.uk/software/figtree). Principal coordinate analysis (PCoA) was performed with GenAlEx 6.5 to evaluate the genetic relationships between populations. The Excel
microsatellite toolkit96 was used to measure polymorphism. DATA AVAILABILITY The datasets generated and analysed during the current study are available in the National Center for
Biotechnology Information (NCBI) Sequence-Read Archive (SRA) database with the accession number of PRJNA926661. REFERENCES * Varshney, R. K. _et al._ Draft genome sequence of chickpea
(_Cicer arietinum_) provides a resource for trait improvement. _Nat. Biotechnol._ 31, 240–246 (2013). Article CAS PubMed Google Scholar * Li, Y. _et al._ Investigating drought tolerance
in chickpea using genome-wide association mapping and genomic selection based on whole-genome resequencing data. _Front. Plant Sci._ 9, 190 (2018). Article ADS PubMed PubMed Central
Google Scholar * Van der Maesen, L. J. G., Maxted, N., Javadi, F., Coles, S. & Davies, A. M. R. Taxonomy of the genus Cicer revisited. _Chickpea Breed. Manag. CAB Int. Wallingford_
14–46 (2007). * Dönmez, A. A. Cicer uludereensis Dönmez: A new species of Cicer (Chickpea) (Fabaceae) from around the Fertile Crescent. SE Turkey. _Turk. J. Bot._
https://doi.org/10.3906/bot-1001-283 (2011). Article Google Scholar * Ozturk, M. Revision of the genus Cicer L. _Turk. Morphol. Palynol. Cytotaxonomical Mol. Phylogenetic Methods Anal.
Seed Protein Elem. Contents Ph Thesis Grad. Sch. Nat. Appl. Sci. Selçuk Univ. Konya Turk._ (2011). * Toker, C., Uzun, B., Ceylan, F. O. & Ikten, C. Chickpea. _Alien Gene Transf. Crop
Plants_ 2, 121–151 (2014). Article Google Scholar * Toker, C. _et al._ _Cicer turcicum_: A new Cicer species and its potential to improve chickpea. _Front. Plant Sci._ 12, 662891 (2021).
Article PubMed PubMed Central Google Scholar * Van der Maesen, L. J. G. Origin, history and taxonomy of chickpea, in _The Chickpea_ 11–34 (1987). * Gupta, S. _et al._ Draft genome
sequence of _Cicer reticulatum_ L., the wild progenitor of chickpea provides a resource for agronomic trait improvement. _Dna Res._ 24, 1–10 (2017). PubMed Google Scholar * Gaur, P. M. _et
al._ Inheritance of protein content and its relationships with seed size, grain yield and other traits in chickpea. _Euphytica_ 209, 253–260 (2016). Article CAS Google Scholar *
Herridge, D. F., Rupela, O. P., Serraj, R. & Beck, D. P. Screening techniques and improved biological nitrogen fixation in cool season food legumes. _Euphytica_ 73, 95–108 (1993).
Article Google Scholar * Mohammed, A., Tana, T., Singh, P., Korecha, D. & Molla, A. Management options for rainfed chickpea (_Cicer arietinum_ L.) in northeast Ethiopia under climate
change condition. _Clim. Risk Manag._ 16, 222–233 (2017). Article Google Scholar * FAOSTAT. FAOSTAT, FAO Statistical Databases (2022). * Bakir, M., Sari, D., Sari, H., Waqas, M. &
Atif, R. M. Chickpea wild relatives: Potential hidden source for the development of climate resilient chickpea varieties, in _Wild Germplasm for Genetic Improvement in Crop Plants_ 269–297
(2021). https://doi.org/10.1016/B978-0-12-822137-2.00006-0. * Chrigui, N. _et al._ Introgression of resistance to leafminer (_Liriomyza cicerina_ Rondani) from _Cicer reticulatum_ Ladiz. to
_C. arietinum_ L. and relationships between potential biochemical selection criteria. _Agronomy _11, 57 (2021). Article CAS Google Scholar * Roorkiwal, M. _et al._ Allele diversity for
abiotic stress responsive candidate genes in chickpea reference set using gene based SNP markers. _Front. Plant Sci._ 5, 248 (2014). Article PubMed PubMed Central Google Scholar * Cui,
C., Mei, H., Liu, Y., Zhang, H. & Zheng, Y. Genetic diversity, population structure, and linkage disequilibrium of an association-mapping panel revealed by genome-wide SNP markers in
sesame. _Front. Plant Sci._ 8, 1189 (2017). Article PubMed PubMed Central Google Scholar * Iruela, M., Rubio, J., Cubero, J. I., Gil, J. & Millan, T. Phylogenetic analysis in the
genus Cicer and cultivated chickpea using RAPD and ISSR markers. _Theor. Appl. Genet._ 104, 643–651 (2002). Article CAS PubMed Google Scholar * Javadi, F. & Yamaguchi, H. RAPD and
seed coat morphology variation in annual and perennial species of the genus Cicer L. _Genet. Resour. Crop Evol._ 51, 783–794 (2004). Article CAS Google Scholar * Talebi, R., Naji, A. M.
& Fayaz, F. Geographical patterns of genetic diversity in cultivated chickpea (_Cicer arietinum_ L.) characterized by amplified fragment length polymorphism. _Plant Soil Environ._ 54,
447–452 (2008). Article CAS Google Scholar * Nguyen, T. T., Taylor, P. W. J., Redden, R. J. & Ford, R. Genetic diversity estimates in Cicer using AFLP analysis. _Plant Breed._ 123,
173–179 (2004). Article CAS Google Scholar * Shan, F., Clarke, H. J., Yan, G., Plummer, J. A. & Siddique, K. H. M. Identification of duplicates and fingerprinting of primary and
secondary wild annual Cicer gene pools using AFLP markers. _Genet. Resour. Crop Evol._ 54, 519–527 (2007). Article CAS Google Scholar * Sethy, N. K., Shokeen, B., Edwards, K. J. &
Bhatia, S. Development of microsatellite markers and analysis of intraspecific genetic variability in chickpea (_Cicer arietinum_ L.). _Theor. Appl. Genet._ 112, 1416–1428 (2006). Article
CAS PubMed Google Scholar * Sudupak, M. A., Akkaya, M. S. & Kence, A. Genetic relationships among perennial and annual Cicer species growing in Turkey assessed by AFLP fingerprinting.
_Theor. Appl. Genet._ 108, 937–944 (2004). Article CAS PubMed Google Scholar * Amirmoradi, B., Talebi, R. & Karami, E. Comparison of genetic variation and differentiation among
annual Cicer species using start codon targeted (SCoT) polymorphism, DAMD-PCR, and ISSR markers. _Plant Syst. Evol._ 298, 1679–1688 (2012). Article CAS Google Scholar * Aggarwal, H. _et
al._ Assessment of genetic diversity among 125 cultivars of chickpea (_Cicer arietinum_ L.) of Indian origin using ISSR markers. _Turk. J. Bot._ 39, 218–226 (2015). Article Google Scholar
* Singh, A., Devarumath, R. M., Ramarao, S., Singh, V. P. & Raina, S. N. Assessment of genetic diversity, and phylogenetic relationships based on ribosomal DNA repeat unit length
variation and Internal Transcribed Spacer (ITS) sequences in chickpea (_Cicer arietinum_) cultivars and its wild species. _Genet. Resour. Crop Evol._ 55, 65–79 (2008). Article CAS Google
Scholar * Millan, T. _et al._ A consensus genetic map of chickpea (_Cicer arietinum_ L.) based on 10 mapping populations. _Euphytica_ 175, 175–189 (2010). Article CAS Google Scholar *
Nayak, S. N. _et al._ Integration of novel SSR and gene-based SNP marker loci in the chickpea genetic map and establishment of new anchor points with _Medicago truncatula_ genome. _Theor.
Appl. Genet._ 120, 1415–1441 (2010). Article CAS PubMed PubMed Central Google Scholar * Gujaria, N. _et al._ Development and use of genic molecular markers (GMMs) for construction of a
transcript map of chickpea (_Cicer arietinum_ L.). _Theor. Appl. Genet._ 122, 1577–1589 (2011). Article PubMed PubMed Central Google Scholar * Thudi, M. _et al._ Novel SSR markers from
BAC-end sequences, DArT arrays and a comprehensive genetic map with 1,291 marker loci for chickpea (_Cicer arietinum_ L.). _PLoS ONE_ 6, e27275 (2011). Article ADS CAS PubMed PubMed
Central Google Scholar * Gaur, R. _et al._ High-throughput SNP discovery and genotyping for constructing a saturated linkage map of chickpea (_Cicer arietinum_ L.). _DNA Res._ 19, 357–373
(2012). Article CAS PubMed PubMed Central Google Scholar * Hiremath, P. J. _et al._ Large-scale development of cost-effective SNP marker assays for diversity assessment and genetic
mapping in chickpea and comparative mapping in legumes. _Plant Biotechnol. J._ 10, 716–732 (2012). Article CAS PubMed PubMed Central Google Scholar * Roorkiwal, M. _et al._ Single
nucleotide polymorphism genotyping for breeding and genetics applications in chickpea and pigeonpea using the BeadXpress platform. _Plant Genome_ 6, plantgenome2013-05 (2013). Article
Google Scholar * Deokar, A. A. _et al._ Genome wide SNP identification in chickpea for use in development of a high density genetic map and improvement of chickpea reference genome
assembly. _BMC Genom._ 15, 708 (2014). Article Google Scholar * Varshney, R. K., Graner, A. & Sorrells, M. E. Genic microsatellite markers in plants: Features and applications. _Trends
Biotechnol._ 23, 48–55 (2005). Article CAS PubMed Google Scholar * Naghavi, M. R., Monfared, S. R. & Humberto, G. Genetic diversity in Iranian chickpea (_Cicer arietinum_ L.)
landraces as revealed by microsatellite markers. _Czech J. Genet. Plant Breed._ 48, 131–138 (2012). Article Google Scholar * Asadi, A. A. & Rashidi Monfared, S. Characterization of
EST-SSR markers in durum wheat EST library and functional analysis of SSR-containing EST fragments. _Mol. Genet. Genom._ 289, 625–640 (2014). Article CAS Google Scholar * Duan, C., Li,
D., Sun, S., Wang, X. & Zhu, Z. Rapid development of microsatellite markers for _Callosobruchus chinensis_ using Illumina paired-end sequencing. _PLoS ONE_ 9, e95458 (2014). Article ADS
PubMed PubMed Central Google Scholar * Davey, J. W. _et al._ Genome-wide genetic marker discovery and genotyping using next-generation sequencing. _Nat. Rev. Genet._ 12, 499–510 (2011).
Article ADS CAS PubMed Google Scholar * Elshire, R. J. _et al._ A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. _PLoS ONE_ 6, e19379 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar * Jaganathan, D., Bohra, A., Thudi, M. & Varshney, R. K. Fine mapping and gene cloning in the post-NGS era: Advances and
prospects. _Theor. Appl. Genet._ 133, 1791–1810 (2020). Article CAS PubMed PubMed Central Google Scholar * Li, Y. _et al._ Association analysis of frost tolerance in rye using candidate
genes and phenotypic data from controlled, semi-controlled, and field phenotyping platforms. _BMC Plant Biol._ 11, 1–14 (2011). Article Google Scholar * Huang, X. _et al._ A map of rice
genome variation reveals the origin of cultivated rice. _Nature_ 490, 497–501 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Wang, M. _et al._ The genome sequence of
African rice (_Oryza glaberrima_) and evidence for independent domestication. _Nat. Genet._ 46, 982–988 (2014). Article CAS PubMed PubMed Central Google Scholar * Mace, E. S. _et al._
Whole-genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum. _Nat. Commun._ 4, 1–9 (2013). Article Google Scholar * Page, J. T. _et al._ Insights
into the evolution of cotton diploids and polyploids from whole-genome re-sequencing. _G3 Genes Genomes Genet._ 3, 1809–1818 (2013). Article Google Scholar * Zhou, Z. _et al._ Resequencing
302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. _Nat. Biotechnol._ 33, 408–414 (2015). Article CAS PubMed Google Scholar * Lin,
T. _et al._ Genomic analyses provide insights into the history of tomato breeding. _Nat. Genet._ 46, 1220–1226 (2014). Article CAS PubMed Google Scholar * Lake, L., Li, Y., Casal, J. J.
& Sadras, V. O. Negative association between chickpea response to competition and crop yield: Phenotypic and genetic analysis. _Field Crops Res._ 196, 409–417 (2016). Article Google
Scholar * Sadras, V. O., Lake, L., Li, Y., Farquharson, E. A. & Sutton, T. Phenotypic plasticity and its genetic regulation for yield, nitrogen fixation and δ13C in chickpea crops under
varying water regimes. _J. Exp. Bot._ 67, 4339–4351 (2016). Article CAS PubMed Google Scholar * Thudi, M. _et al._ Whole genome re-sequencing reveals genome-wide variations among
parental lines of 16 mapping populations in chickpea (_Cicer arietinum_ L.). _BMC Plant Biol._ 16, 53–64 (2016). Article Google Scholar * Li, Y. _et al._ Genome analysis identified novel
candidate genes for ascochyta blight resistance in chickpea using whole genome re-sequencing data. _Front. Plant Sci._ 8, 359 (2017). PubMed PubMed Central Google Scholar * Nei, M.
_Molecular Evolutionary Genetics_ (Columbia University Press, 1987). Book Google Scholar * Khajuria, Y. P. _et al._ Development and integration of genome-wide polymorphic microsatellite
markers onto a reference linkage map for constructing a high-density genetic map of chickpea. _PLoS ONE_ 10, e0125583 (2015). Article PubMed PubMed Central Google Scholar * Winter, P.
_et al._ A linkage map of the chickpea (Cicer arietinum L.) genome based on recombinant inbred lines from a _C. arietinum_ × _C. reticulatum_ cross: Localization of resistance genes for
fusarium wilt races 4 and 5. _Theor. Appl. Genet._ 101, 1155–1163 (2000). Article CAS Google Scholar * Gaur, R. _et al._ Advancing the STMS genomic resources for defining new locations on
the intraspecific genetic linkage map of chickpea (_Cicer arietinum_ L.). _BMC Genom._ 12, 117 (2011). Article CAS Google Scholar * Choudhary, P. _et al._ Genetic structure and diversity
analysis of the primary gene poolof chickpea using SSR markers. _Genet. Mol. Res._ 891–905 (2012). * Vadez, V. _et al._ Assessment of ICCV 2 x JG 62 chickpea progenies shows sensitivity of
reproduction to salt stress and reveals QTL for seed yield and yield components. _Mol. Breed._ 30, 9–21 (2012). Article Google Scholar * Wei, W. _et al._ Characterization of the sesame
(_Sesamum indicum_ L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. _BMC Genom._ 12, 1–13 (2011). Article Google Scholar * Yates, S. A. _et
al._ De novo assembly of red clover transcriptome based on RNA-Seq data provides insight into drought response, gene discovery and marker identification. _BMC Genomics_ 15, 1–15 (2014).
Article Google Scholar * Zhang, J. _et al._ De novo assembly and characterisation of the transcriptome during seed development, and generation of genic-SSR markers in peanut (_Arachis
hypogaea_ L.). _BMC Genom._ 13, 1–6 (2012). Article Google Scholar * Wang, Z. _et al._ De novo assembly and characterization of root transcriptome using Illumina paired-end sequencing and
development of cSSR markers in sweetpotato (_Ipomoea batatas_). _BMC Genom._ 11, 1–14 (2010). Article Google Scholar * Suresh, S. _et al._ Development and molecular characterization of 55
novel polymorphic cDNA-SSR markers in faba bean (_Vicia faba_ L.) using 454 pyrosequencing. _Molecules_ 18, 1844–1856 (2013). Article CAS PubMed PubMed Central Google Scholar * Verma,
P., Shah, N. & Bhatia, S. Development of an expressed gene catalogue and molecular markers from the de novo assembly of short sequence reads of the lentil (_Lens culinaris_ M edik.)
transcriptome. _Plant Biotechnol. J._ 11, 894–905 (2013). Article CAS PubMed Google Scholar * Varshney, R. K. _et al._ A comprehensive resource of drought-and salinity-responsive ESTs
for gene discovery and marker development in chickpea (_Cicer arietinum_ L.). _BMC Genom._ 10, 1–18 (2009). Article Google Scholar * Hiremath, P. J. _et al._ Large-scale transcriptome
analysis in chickpea (_Cicer arietinum_ L.), an orphan legume crop of the semi-arid tropics of Asia and Africa. _Plant Biotechnol. J._ 9, 922–931 (2011). Article CAS PubMed PubMed Central
Google Scholar * Singh, R., Singhal, V. & Randhawa, G. J. Molecular analysis of chickpea (_Cicer arietinum_ L) cultivars using AFLP and STMS markers. _J. Plant Biochem. Biotechnol._
17, 167–171 (2008). Article CAS Google Scholar * Choudhary, S., Sethy, N. K., Shokeen, B. & Bhatia, S. Development of sequence-tagged microsatellite site markers for chickpea (_Cicer
arietinum_ L.). _Mol. Ecol. Notes_ 6, 93–95 (2006). Article CAS Google Scholar * Sefera, T., Abebie, B., Gaur, P. M., Assefa, K. & Varshney, R. K. Characterisation and genetic
diversity analysis of selected chickpea cultivars of nine countries using simple sequence repeat (SSR) markers. _Crop Pasture Sci._ 62, 177–187 (2011). Article Google Scholar * Fayaz, H.
_et al._ Assessment of molecular genetic diversity of 384 chickpea genotypes and development of core set of 192 genotypes for chickpea improvement programs. _Genet. Resour. Crop Evol._ 69,
1193–1205 (2022). Article CAS Google Scholar * Keneni, G. _et al._ Genetic diversity and population structure of Ethiopian chickpea (_Cicer arietinum_ L.) germplasm accessions from
different geographical origins as revealed by microsatellite markers. _Plant Mol. Biol. Rep._ 30, 654–665 (2012). Article Google Scholar * Mohan, S. & Kalaimagal, T. Genetic diversity
studies in chickpea (_Cicer arietinum_ L.) genotypes using SSR markers. _J. Food Legum._ 33, 1–5 (2020). Google Scholar * Upadhyaya, H. D. _et al._ Genetic structure, diversity, and allelic
richness in composite collection and reference set in chickpea (_Cicer arietinum_ L.). _BMC Plant Biol._ 8, 1–12 (2008). Article Google Scholar * Hajibarat, Z., Saidi, A., Hajibarat, Z.
& Talebi, R. Genetic diversity and population structure analysis of landrace and improved chickpea (_Cicer arietinum_) genotypes using morphological and microsatellite markers. _Environ.
Exp. Biol._ 12, 161–166 (2014). Google Scholar * Farahani, S. _et al._ Whole genome diversity, population structure, and linkage disequilibrium analysis of chickpea (_Cicer arietinum_ L.)
genotypes using genome-wide DArTseq-based SNP markers. _Genes_ 10, 676 (2019). Article CAS PubMed PubMed Central Google Scholar * Suthar, K. P. _et al._ Genetic diversity assessment in
chickpea genotypes using STMS. _Legume Res. Int. J._ 35, 285–293 (2012). Google Scholar * Kumar, A. _et al._ Identification of highly polymorphic molecular markers and potential genotypes
for harnessing chickpea breeding strategies. _Legume Res. Int. J._ 45, 804–814 (2022). Google Scholar * Seyedimoradi, H., Talebi, R., Kanouni, H., Naji, A. M. & Karami, E. Genetic
diversity and population structure analysis of chickpea (_Cicer arietinum_ L.) advanced breeding lines using whole-genome DArTseq-generated SilicoDArT markers. _Braz. J. Bot._ 43, 541–549
(2020). Article Google Scholar * Botstein, D., White, R. L., Skolnick, M. & Davis, R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms.
_Am. J. Hum. Genet._ 32, 314–331 (1980). CAS PubMed PubMed Central Google Scholar * Meszaros, K. _et al._ Efficiency of different marker systems for genotype fingerprinting and for
genetic diversity studies in barley (_Hordeum vulgare_ L.). _S. Afr. J. Bot._ 73, 43–48 (2007). Article CAS Google Scholar * Mir, A. H. _et al._ SSR markers in revealing extent of genetic
diversity and phylogenetic relationships among chickpea core collection accessions for Western Himalayas. _Mol. Biol. Rep._ 49, 11469–11479 (2022). Article CAS PubMed Google Scholar *
Sari, D. _et al. _Intraspecific versus interspecific crosses for superior progeny in _Cicer_ species. _Crop Sci._ 2, 2122–2137 (2022). Article Google Scholar * Doyle, J. J. & Doyle, J.
L. A rapid total DNA preparation procedure for fresh plant tissue. _Focus_ 12, 13–15 (1990). Google Scholar * Girardot, C., Scholtalbers, J., Sauer, S., Su, S.-Y. & Furlong, E. E. Je,
a versatile suite to handle multiplexed NGS libraries with unique molecular identifiers. _BMC Bioinform._ 17, 1–6 (2016). Article Google Scholar * Chen, S., Zhou, Y., Chen, Y. & Gu, J.
fastp: an ultra-fast all-in-one FASTQ preprocessor. _Bioinformatics_ 34, i884–i890 (2018). Article PubMed PubMed Central Google Scholar * Kizil, S. _et al._ Genome-wide discovery of
InDel markers in sesame (_Sesamum indicum_ L.) using ddRADSeq. _Plants_ 9, 1262 (2020). Article CAS PubMed PubMed Central Google Scholar * Langmead, B. & Salzberg, S. L. Fast
gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar * Garrison, E. & Marth, G. Haplotype-based variant detection
from short-read sequencing. Preprint at http://arxiv.org/abs/1207.3907 (2012). * Koressaar, T. & Remm, M. Enhancements and modifications of primer design program Primer3.
_Bioinformatics_ 23, 1289–1291 (2007). Article CAS PubMed Google Scholar * Untergasser, A. _et al._ Primer3Plus, an enhanced web interface to Primer3. _Nucleic Acids Res._ 35, W71–W74
(2007). Article PubMed PubMed Central Google Scholar * Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. _Nat. Biotechnol._ 18, 233–234 (2000). Article CAS
PubMed Google Scholar * Peakall, R. O. D. & Smouse, P. E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. _Mol. Ecol. Notes_ 6, 288–295
(2006). Article Google Scholar * Perrier, X. & Jacquemoud-Collet J. P. DARwin Software, accessed 23 May 2023. http://darwin.cirad.fr/darwin. * Sokal, R. R. & Michener, C. D. A
statistical method for evaluating systematic relationships. _Univ. Kans. Sci. Bull._ 28, 1409–1438 (1958). Google Scholar * Park, S. D. E. Trypanotolerance in West African cattle and the
population genetic effects of selection. Ph.D. Thesis University Dublin (2001). Download references ACKNOWLEDGEMENTS This study was produced PhD thesis of the first author, DS. Authors are
also grateful to the anonymous reviewers for their thoughtful input on earlier versions of this manuscript. FUNDING Authors are thankful to the funding provided by Akdeniz University
Scientific Research Project Coordination Unit with the project no: FDK-2019-4122. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Field Crops, Faculty of Agriculture, Akdeniz
University, 07070, Antalya, Turkey Duygu Sari, Hatice Sari & Cengiz Toker * Department of Plant Protection, Faculty of Agriculture, Akdeniz University, 07070, Antalya, Turkey Cengiz
Ikten Authors * Duygu Sari View author publications You can also search for this author inPubMed Google Scholar * Hatice Sari View author publications You can also search for this author
inPubMed Google Scholar * Cengiz Ikten View author publications You can also search for this author inPubMed Google Scholar * Cengiz Toker View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS C.T. and D.S. designed the research and methodology. D.S. and H.S. conducted laboratory studies and C.I. analyzed the sequence data. C.T.
and D.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Duygu Sari. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise
in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the
permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sari, D., Sari, H., Ikten, C. _et al._ Genome-wide discovery of di-nucleotide SSR markers based on whole genome re-sequencing data of _Cicer
arietinum_ L. and _Cicer reticulatum_ Ladiz. _Sci Rep_ 13, 10351 (2023). https://doi.org/10.1038/s41598-023-37268-w Download citation * Received: 19 January 2023 * Accepted: 19 June 2023 *
Published: 26 June 2023 * DOI: https://doi.org/10.1038/s41598-023-37268-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
