Therapeutic vaccination following early antiretroviral therapy elicits highly functional t cell responses against conserved hiv-1 regions
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT ‘Kick and kill’ cure strategies aim to induce HIV protein expression in latently infected cells (kick), and thus trigger their elimination by cytolytic T cells (kill). In the
Research in Viral Eradication of HIV Reservoirs trial (NCT02336074), people diagnosed with primary HIV infection received immediate antiretroviral therapy (ART) and were randomised 24 weeks
later to either a latency-reversing agent, vorinostat, together with ChAdV63.HIVconsv and MVA.HIVconsv vaccines, or ART alone. This intervention conferred no reduction in HIV-1 reservoir
size over ART alone, despite boosting virus-specific CD4+ and CD8+ T cells. The effects of the intervention were examined at the cellular level in the two trial arms using unbiased
computational analysis of polyfunctional scores. This showed that the frequency and polyfunctionality of virus-specific CD4+ and CD8+ T cell populations were significantly increased over 12
weeks post-vaccination, compared to the ART-only arm. HIV-specific IL-2-secreting CD8+ T cells also expanded significantly in the intervention arm and were correlated with antiviral activity
against heterologous HIV in vitro. Therapeutic vaccination during ART commenced in primary infection can induce functional T cell responses that are phenotypically similar to those of HIV
controllers. Analytical therapy interruption may help determine their ability to control HIV in vivo. SIMILAR CONTENT BEING VIEWED BY OTHERS SARS COV-2 MRNA VACCINATION EXPOSES LATENT HIV TO
NEF-SPECIFIC CD8+ T-CELLS Article Open access 19 August 2022 IMMUNOGENICITY OF 2 THERAPEUTIC MOSAIC HIV-1 VACCINE STRATEGIES IN INDIVIDUALS WITH HIV-1 ON ANTIRETROVIRAL THERAPY Article Open
access 23 May 2024 DYNAMICS AND DURABILITY OF HIV-1 NEUTRALIZATION ARE DETERMINED BY VIRAL REPLICATION Article Open access 13 November 2023 INTRODUCTION Antiretroviral therapy (ART) has
dramatically improved survival for people living with HIV (PLWH) but alone is insufficient to cure HIV-1 (hereafter referred to as HIV) infection. Additional interventions are needed to
eliminate long-lived CD4+ cells harbouring quiescent replication-competent proviruses, which are responsible for virological relapse if ART is interrupted1. HIV-specific T cell responses
curtail primary viremia and modulate the provirus landscape during ART, therefore, therapeutic vaccination has been extensively explored as a strategy to amplify these responses, with the
aim of purging reservoirs2,3. However, few therapeutic vaccine regimens have provided clinical benefit, likely reflecting both limitations in the design of HIV immunogens and underlying
immunological impairment. Initiation of ART during primary HIV infection (PHI) mitigates immune damage, restricts the formation of reservoirs and increases the potential to achieve
post-treatment control (PTC)4,5. Rationally designed immunogens have therefore been tested in the context of early ART, either alone or to deliver a ‘kick and kill’, a strategy in which a
latency-reversing agent is used to ‘kick’ HIV antigen expression, with vaccine-induced CD8+ T cells delivering the ‘kill’. Results have been mixed. Chimpanzee adenovirus (ChAdV63) and
modified vaccinia Ankara (MVA) vectors encoding an HIV conserved immunogen comprising the 14 most conserved sub-protein regions of HIV (ChAdV63.HIVconsv + MVA.HIVconsv) were administered
together with romidepsin, a histone deacetylase inhibitor (HDACi) in the BCN01/02 studies6,7,8. Nearly a quarter of participants showed PTC for 32 weeks during an analytical therapy
interruption (ATI). Furthermore, vaccination with a ChAdV63- / MVA-vectored immunogen targeting regions of vulnerability within the HIV proteome was associated with PTC for up to 22 weeks in
a subset of participants9. By contrast, vaccination with human adenovirus type 26 (Ad26) and MVA vectors encoding HIV mosaic immunogens did not lead to control of viremia after ATI10. In
the Research in Viral Eradication of HIV Reservoirs (RIVER) trial, the first randomised placebo-controlled study of a kick and kill strategy, individuals who had initiated ART shortly after
a diagnosis of PHI and had received at least 24 weeks’ continuous therapy were randomly allocated to an intervention arm involving administration of ChAdV63.HIVconsv and MVA.HIVconsv
vaccines together with the HDACi, vorinostat (ART + V + V) or placebo (ART-only). The intervention did not reduce HIV DNA or RNA in CD4+ T cells, despite a significant amplification of
HIVconsv-specific CD4+ and CD8+ T cell responses11. These results led us to investigate the functionality of expanded HIV-specific T cells. RESULTS The RIVER study design has been described
previously and is summarised in Methods11. In brief, participants started ART within 1 month of a confirmed diagnosis of PHI and were treated for at least 24 weeks at the time of
randomisation. Provided that plasma HIV RNA was < 50 copies/ml, participants were randomised 1:1 to receive either ART + V + V or ART alone for a further 18 weeks. The ChAdV63.HIVconsv
vaccine (5 × 1010 vp) was administered on the day of randomisation and MVA.HIVconsv (2 × 108 pfu) at week 8 post-randomisation. Vorinostat (400 mg) was administered every 3 days during
post-randomisation weeks 0–4, up to a total of 10 doses. Sample availability permitting, HIVconsv-specific T cell responses were evaluated at the following time-points: enrolment (pre-ART
and close to the diagnosis of PHI if enrolled with a prospective diagnosis, ie.stratum 1 participants only, see Methods), randomisation (ie. pre-vaccination), week 9 and week 12
post-randomisation (ie. 1 week and 4 weeks after MVA.HIVconsv vaccination, and denoted PR-W9 and PR-W12 respectively). The baseline characteristics of the participants who were included in
this analysis are summarised in Table 1. POLYFUNCTIONALITY OF HIV-SPECIFIC CD4+ T CELL RESPONSES IS INCREASED FOLLOWING THERAPEUTIC VACCINATION We have previously reported that participants
in the ART + V + V arm showed a significant increase in the frequency of HIVconsv-specific CD4+ T cells that co-expressed CD154, a marker of T cell activation, and IFN-γ (median 15.1-fold;
0.0064 to 0.097% CD4+ T cells) from randomisation to week 9 post-randomisation (PR-W9)11. To further investigate the functional phenotype of the responding cells, we first analysed the
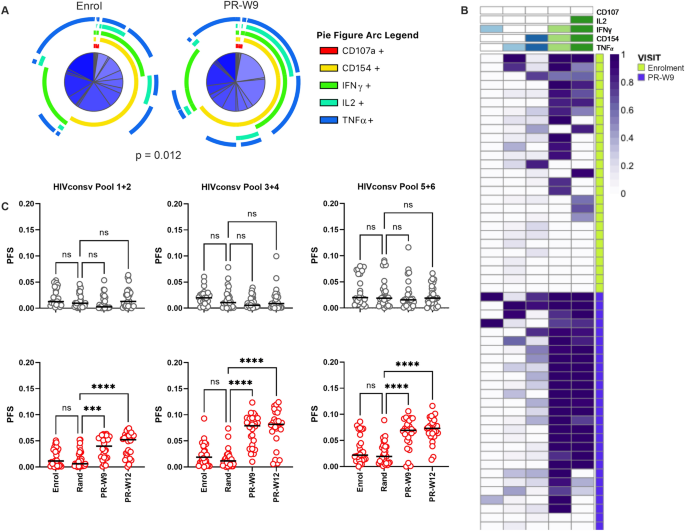
HIVconsv-specific T cell populations for expression of combinations of CD107a, CD154, IFN-γ, TNF-α and IL-2, at enrolment and at PR-W9. This enabled a comparison of T cell populations that
were primed by HIV infection with those that were induced or boosted by therapeutic vaccination. Simplified Presentation of Incredibly Complex Evaluations (SPICE) analysis showed a
significant difference in the responding phenotypes at these two time-points (Permutation test, _p_ = 0.012; Fig. 1A). The largest difference was seen in CD4+ T cells displaying the CD154+
/IFN-γ + /IL2+ /TNF-α + phenotype, which were significantly expanded at PR-W9 (Wilcoxon rank sum test, _p_ = 0.001, Supplementary Figs. 1 and 2). While ART-only participants also had
detectable HIVconsv-specific CD4+ T cells at enrolment, their phenotype as determined by SPICE was unchanged at PR-W9 (data not shown)11. Polyfunctionality of HIVconsv-specific T cells
responding was next assessed using COMPASS, a Bayesian hierarchical framework algorithm that allows the definition of a functional score (FS) and polyfunctionality score (PFS); the latter
provides summary of functionality after weighting according to the individual functionalities and relative dominance of the sub-populations of cells within the defined parental (CD8+ or CD4
+) responder population12. The posterior probabilities of antigen-specific responses across all functional subsets were assessed for each arm of the study at all visits up to PR-W12.
Responses were resolved to antigenic sub-regions within the HIVconsv immunogen using pools of overlapping peptides. PFS were significantly higher post-randomisation in the ART + V + V arm
for each peptide pool tested (repeated measures mixed effects model with Sidak’s multiple comparison test; Fig. 1B and C). This was due primarily to higher frequencies of 3- or 4-functional
CD4+ cells, chiefly expressing CD154, IFN-γ, TNF-α and IL-2 (Supplementary Fig. 2). By contrast, PFS did not change significantly over time in the ART-only arm (Fig. 1C). POLYFUNCTIONALITY
OF HIV-SPECIFIC CD8+ T CELL RESPONSES IS INCREASED FOLLOWING THERAPEUTIC VACCINATION The majority of participants in both study arms had detectable HIV-specific CD107+ IFN-γ + CD8+ T cell
responses at enrolment or randomisation, as shown by Fidler et al.11; these were also amplified in the ART + V + V arm following vaccination, with a > threefold increase in median
frequency between randomisation and PR-W9 (0.0518–0.194% CD8+ T cells). To determine whether vaccine-expanded responses were qualitatively different from those primed by HIV, we assessed the
functional phenotype of CD8+ T cells using the same methodology as for CD4+ T cells. Nine weeks post-randomisation, the overall CD8+ T cell response phenotype had changed significantly in
the ART + V + V arm (PR-W9 vs. enrolment; Permutation test, _p_ = 0.005; Fig. 2A). Vaccinated individuals showed an increase in the frequency of several sub-populations, most of which
co-expressed CD107a and IFN-γ in combination with either of TNF-α and IL2, with the increase in the 4-functional CD107a+ /IFN-γ+ /IL2+ /TNF-α + population being most significant (Wilcoxon
Rank Sum test, _p_ = 0.0001; Supplementary Figs. 3 and 4). These changes were corroborated by COMPASS analysis of PFS (Fig. 2B). The differences between pre- and post-randomisation visits
were strongest for responses to Pools 1 + 2 and 5 + 6, with ART + V + V recipients showing an increased proportion of CD107a+ /IFN-γ + CD8+ T cells that co-expressed IL-2, while ART-only
recipients showed no change in the responses to Pools 1–4 and a slight decrease in responses to Pool 5 + 6 (Fig. 2B and C). We excluded the possibility that ART + V + V recipients had higher
frequencies of HIVconsv-specific CD8+ IFN-γ + TNF-α + cells prior to randomisation (they were marginally higher in the ART-only arm than the ART + V + V arm at enrolment; Wilcoxon Rank sum
test, _p_ = 0.045) or more polyfunctional CD8 + T cells at this time-point (Permutation test for overall phenotype difference between study arms, _p_ = 0.6; Supplementary Fig. 5).
POLYFUNCTIONALITY OF CD4+ AND CD8+ SUBSETS OF HIVCONSV-SPECIFIC T CELLS PRE-AND POST-RANDOMISATION ARE STRONGLY CORRELATED Next, a possible correlation between PFS for HIVconsv-specific CD4+
and CD8+ T cells was explored. ART-only and ART + V + V participants formed distinct clusters after randomisation, consistent with an increase in PFS in the latter group (Fig. 3). The PFS
for total HIVconsv-specific CD4+ and CD8+ T cells were positively correlated at randomisation, PR-W9 and PR-W12 but not at enrolment, with the strongest association being observed at PR-W9
(r = 0.59, _p_ < 0.0001; Fig. 3). Of note, median CD4/CD8 ratio was > 1 from randomisation onwards but < 1 at screening (Table 1). CD8+ T CELL ANTIVIRAL ACTIVITY IN VACCINEES IS
POSITIVELY ASSOCIATED WITH THE FREQUENCY OF HIVCONSV-SPECIFIC CD8+ T CELLS EXPRESSING IL-2 The primary analysis of the RIVER trial showed that CD8+ T cell antiviral activity, as indicated by
the capacity to eliminate HIV-infected CD4+ T cells in vitro, waned over time in ART-only recipients yet was preserved in ART + V + V recipients at PR-W9 and PR-W1211. Several possible
explanations were considered. Certain HLA class I alleles have been previously been associated with potent antiviral activity (HLA-B*27:05, HLA-B*57:01 and B*58:01)13,14. RIVER participants
were not significantly enriched for these alleles in the ART + V + V arm (Fisher’s exact test, _p_ = 0.7)15. Furthermore, antiviral activity was not associated with superior
polyfunctionality scores per se, as defined by the PFS of aggregated HIVconsv-specific CD4+ and CD8+ T cell populations, at pre- or post-randomisation time points in either study arm (Data
available on request). On the other hand, HIVconsv-specific 3- or 4-functional CD8+ T cells that expressed IL-2 were significantly elevated post-randomisation in vaccinees and were found to
be positively correlated with infected cell elimination (r = 0.41, _p_ = 0.045; Fig. 4A). To rule out the possibility that antiviral activity was affected by preferential expansion of
specific effector cell subsets in the ART + V + V arm, or progression to a state of exhaustion in the ART-only arm, we characterised HIVconsv-specific CD8+ T cells post-randomisation
according to several markers of antigen experience (Fig. 4B and C; Supplementary Fig. 6). Despite the difference in frequency of HIVconsv-specific CD8+ T cells between the two study arms,
effector memory cells (CD45RA- CCR7-) were the dominant sub-population in both. Terminally differentiated effectors (CD45RA+ CCR7- CD57+/-) were preferentially expanded in ART-only
participants whereas the proportion of central memory cells (CD45RA- CCR7+) was significantly greater in ART + V + V participants. The groups did not differ with respect to the EOMES / T-bet
ratio nor the expression of PD-1 or TIGIT in HIVconsv-specific CD8+ T cells. DISCUSSION Polyfunctional virus-specific CD4+ and CD8+ T cells that secrete IL-2 and are capable of lysing
HIV-infected cells in vitro are typically detected in HIV controllers and rarely observed in chronic HIV infection, even after prolonged ART14,16,17,18. This study shows that therapeutic
vaccination in people with HIV who receive very early ART can elicit HIV-specific T cell responses with a similar phenotype to those observed in HIV controllers, exemplified by sustained
cytokine-secreting and cytolytic functions and by the establishment of a central memory pool19. The positive association between PFS for CD4+ and CD8+ T cells from randomisation through
PR-W12 indicates that control of viremia facilitates the maintenance of CD4+ and CD8+ T cell functionality to a similar extent. Moreover, the increased CD4+ and CD8+ T cell PFS to HIVconsv
Pool 1 + 2, which covers Gag sequences within HIVconsv, suggest that Gag-specific T cell functionality was enhanced by vaccination. We have shown previously that CD8+ T cell targeting of
functionally constrained regions within Gag proteins is a major driver of CD8+ T cell antiviral activity14,20. We surmise, therefore, that the superior CD8+ T cell antiviral effect in the
ART + V + V arm was due to a combination of re-focusing, ie. higher frequencies of functional Gag-specific CD8+ T cells, and of HIV-specific IL-2-secreting CD4+ and CD8+ T cells, since IL-2
is essential for HIV-specific T cell proliferation and survival21,22. Taken together, the results presented here indicate that a fully functional immune response to conserved viral epitopes
was elicited by the vaccination strategy. Other factors must therefore underlie the apparent lack of impact of the study intervention on viral reservoirs in RIVER trial participants. For
example, the reservoir in early treated PLWH is very low to begin with and therefore to achieve any further statistically significant decrease is extremely challenging4,23. It has been noted
previously that vorinostat administration had minimal effect on viral reactivation in this study, therefore, it is possible that cellular reservoirs were not sensitised to cytolytic T cells
following vaccination or not accessed by the vaccine-elicited T cell effectors. In contrast to the BCN01/02 studies, which evaluated the ChAdV63.HIVconsv and MVA.HIVconsv vaccines together
with the HDACi, romidepsin in a similar protocol, the RIVER trial did not include an ATI, therefore, we do not know whether the responses induced in this study would have been sufficient to
control recrudescent virus and delay rebound. PTC has hitherto been observed infrequently after therapeutic vaccination. Colby et al. observed a delay of < 1 week after administration of
HIV mosaic immunogens in a prime-boost strategy, and it is noteworthy that the vaccination regimen in this study enhanced the functionality of Pol- and Env-, but not Gag-specific T cells,
possibly as a consequence of antigenic competition and lack of re-focusing towards vulnerable regions of the viral proteome10,24. We and others have shown previously that viral load
set-point after PHI is strongly influenced by CD8+ T cell responses to the most functionally constrained regions within the HIV proteome, the majority of which are found within Gag
proteins14,25. This may explain the longer period of PTC observed following vaccination strategies targeted to such regions9. Nevertheless, the lack of sustained PTC observed with most
therapeutic vaccines tested to date raises the question of whether vaccine-induced or boosted T cells traffic to or access tissue reservoirs, including sanctuary sites such as B cell
follicles, and whether anamnestic responses elicited by these vaccines may be too slow to eliminate infected cells immediately upon viral reactivation8,26,27. If so, then alternative
approaches will be needed for effective targeting of viral reservoirs. Access to B cell follicles may require treatment with IL-15 or an IL-15 superagonist28,29. Adoptive T cell therapies or
T cell redirecting agents such as bispecific T cell engagers may be better able to overcome the temporal and spatial barriers that limit the effectiveness of vaccine-boosted HIV-specific T
cells30. Accumulating evidence supports initiation of ART as early as possible after HIV acquisition, in order to prevent seeding of immune-privileged sites and acute inflammation, which
drives the destruction of lymphoid architecture, early loss of CD4+ T cell help and progressive T cell dysfunction31,32,33. Our data provide evidence to suggest that early ART enables the
generation of highly functional virus-specific T cells; however, this cannot be definitely established due to the lack of a comparator arm comprising vaccinees who initiated ART during the
chronic phase of infection. Nevertheless, early ART may not only increase the potential for immune-based therapies to achieve PTC but may also ensure that PLWH can be adequately protected by
vaccination against diverse infectious pathogens. This has been highlighted recently by Frater et al. who showed that ART-experienced people living with HIV were capable of mounting
functional humoral and cellular responses to SARS-CoV-2 vaccination that were equivalent to those in people without HIV34. METHODS STUDY APPROVAL All individuals gave written informed
consent to participate in the RIVER study (NCT02336074), a phase 2, openlabel randomised controlled trial that was conducted at 6 clinical sites in the UK during December 2015 to November
2017. Approval for the study was obtained from the South Central—Oxford A Research Ethics Committee, UK (reference: 14/SC/1372) from all participating centres in accordance with the
principles of the Declaration of Helsinki. STUDY PARTICIPANTS Participants aged 18–60 years who had acquired HIV infection within the preceding ≤ 6 months and had started ART within 1 month
from confirmed PHI diagnosis were enrolled in the RIVER trial (NCT02336074) if they fulfilled pre-specified recent infection criteria. Eligible consenting participants were recruited either
to stratum 1 (ART initiation at enrolment and randomisation 24 weeks later) or stratum 2 (previously initiated on ART within 4 weeks of a PHI diagnosis, with ART duration of at least 6
months and up to 2 years after PHI). Sixty men were randomly assigned to receive ART + V + V or ART alone. Full details, including baseline characteristics for the entire study population,
have been reported previously11. CD4+ cell counts, CD4/CD8 ratio and plasma viral load were measured at screening, randomisation and post-randomisation week 8, day 3 (PR-W8 + 3). These were
the closest time-points to those visits when the HIVconsv-specific T cell analyses were performed, therefore, the evaluated participants’ results at these time-points are shown in Table 1.
Peripheral blood mononuclear cells (PBMC) were obtained from the RIVER study participants and cryopreserved immediately. For each participant, cryopreserved PBMC from all study visits were
thawed and analysed simultaneously. For the analyses described here, samples from the randomisation visit were available in 27 participants in the ART-only arm, and 30 participants in the
ART + V + V arm. Samples were missing from other time-points in 2–3 participants in the ART-only arm and 2–4 participants in the ART + V + V arm. INTRACELLULAR CYTOKINE STAINING (ICS)
Cryopreserved PBMC were thawed, washed and rested overnight in RPMI medium supplemented with 10% fetal calf serum (FCS), 1% (v/v) penicillin/streptomycin and 2mM L-glutamine (R10 medium) at
37 °C, then stimulated with peptide pools (15-mers with 11aa overlap) corresponding to the HIVconsv vaccine transgene; 2 μg/ml), mock control (0.45% DMSO) and positive controls (SEB, 5
μg/ml; CMV pp65, 2 μg/ml, NIH AIDS Reagent Repository) at 37 °C for 6 h in the presence of Golgiplug, Golgistop (BD Biosciences) and CD107a BV42135. Following viability and surface staining,
cells were fixed using BD cytofix/cytoperm solution according to the manufacturer's instructions and intracellularly stained for multiparameter flow cytometric analysis using reagents
as listed in Supplementary Table 1. At least 10,000 viable singlet CD3+ CD4+ and CD8+ lymphocyte events were acquired using a BD Fortessa X20 cytometer. MULTIPARAMETER FLOW CYTOMETRY
ANALYSIS Initial analysis was performed using FlowJo v9.9.7. T cell functionality was analysed using COMPASS12. The polyfunctional score (PFS) for HIVconsv-specific responses was assessed
using COMbinatorial Polyfunctionality analysis of Antigen-Specific T-cell Subsets, an open-sourced platform employing Bayesian hierarchical frameworks to model observed functional cell
subsets and select those most likely to be antigen-specific responses12. The PFS weighs the different responding subsets by their degree of functionality and the posterior probabilities were
reported in both CD4+ and CD8+ T cell subsets expressing combinations of IFN-γ, IL2, TNF-α, CD107a and CD154. Analysis of cell surface phenotype and transcriptional profile was performed
using PESTLE and SPICE36. Individuals with HIVconsv-specific T cell CD8+ responses (> 10 IFN-γ + CD107a + events within the CD3+ CD8+ population) were selected for further analysis using
a panel of antibodies to the following: CCR7, CD45RA, CD57, PD-1, TIGIT, Eomesodermin (EOMES) and T-bet (Supplementary Table 1). CD8+ T CELL INFECTED CELL ELIMINATION ASSAY CD8+ T cell
antiviral activity was assessed in an infected cell elimination assay, as previously described11. Briefly, cryopreserved PBMC were thawed and expanded using anti-human CD3 (OKT3) antibody at
100ng/ml (BD Pharmingen) and IL-2 at 100 IU/ml (Proleukin) for 7 days. CD4+ and CD8+ populations were subsequently purified by positive selection with immunomagnetic beads (MACS, Miltenyi
Biotec) according to the manufacturer’s instructions. CD4+ T cells were super-infected with HIV IIIB (National Institute for Biological Standards and Control, United Kingdom) by
spinoculation at a multiplicity of infection (MOI) of 0.01 and cultured alone or together with autologous CD8+ T cells at CD4:CD8 ratios of 1:1 or 10:1 for 7 days. On day 7, cells from
replicate wells were pooled and stained for viability, CD3, CD4, CD8 and intracellular Gag p2437. Samples were acquired on a Fortessa X20 cytometer (BD) and analysed by FlowJo v9.9.6. Assay
acceptance required acquisition of at least 2000 viable CD3+ /CD8- lymphocyte events. Reduction in p24 + cells was expressed as percent infected cell elimination and determined as follows:
[(fraction of p24 + cells in CD4 + T cells cultured alone) − (fraction of p24 + in CD4+ T cells cultured with CD8+ cells)]/(fraction of p24 + cells in CD4+ T cells cultured alone) × 100.
STATISTICAL ANALYSIS HIVconsv peptide pool-specific PFS in T cell populations was assessed longitudinally using repeated measures mixed effects model with Sidak’s test for multiple
comparisons. Correlations were assessed using simple linear regression analysis. SPICE 6 was used to determine statistically significant differences in T cell phenotypes using permutation
tests, t tests and Wilcoxon rank sum tests. All other statistical analyses were performed using GraphPad Prism v9.1.2. DATA AVAILABILITY The data supporting the results in this manuscript
are provided within the main figures and supplementary material or, in the case of polyfunctional scores for aggregated T cell populations, will be made available on request. Please contact:
[email protected]. REFERENCES * Chun, T. W. _et al._ Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. _Proc. Natl. Acad. Sci. U.
S. A._ 94, 13193–13197 (1997). Article ADS CAS PubMed PubMed Central Google Scholar * Pitman, M. C., Lau, J. S. Y., McMahon, J. H. & Lewin, S. R. Barriers and strategies to achieve
a cure for HIV. _Lancet HIV_ 5, e317–e328 (2018). Article PubMed PubMed Central Google Scholar * Warren, J. A. _et al._ The HIV-1 latent reservoir is largely sensitive to circulating T
cells. _Elife_ 9, e57246 (2020). Article CAS PubMed PubMed Central Google Scholar * Ananworanich, J. _et al._ HIV DNA set point is rapidly established in acute HIV infection and
dramatically reduced by early ART. _EBioMedicine_ 11, 68–72 (2016). Article PubMed PubMed Central Google Scholar * Namazi, G. _et al._ The control of HIV after antiretroviral medication
pause (CHAMP) study: Posttreatment controllers identified from 14 clinical studies. _J. Infect. Dis._ 218, 1954–1963 (2018). Article PubMed PubMed Central Google Scholar * Letourneau, S.
_et al._ Design and pre-clinical evaluation of a universal HIV-1 vaccine. _PLoS One_ 2, e984 (2007). Article ADS PubMed PubMed Central Google Scholar * Mothe, B. _et al._ Therapeutic
vaccination refocuses T-cell responses towards conserved regions of HIV-1 in early treated individuals (BCN 01 study). _EClinicalMedicine_ 11, 65–80 (2019). Article PubMed PubMed Central
Google Scholar * Mothe, B. _et al._ HIVconsv vaccines and romidepsin in early-treated HIV-1-infected individuals: Safety, immunogenicity and effect on the viral reservoir (Study BCN02).
_Front. Immunol._ 11, 823 (2020). Article CAS PubMed PubMed Central Google Scholar * Bailón, L. _et al._ Safety, immunogenicity and effect on viral rebound of HTI vaccines in early
treated HIV-1 infection: A randomized, placebo-controlled phase 1 trial. _Nat. Med._ https://doi.org/10.1038/s41591-022-02060-2 (2022). Article PubMed Google Scholar * Colby, D. J. _et
al._ Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. _Nat. Med._ 26, 498–501 (2020). Article
CAS PubMed Google Scholar * Fidler, S. _et al._ Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in
participants with recent HIV infection (the RIVER trial): A phase 2, randomised trial. _Lancet (London, England)_ 395, 888–898 (2020). Article CAS PubMed Google Scholar * Lin, L. _et
al._ COMPASS identifies T-cell subsets correlated with clinical outcomes. _Nat. Biotechnol._ 33, 610–616 (2015). Article CAS PubMed PubMed Central Google Scholar * Saez-Cirion, A. _et
al._ HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. _Proc. Natl. Acad. Sci. U. S. A._ 104,
6776–6781 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Hancock, G. _et al._ Identification of effective subdominant anti-HIV-1 CD8+ T cells within entire
post-infection and post-vaccination immune responses. _PLoS Pathog._ 11, e1004658 (2015). Article CAS PubMed PubMed Central Google Scholar * Migueles, S. A. _et al._ Trivalent
adenovirus type 5 HIV recombinant vaccine primes for modest cytotoxic capacity that is greatest in humans with protective HLA class I alleles. _PLoS Pathog._ 7, e1002002 (2011). Article CAS
PubMed PubMed Central Google Scholar * Emu, B. _et al._ HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such
responses are not always necessary for long-term virus control. _J. Virol._ 82, 5398–5407 (2008). Article CAS PubMed PubMed Central Google Scholar * Betts, M. R. _et al._ HIV
nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. _Blood_ 107, 4781–4789 (2006). Article CAS PubMed PubMed Central Google Scholar * Migueles, S. A. _et
al._ Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. _J. Virol._ 83, 11876–11889
(2009). Article CAS PubMed PubMed Central Google Scholar * Burgers, W. A. _et al._ Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral
set point. _J. Immunol._ 182, 4751–4761 (2009). Article CAS PubMed Google Scholar * Yang, H. _et al._ Incoming HIV virion-derived Gag spacer peptide 2 (p1) is a target of effective
CD8(+) T cell antiviral responses. _Cell Rep._ 35, 109103 (2021). Article CAS PubMed Google Scholar * Younes, S. A. _et al._ HIV-1 viremia prevents the establishment of interleukin
2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. _J. Exp. Med._ 198, 1909–1922 (2003). Article CAS PubMed PubMed Central Google Scholar * Trautmann, L.
_et al._ Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. _Blood_ 120, 3466–3477 (2012). Article CAS
PubMed PubMed Central Google Scholar * Leyre, L. _et al._ Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. _Sci.
Transl. Med._ 12, eaav3491 (2020). Article CAS PubMed PubMed Central Google Scholar * Kallas, E. G. _et al._ Antigenic competition in CD4(+) T cell responses in a randomized,
multicenter, double-blind clinical HIV vaccine trial. _Sci. Transl. Med._ 11(519), eaaw1673 (2019). Article CAS PubMed PubMed Central Google Scholar * Mothe, B. _et al._ Definition of
the viral targets of protective HIV-1-specific T cell responses. _J. Transl. Med._ 9, 208 (2011). Article CAS PubMed PubMed Central Google Scholar * Banga, R. _et al._ PD-1(+) and
follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. _Nat. Med._ 22, 754–761 (2016). Article CAS PubMed Google Scholar * Hansen,
S. G. _et al._ Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. _Nature_ 473, 523–527 (2011). Article ADS CAS PubMed PubMed Central Google Scholar
* Watson, D. C. _et al._ Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. _PLoS Pathog._ 14, e1006902 (2018). Article PubMed
PubMed Central Google Scholar * Webb, G. M. _et al._ The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. _Blood Adv._ 2, 76–84 (2018). Article
CAS PubMed PubMed Central Google Scholar * Yang, H., Wallace, Z. & Dorrell, L. Therapeutic targeting of HIV reservoirs: How to give T cells a new direction. _Front. Immunol._ 9, 2861
(2018). Article CAS PubMed PubMed Central Google Scholar * Macías, J. _et al._ Structural normalization of the lymphoid tissue in asymptomatic HIV-infected patients after 48 weeks of
potent antiretroviral therapy. _AIDS_ 15, 2371–2378 (2001). Article PubMed Google Scholar * Schuetz, A. _et al._ Initiation of ART during early acute HIV infection preserves mucosal Th17
function and reverses HIV-related immune activation. _PLoS Pathog._ 10, e1004543 (2014). Article PubMed PubMed Central Google Scholar * Jain, V. _et al._ Antiretroviral therapy initiated
within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. _J. Infect. Dis._ 208, 1202–1211 (2013). Article CAS PubMed PubMed Central
Google Scholar * Frater, J. _et al._ Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical
trial. _Lancet HIV_ https://doi.org/10.1016/S2352-3018(21)00103-X (2021). Article PubMed PubMed Central Google Scholar * Borthwick, N. _et al._ Vaccine-elicited human T cells recognizing
conserved protein regions inhibit HIV-1. _Mol. Ther._ 22, 464–475 (2014). Article CAS PubMed PubMed Central Google Scholar * Roederer, M., Nozzi, J. L. & Nason, M. C. SPICE:
Exploration and analysis of post-cytometric complex multivariate datasets. _Cytometry A_ 79, 167–174 (2011). Article PubMed PubMed Central Google Scholar * Yang, H. _et al._ Improved
quantification of HIV-1-infected CD4+ T cells using an optimised method of intracellular HIV-1 gag p24 antigen detection. _J. Immunol. Methods_ 391, 174–178 (2013). Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in part by UK National Institute for Health Research (NIHR) infrastructure through the National Institute for
Health Research (NIHR) Biomedical Research Centres based at University of Oxford, Imperial College Healthcare NHS Trust and Imperial College London, University of Cambridge and King’s
College London. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. GSK provided the ChAd63 vector used
in the RIVER study. We are grateful to Dr Greg Finak, Fred Hutchinson Cancer Research Centre, for assistance with coding. GlaxoSmithKline Biologicals SA was provided the opportunity to
review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Nuffield Department of Medicine, University of Oxford, Oxford, UK Jakub Kopycinski, Hongbing Yang, Gemma Hancock, Matthew Pace, Ellen Kim, John Frater, Tomás Hanke & Lucy Dorrell *
Medical Research Council Clinical Trials Unit, University College London, London, UK Wolfgang Stöhr * Joint Research Centre for Human Retrovirus Infection, Kumamoto University, Kumamoto,
Japan Tomás Hanke * Department of Infectious Disease, Imperial College London, and National Institute for Health Research Imperial Biomedical Research Centre, London, UK Sarah Fidler *
Immunocore Ltd, 93 Park Drive, Milton Park, Abingdon, OX14 4RY, Oxon, UK Lucy Dorrell Authors * Jakub Kopycinski View author publications You can also search for this author inPubMed Google
Scholar * Hongbing Yang View author publications You can also search for this author inPubMed Google Scholar * Gemma Hancock View author publications You can also search for this author
inPubMed Google Scholar * Matthew Pace View author publications You can also search for this author inPubMed Google Scholar * Ellen Kim View author publications You can also search for this
author inPubMed Google Scholar * John Frater View author publications You can also search for this author inPubMed Google Scholar * Wolfgang Stöhr View author publications You can also
search for this author inPubMed Google Scholar * Tomás Hanke View author publications You can also search for this author inPubMed Google Scholar * Sarah Fidler View author publications You
can also search for this author inPubMed Google Scholar * Lucy Dorrell View author publications You can also search for this author inPubMed Google Scholar CONSORTIA RIVER TRIAL STUDY GROUP
* Jakub Kopycinski * , Hongbing Yang * , Matthew Pace * , John Frater * , Tomás Hanke * , Lucy Dorrell * , Wolfgang Stöhr * & Sarah Fidler CONTRIBUTIONS J.K. and L.D. designed the
experimental plans for this sub-study of RIVER. J.K., H.Y., G.H., M.P. and E.K. conducted experiments. T.H. designed and provided the HIVconsv vaccines. J.F., W.S. and S.F. designed and
executed the RIVER study. J.K. and L.D. analysed data and wrote the manuscript. All authors provided critical review of the manuscript. CORRESPONDING AUTHOR Correspondence to Lucy Dorrell.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in
the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your
intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence,
visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kopycinski, J., Yang, H., Hancock, G. _et al._ Therapeutic vaccination
following early antiretroviral therapy elicits highly functional T cell responses against conserved HIV-1 regions. _Sci Rep_ 13, 17155 (2023). https://doi.org/10.1038/s41598-023-42888-3
Download citation * Received: 23 November 2022 * Accepted: 15 September 2023 * Published: 11 October 2023 * DOI: https://doi.org/10.1038/s41598-023-42888-3 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative
