Cross-sectional and longitudinal analyses of urinary extracellular vesicle mrna markers in urothelial bladder cancer patients
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT We designed this multi-center prospective study with the following objectives: (1) the cross-sectional validation of extracellular vesicles (EV) mRNA markers to detect urothelial
bladder cancer (UBC) before transurethral resection of bladder cancer (TURBT), and (2) the longitudinal validation of EV mRNA markers to monitor non-muscle invasive bladder cancer (NMIBC)
recurrence after TURBT. EV mRNA markers evaluated in this study were _KRT17_, _GPRC5A_, and _SLC2A1_ in addition to two additional markers from literatures, _MDK_ and _CXCR2_, and measured
by quantitative RT-PCR with normalization by a reference gene (_ALDOB_). Diagnostic performances of EV mRNA markers were compared to conventional markers. Regarding the first objective, we
confirmed that EV mRNA biomarkers in urine were higher in UBC patients, particularly those with higher stage/grade tumors, than in those without UBC (n = 278 in total) and the diagnostic
performance of EV mRNA _MDK_ and _KRT17_ outperformed conventional biomarkers with AUC 0.760 and 0.730, respectively. Concerning the second objective, we prospectively analyzed the time
courses of EV mRNA markers while NMIBC patients (n = 189) (median follow-up 19 months). The expression of EV mRNA _KRT17_ was significantly high in patients with recurrence, while it
gradually decreased over time in those without recurrence (p < 0.01). SIMILAR CONTENT BEING VIEWED BY OTHERS POTENTIAL OF MIRNAS IN URINARY EXTRACELLULAR VESICLES FOR MANAGEMENT OF ACTIVE
SURVEILLANCE IN PROSTATE CANCER PATIENTS Article Open access 22 November 2021 URINE CELL-FREE DNA MULTI-OMICS TO DETECT MRD AND PREDICT SURVIVAL IN BLADDER CANCER PATIENTS Article Open
access 19 January 2023 URINE BIOMARKERS IN BLADDER CANCER — CURRENT STATUS AND FUTURE PERSPECTIVES Article 24 May 2023 INTRODUCTION The National Cancer Institute Japan estimated about 23,000
new cases of bladder cancer in Japan in 20191 and the American Cancer Society estimated about 82,000 cases of bladder cancer in 20232. Bladder cancer is associated with subjective symptoms,
including frequent urination and gross hematuria, and is frequently detected by imaging techniques, such as cystoscopy, ultrasonography, and CT. Between 70 and 80% of all bladder cancer
patients are diagnosed with non-muscle invasive bladder cancer (NMIBC) and continue to receive surveillance following transurethral resection of bladder tumor (TURBT). Since the recurrence
and progression rate of NMIBC is as high as 50 to 70%, those with bladder cancer history require lifelong monitoring of recurrence, which makes bladder cancer the most expensive cancer from
diagnosis to treatment3. The gold standard surveillance method is cystoscopy, which is invasive and uncomfortable for patients. Urine cytology is a well-established non-invasive test due to
its high specificity; however, its low sensitivity does not effectively exclude the presence of bladder cancer. Under these circumstances, there have been several reports of new non-invasive
and sensitive tests using non-invasive biomarkers, but they are only used as an adjunctive diagnostic test to cystoscopy due to their low sensitivity and specificity4,5. Therefore, a
combination of several methods might be a key to improve and personalize the surveillance strategy for patients with NMIBC. Exosomes and microvesicles are membrane vesicles secreted at an
elevated level in cancer patients6. These extracellular vesicles (EV) are known to be involved with intercellular communication through transporting biologically active molecules including
mRNA, miRNA, DNA, and proteins between cells7. There are several studies using whole urine, urinary cells and exosomes for bladder cancer mRNA biomarkers5,8. One of the advantages to use EV
mRNA as a diagnostic marker is that much knowledge has already been obtained regarding the expression patterns of mRNA in cancer tissues, urinary system organs, and immune system cells
thanks to the recent multi-omics studies9,10. Therefore, when evaluating mRNA candidates in urinary EV as diagnostic markers, a selection process can be simple because there exists much data
regarding putative biological functions, pathways, and expression patterns in various organs. In addition, assay development for mRNA is much easier than microRNA and protein due to the
high sensitivity and specificity of real-time PCR method, which can greatly minimize a risk of non-specific amplification. In our previous cross-sectional study11, EV mRNA marker screening
was conducted using urine from the patients with bladder cancer, and three EV mRNA markers, _SLC2A1_, _KRT17_, and _GPRC5A,_ were identified and validated in a small single-center study. In
particular, these three EV mRNA markers were 29.5, 20.6, and 18.2 times more highly expressed in urine from urothelial cancer patients than those in healthy controls, respectively. In this
prospective multi-center study, urinary EV mRNA markers were longitudinally evaluated before and after TURBT. Using the samples obtained before TURBT, we performed cross-sectional validation
of the mRNA markers that we previously identified and analyzed11. Subsequently, the performance of these mRNA markers was validated as a predictor of tumor recurrence using longitudinal
clinical data and urine samples. In addition, the performance of these EV mRNA-based urine markers was compared to cytology and other approved urinary markers including bladder tumor antigen
(BTA), nuclear matrix protein 22 (NMP22) and UroVysion fluorescence in situ hybridization (FISH) assay12. We also developed the model for predicting tumor recurrence by combination of these
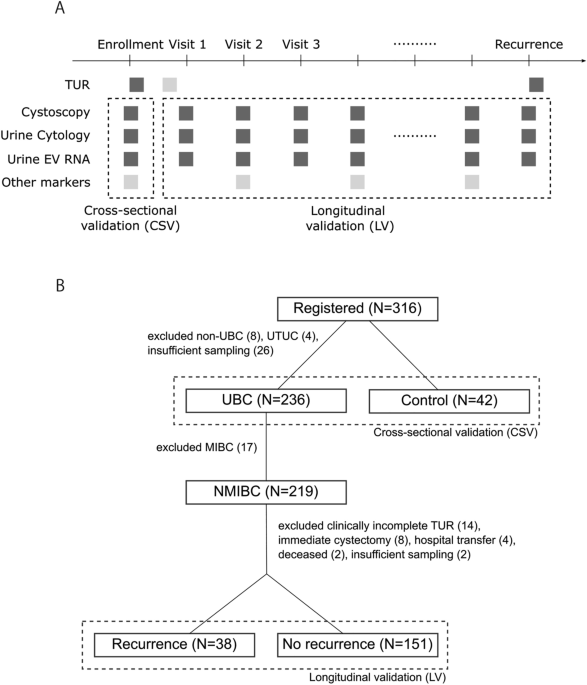
several methods. MATERIALS AND METHODS STUDY DESIGN, CLINICAL RECORD AND PATIENT ENROLLMENT We designed this multicenter prospective study with the following two objectives: (1)
cross-sectional validation (CSV) of the EV mRNA markers for urothelial bladder cancer (UBC) detection and (2) longitudinal validation (LV) of the EV mRNA markers for NMIBC recurrence
monitoring (Fig. 1A). To achieve these objectives, we registered patients at our institutes prior to their scheduled TUR surgeries and collected urine samples for marker validation (CSV
phase) (Fig. 1A). Once pathologically confirmed as their tumors are NMIBC (i.e., LGTa, HGTa, T1, any Tis by the NCCN risk categories), we enrolled and followed up these patients for
recurrence monitoring by the EV mRNA markers (LV phase) (Fig. 1A). After the initial TURBT, indications for a second TURBT, intravesical therapy (Bacillus Calmette–Guérin (BCG) or
intravesical chemotherapy (IVC)) after TURBT, and surveillance frequency until recurrence were based on each institution and urologist’s discretion according to the current clinical
guideline13. Patients’ clinical data were prospectively recorded by urologists using a clinical record form including height, weight, gender, smoking status, previous UBC and other cancer
history, clinical data (blood, urine, cystoscopy, urine cytology), pathology result, intravesical therapy information including a type of therapy (BCG and/or IVC), etc. The records were
maintained and updated at every outpatient visit through the study and its integrity was reviewed by board certified urologists. Urine samples were collected during office visits for
biomarker measurement and stored at −80 °C and shipped to our laboratory with dry ice unless otherwise noted. Bladder tumor was diagnosed by cystoscopy, urine cytology and pathological
diagnosis of resected tumors. Pathologic staging was reported according to the Union for International Cancer Control (UICC) staging system (7th edition)14. Tumor grade was determined by the
World Health Organization 2004 criteria15. The patients were categorized into four risk groups using the NCCN guideline: LGTa, HGTa, T1, and any Tis16. Urine cytology specimens were
evaluated by a cytopathologist in each institution following its standard operational procedure according to the Papanicolaou procedure17 or the Paris System18. Cytopathological findings
were divided into negative cytology, suspicious cytology, or positive cytology as follows: (1) Negative cytology: Classes I and II in the Papanicolaou procedure or “Negative for high-grade
urothelial carcinoma”, “Atypical urothelial cells” in the Paris System, (2) Suspicious cytology: Classes III and IV in the Papanicolaou procedure or “Suspicious for high-grade papillary
urothelial carcinoma” in the Paris System, and (3) Positive cytology: Class V in the Papanicolaou procedure or “Low-grade urothelial neoplasm” and “High-grade urothelial carcinoma” in the
Paris System. Microhematuria was defined as positive for greater than or equal to 5 red blood cells per high power field and negative for less than 5 cells. We registered 316 patients
including control (42 subjects) between October 2017 and November 2021 (Fig. 1B). Non-UBC (benign tumor) (N = 8) and upper tract urothelial carcinoma (UTUC) (N = 4) were excluded due to out
of study scope, and 26 UBC patients were excluded due to insufficient urine sampling, which resulted in 236 UBC patients. Following three groups of subjects were enrolled as control: (1)
healthy subjects who had no sign for bladder and other cancer through routine checkups (N = 11), (2) patients who had previous BC history but no recurrence at least for 17 months (median 4
years) (N = 17), and (3) patients who were suspected for UBC but later pathologically confirmed as benign tumors or no malignant disease (N = 14). In total, we enrolled 236 UBC patients and
42 control subjects and collected urine samples prior to TUR for the CSV phase. For the LV phase, after excluding 17 muscle invasive bladder cancer (MIBC) patients, 219 patients with NMIBC
were followed up for cancer recurrence. In addition, 30 patients were excluded due to clinically incomplete TUR (N = 14), immediate cystectomy (N = 8), hospital transfer (N = 4), deceased (N
= 2), and insufficient sampling (N = 2). As a result, we obtained clinical data and samples from 189 patients in total (38 cancer recurrence and 151 no recurrence cases) for the LV phase.
CENTRAL EUROPE COHORT For additional CSV, an independent validation cohort of 30 UBC patients was recruited prospectively in Central Europe between June 2021 and November 2021 through a
commercial biospecimen procurement service (Discovery Life Sciences, IRB approval no. DLS-BB044-V.1). Clinical data including pathology and urine cytology results and urine samples were
obtained as described above. EV MRNA ASSAY EV mRNA assay was performed as described previously11,19. Briefly, after being thawed, urine was centrifuged at 800xg for 15 min to remove cells
and large debris. Ten mL urine supernatant was used for EV isolation and mRNA extraction by an ExoComplete tube kit (Showa Denko Materials, Tokyo, Japan) following the manufacturer’s
protocol. cDNA was synthesized by qScript XLT cDNA SuperMix (Quantabio, MA, USA) with the following reaction protocol: 5 min at room temperature, followed by 1 h at 42 °C and 5 min at 85 °C.
Quantitative PCR (qPCR) was done by SsoAdvanced Universal SYBR Green Supermix (Bio-rad, CA, USA). Primer sequences used in this study is available in Supplementary Table 1. qPCR was
performed in a ViiA 7 Real-Time PCR System (Thermo Fisher Scientific, CA, USA) with the following protocol: 95 °C 10 min, followed by 40 cycles of 95 °C 30 s and 65 °C for 1 min and a
melting curve analysis. Threshold cycle values were obtained from the instrument software and normalized by that of reference gene (_ALDOB_) using the delta Ct method. FDA CLEARED BIOMARKER
ASSAYS UroVysion assay (Abbott, IL, USA) was performed by a commercial clinical laboratory (SRL, Tokyo, Japan) following its sample collection protocol: isolating urine sediments and
shipping to the laboratory at 4 °C. NMP22 ELISA kit (Abbott, IL, USA) and BTA stat kit (Polymedoco, NY, USA) were purchased and the measurement was conducted following the manufacturers’
protocols. DATA ANALYSIS Data analysis was conducted by R unless otherwise noted20. Kaplan–Meier survival curve analysis was conducted by R. Statistical significance was determined by a
log-rank test. Censored patients were indicated by crosses in the curves. ROC curve analysis including area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV)
and negative predicative value (NPV) calculation was done by pROC21. Optimum threshold for each marker was obtained at the nearest point of the ROC curve to the top-left corner in the CSV
phase and applied to the following analysis in the LV phase. EV mRNA expression and other markers were analyzed by Welch’s t-test. Time point comparisons for each group were analyzed with
mixed model repeat measure model by JMP Pro 16 (SAS Institute Inc., NC, USA). p value of < 0.05 was considered statistically significant. Graphs were prepared by ggplot222. STUDY REVIEW,
APPROVAL AND CONSENT TO PARTICIPATE This study was reviewed and approved by institution review boards at Hokkaido University Hospital (IRB approval no. 017-0036), Sapporo City General
Hospital (IRB approval no. R02-059–704), Hokkaido Cancer Center (IRB approval no. 017-0036), Teine Keijinkai Hospital (IRB approval no. 2-017135-01), and Sapporo Keiyukai Hospital (IRB
approval no. H29-26). Informed consent was obtained from all individual participants included in the study. All methods were performed in accordance with the relevant guidelines and
regulations. RESULTS PATIENT ENROLLMENT AND CLINICAL OUTCOMES UBC patients (N = 236) enrolled in this study include LGTa (27%), HGTa (29%), T1 (22%), any Tis (14%), and MIBC (7%) by the NCCN
risk categories and their clinical characteristics were summarized in Table 1. After our guideline-based surveillance during this study period (median follow up: 19 months), their 1-, 2-
and 3-year recurrence-free survival were 87.7%, 75.4% and 66.9%, respectively (Table 2), and the NCCN risk category, UBC history, tumor size, tumor number, sex, and smoking status were not
significant predictors for recurrence-free survival (Fig. 2A, B, Suppl Fig. 1A to 1D). The breakdown of initial TUR pathology and recurrent pathology are shown in Suppl Table 2.
CROSS-SECTIONAL MARKER VALIDATION For the first study objective, we aimed for a CSV of the EV mRNA markers we identified in our previous study11. Although the previous study cohort was
obtained in one of our institutes, the patients were recruited independently from the previous study. First, we investigated if the previously identified reference gene, _ALDOB_, is useful
to normalize the EV mRNA markers in urine in this study cohort. _ALDOB_ and other candidate reference genes including _GAPDH_ and _ACTB_ were analyzed by ANOVA in the CSV phase (Suppl Table
2). _ALDOB_ was not only one of the most highly expressed genes among the tested genes but also no change of its expression levels was observed among absence and presence of NMIBC/MIBC or
among the NCCN risk categories, which satisfy the requirements of a reference gene. On the other hand, _GAPDH_ and _ACTB_, were differentially expressed in urinary EV mRNA although their
expression levels in urine was high, therefore those conventional reference genes were not ideal for normalization in this urinary EV mRNA analysis. These data corroborated our previous
study result and re-confirmed that _ALDOB_ expression is high and stable in urine EV independent of bladder tumor status and can be used to normalize the EV mRNA marker expression levels as
a reference gene. Next, the EV mRNA marker expression, which was normalized by _ALDOB_, was analyzed in the CSV phase (Fig. 3). In this study, we focused on the three markers we identified
in our previous study, _KRT17_, _GPRC5A_ and _SLC2A1_11, in addition to the two additional markers from literatures, _MDK_ and _CXCR2_23. These EV mRNA markers were highly expressed in UBC
especially with higher stage/grade tumors such as T1, any Tis and MIBC compared to the control. In LGTa and HGTa, the EV mRNA expression especially _MDK_ and _KRT17_ was not as high as in
T1, any Tis and MIBC however it was statistically significant compared to that in the control (Fig. 3). The NCCN risk categories do not include risk factors used in the AUA, EUA or EORTC
risk categories, such as previous UBC history, tumor size and number. Therefore, the EV mRNA expression was further analyzed against these risk factors (Suppl Fig. 2). The EV mRNA expression
was higher in those without previous UBC history, with larger tumors or with larger number of tumors than their counterparts. The result with UBC history was counterintuitive however this
is because those without UBC history tend to have higher stage/grade tumors compared to those with UBC history who are under our surveillance for cancer recurrence. These data suggest that
the EV mRNA expression level was associated with UBC stage, grade, size and number. Diagnostic performance of these EV mRNA markers was estimated by ROC curve analysis and compared against
that of urine cytology, microhematuria, and FDA cleared biomarkers (BTA and NMP22) (Table 3). Among the biomarkers, _MDK_ showed the best AUC, 0.760, for the detection of UBC, followed by
_KRT17_ with AUC 0.730, and both EV mRNA markers outperformed conventional markers such as cytology (AUC 0.721), NMP22 (AUC 0.692), microhematuria (AUC 0.669) and BTA (AUC 0.659). MARKER
VALIDATION IN THE CENTRAL EUROPE COHORT To validate these EV mRNA markers further, we recruited a validation cohort of 30 subjects with prior UBC history in Central Europe. The patient
characteristics of this cohort was summarized in Suppl Table 2. This cohort consists of 12 UBC subjects with LGHa (25%), HGTa (25%), T1 (42%) and MIBC (8%) as well as 18 control subjects who
had previous UBC history and were at least six-month recurrence free. The EV mRNA markers as well as the other biomarkers were measured as described in the “Material and methods” section.
In the Central Europe cohort, the EV mRNA markers were highly expressed in UBC patients compared to the control subjects (Suppl Fig. 3), which support the above CSV phase results (Fig. 3) as
well as our previous study results5. In terms of marker performance, _MDK_ and _KRT17_ outperformed the other markers with AUC 0.824 and 0.736, respectively (Suppl Table 5), although
statistical significance was not obtained in differential gene expression analysis among the NCCN risk categories (Suppl Fig. 3). Additionally, it is notable that BTA performed better in
this cohort than in the CSV phase. Taken together, these two CSV suggested that the urinary EV mRNA markers, especially _KRT17_ and _MDK_, are promising biomarkers for the detection of UBC
and may be superior to traditional biomarkers such as cytology, microhematuria and other FDA-cleared biomarkers although further validation is required. LONGITUDINAL VALIDATION (LV) OF THE
EV MRNA MARKERS For the second study objective, we aimed for a LV of the EV mRNA markers in a surveillance setting after TURBT, assuming such markers will help earlier detection of cancer
recurrence and/or reduce a frequency of cystoscopy procedures eventually. In this LV phase, urine samples were collected for the EV mRNA marker measurement (median 5 samples per patient)
while the enrolled patients were under our guideline-based surveillance for cancer recurrence (Fig. 1A). Time courses of these marker expression in the LV phase were analyzed by Loess
trend-line analysis (Fig. 4). For those without cancer recurrence during the study period (N = 151, “No Rec”), the expression of the _KRT17_, _GPRC5A_, and _MDK_ remained similar to the
thresholds for UBC detection, which was predetermined in the CSV phase of the study (Table 3), almost for 12 months after the TURBT, and gradually decreased over time and went below the
thresholds only after 12–24 months. On the other hand, the time courses of _SLC2A1_ and _CXCR2_ indicated disappointing results indicating those markers may not be helpful to monitor the
cancer recurrence. _SLC2A1_ expression continuously increased over time. _CXCR2_ expression was at least fourfold higher than the threshold for UBC detection immediately after the TURBT and
decreased over time and required 30 months to go below the threshold. To understand why these EV mRNA markers were expressed at the levels near the best thresholds for UBC detection for more
than 12 months after TUR when tumors were supposedly eradicated, “No Rec” group was split into sub-groups by TUR pathology results, tumor size, tumor number or post-TUR therapies and time
courses of the EV mRNA expression was compared among the sub-groups (Suppl Fig. 4). Although overall trends are similar to each other, several distinct patterns were observed. For example,
_KRT17_ and _CXCR2_ expression levels were elevated in those who had more aggressive tumors during the first 12 months after TUR, i.e., LGTa < HGTa = any Tis < T1. Similarly, the EV
mRNA expression was elevated in those with larger or greater number of tumors than those with smaller or less (Suppl Fig. 4B, 4C). These observations are interesting because the EV mRNA
expression seems to be affected by tumor status even after tumors were eradicated, and more aggressive tumors leave the EV mRNA elevated even after TUR. Lastly, second TUR and intravesical
therapy, which are generally provided during the first 6 months after initial TUR, seem to increase the EV mRNA expression levels higher may be due to more aggressive tumors or intravesical
immune response, though it is inconclusive whether these therapies directly affect the expression levels (Suppl Fig. 4D). On the other hand, for those with cancer recurrence in the LV phase
(N = 38, “Rec”), _KRT17_ expression was significantly elevated compared to not only the best threshold for UBC detection but also the expression level in the “No Rec” throughout the study
period (p < 0.0001) (Fig. 4A). The difference of _KRT17_ expression between “Rec” and “No Rec” increased over time especially after about 12 months. In addition, _MDK_ was also expressed
significantly higher in the “Rec” group than in the “No Rec”, time to time (p = 0.0224). For comparison, time courses of the cytology and microhematuria results in categorical scores (0 for
negatives and 1 for positives) were analyzed (Fig. 4B). In the “No Rec” group, both cytology and microhematuria were close to score 0, or mostly negative, throughout the time, while BTA
results were close to score 0.5, a mixture of positives and negatives, during the first 6 months after TUR and gradually decreased to score 0 over time. In the “Rec” group, on the other
hand, cytology, microhematuria and BTA scores exceeded those in the “No Rec” group throughout the time. Diagnostic performance of the EV mRNA markers for cancer recurrence detection was
evaluated in the LV phase (Table 4A). _KRT17_ outperformed the other markers with AUC 0.653 and NPV 91.6%. Compared to the cytology (AUC 0.603 and NPV 86.6%), _KRT17_ could be a good
alternative and may be useful to rule out those with no risk of cancer recurrence because of the relatively high NPV. Other EV mRNA such as _CXCR2_ and _MDK_ performed better than cystoscopy
with AUC 0.628 and 0.606, respectively, while BTA and UroVysion were less promising with AUC 0.565 and 0.488, respectively. Since the EV mRNA expression remained close to the threshold
levels for 12–24 months after the TUR, diagnostic performance was evaluated in the LV phase excluding the first 6 or 12 months after the TUR (Table 4B, C). Diagnostic performance of _KRT17_
improved to AUC 0.667 and NPV 93.8% by excluding the first 6 months and AUC 0.700 and NPV 96.6% by excluding the first 12 months. Thus, _KRT17_ could be used to rule out those without cancer
recurrence more accurately especially after 6 to 12 months after TUR. To further improve the diagnostic performance, we employed a simple algorithmic analysis of _KRT17_ in combination with
cytology and/or microhematuria results. For _KRT17_, we assigned score 1 (positive) when its expression level is above the threshold determined in the CSV phase and score 0 (negative) when
the expression is below the threshold. Averaged scores of _KRT17_, cytology and/or microhematuria were analyzed, which represents the average number of positive markers out of each
combination (Fig. 4C). Diagnostic performance was improved to AUC 0.697, 0.697 and 0.718 by adding cytology and hematuria results to _KRT17_ although NPV wasn’t improved (Table 4). Lastly,
predictive value of _KRT17_ was investigated. First, _KRT17_ in urine obtained prior to TUR (CSV phase) was not predictive for the post-TUR recurrence-free survival (Fig. 5A). _KRT17_ during
the first 6 months after TUR (LV phase) was not predictive either (log rank p > 0.05), however at least those with _KRT17_ positives indicated slightly poor prognosis compared to those
with _KRT17_ negatives (Fig. 5B). On the other hand, the average score of _KRT17_, cytology and microhematuria during the first 6 months after TUR was clearly able to predict recurrence-free
survival (Fig. 5C) while each marker alone did not (Fig. 5B, Suppl Fig. 5A, B). Therefore, _KRT17_ in combination with other conventional biomarkers may have a predictive value of cancer
recurrence although further validations are necessary. DISCUSSION We designed this study with the two objectives: 1. CSV of the EV mRNA markers for UBC detection and 2. LV of the EV mRNA
markers for NMIBC recurrence monitoring. For the first objective, we were able to confirm the EV mRNA markers were highly expressed in urine from UBC patients especially with higher
stage/grade tumors compared to those without UBC in the CSV phase (N = 278 in total) and Central Europe cohorts (N = 30 in total) (Fig. 3, Suppl Fig. 1). We also confirmed that the EV mRNA
markers especially _MDK_ and _KRT17_ outperformed urine cytology and other conventional markers in both cohorts (Table 3, Suppl Table 4). Those data support the validity of the EV mRNA
markers for UBC detection. For the second objective, we investigated the time courses of the EV mRNA markers prospectively while NMIBC patients (N = 189) were under our surveillance in the
LV phase. One of the EV mRNA markers, _KRT17_, was confirmed to be expressed higher in those with cancer recurrence after TURBT until recurrence, while its expression gradually decreased
over a time for those without cancer recurrence during the study period (Fig. 4A). Indeed, NPV of _KRT17_ obtained in this study was 91.6%, 93.8% and 96.6% for > 0 month, > 6 months
and > 12 months after TURBT, respectively, thus it may be helpful to reduce surveillance frequency/duration using this marker although further validation studies are necessary (Table 4).
These data suggest that the EV mRNA markers especially, _KRT17_, is a promising biomarker for UBC detection and recurrence monitoring. It is intriguing that _KRT17_, and other mRNA
expression remained high for more than 12 months after TURBT in those with no recurrence despite tumors were eradicated by TURBT (Fig. 4A), which may hamper the use of these markers
immediately after TURBT. Our analysis indicated that those who had more aggressive tumors or those who received second TURBT tend to have high EV mRNA expression even after TURBT (Suppl Fig.
4). In those who had cancer recurrence, _KRT17_ expression was high already before cancer recurrence was detected by cystoscopy. Thus, the source of _KRT17_ and other markers to be further
investigated in the future study. On the other hand, these data suggest _KRT17_ may be clinically more useful for selected patient populations such as those with less aggressive tumors.
_KRT17_ has been reported to be overexpressed in many types of cancer including UBC, breast cancer, colon cancer, non-small lung cancer, cervical cancer, oral cancer, esophagus cancer,
pancreatic cancer, etc. _KRT17_ overexpression is associated with poor prognosis and cancer progression in non-small lung cancer24, colon cancer25 and other types of cancer26. To the
contrary, Wu et al. reported that _KRT17_ low expression in UBC is associated with poor overall and progression-free survivals by histochemistry analysis. Also, _KRT17_ is expressed higher
in less aggressive tumors than more aggressive ones27. In this study, EV _KRT17_ and cytology/microhematuria scores during the first 6 months after TURBT (LV phase) were able to predictive
recurrence-free survival (Fig. 5C) and at least those with high EV _KRT17_ expression indicated a relatively poor survival though statistical significance wasn’t obtained (Fig. 5B). The
discrepancy between the study by Wu et al.27 and this study may be due to the difference between tumor _KRT17_ and urinary EV _KRT17_. Babu et al.28 recently reported that KRT17 protein is
over expressed in urinary cells and tumors in bladder cancer patients and is a highly accurate biomarker for bladder cancer. These accumulating data support the validity of _KRT17_ as a
marker for bladder cancer. Achieving the good performance for predicting recurrence is challenging, because the recurrent lesions under the follow-ups are usually very small to discharge EVs
into urine. This is consistent with the result in which most of tumor size was less than 3 cm at the time of the recurrence. However, _KRT17_ can discriminate the tumor recurrence after 12
months after surgery. The discrimination ability was improved as the follow-up was prolonged. This discrimination ability might be due to the elimination of insignificant perioperative
effect. Additionally, we created a simple algorithm combining _KRT17_ and conventional biomarkers such as urine cytology and microhematuria. We chose these two markers because of the high
sensitivity of microscopic hematuria and the high specificity of urine cytology. In fact, this combination improved the diagnostic performance. Furthermore, with the recent advancement in
this field, recurrence monitoring could be further improved in combination with other biomarkers such as urinary cells and circulating tumor DNA. Urinary cells could be analyzed from whole
urine together with EV RNA, therefore new urinary cell RNA assay23 and KRT17 immunohistochemistry28 could be complementary to EV RNA assay though further clinical validation is necessary.
There are several limitations in this study. First, despite overall trend of the EV mRNA expression time course looks promising, the time course in each case looks noisy and difficult to
take actual clinical actions based on the biomarker data yet. In the future, larger scale and long-term validation studies are necessary. In addition, new laboratory technologies, such as
droplet digital PCR, which allow more accurate quantification, especially at very low concentrations, may overcome this problem, as mRNA expression levels in urinary EV are much lower than
in tissue. In addition, the predictors with significant differences in the other reports were not necessarily significant factors in our study because a portion of patients were excluded due
to radical cystectomy. Diagnostic performance was compared to UroVysion and NMP22, however sample sizes of these assays especially in the LV phase were limited (N = 46 and N = 12,
respectively). EV _ALDOB_ was selected for a reference gene to normalize EV mRNA marker expression levels because we confirmed EV _ALDOB_ level was stable in our studies. However, _ALDOB_ is
highly expressed in kidney therefore EV _ALDOB_ levels in urine may fluctuate depending on the patients’ kidney functions, which we were not able to investigate in this study because of
limited clinical data. Taken together, these data suggest that the EV mRNA markers especially, _KRT17_, is a promising biomarker for UBC detection and recurrence monitoring though further
validations especially in real-life clinical settings are necessary. DATA AVAILABILITY All data generated or analysed during this study are included in this published article and its
supplementary information files. ABBREVIATIONS * EV: Extracellular vesicles * TUR: Transurethral resection of bladder tumor * AUC: Area under the curve * UBC: Urothelial bladder cancer *
qPCR: Quantitative polymerase chain reaction REFERENCES * Cancer Information Service in Japan. https://ganjoho.jp/reg_stat/statistics/stat/cancer/21_bladder.html (2022). * American Cancer
Society, Key Statistics for Bladder Cancer. https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html (2022). * Botteman, M. F., Pashos, C. L., Redaelli, A., Laskin, B.
& Hauser, R. The health economics of bladder cancer: A comprehensive review of the published literature. _Pharmacoeconomics_ 21, 1315–1330 (2003). Article PubMed Google Scholar *
Lenis, A. T., Lec, P. M., Chamie, K. & Mshs, M. Bladder cancer: A review. _JAMA_ 324, 1980–1991. https://doi.org/10.1001/jama.2020.17598 (2020). Article CAS PubMed Google Scholar *
Ng, K., Stenzl, A., Sharma, A. & Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. _Urol. Oncol.: Semin. Orig. Investig._ 39,
41–51. https://doi.org/10.1016/j.urolonc.2020.08.016 (2021). Article CAS Google Scholar * Yang, J., Wei, F., Schafer, C. & Wong, D. T. W. Detection of tumor cell-specific mRNA and
protein in exosome-like microvesicles from blood and saliva. _PLOS ONE_ 9, e110641. https://doi.org/10.1371/journal.pone.0110641 (2014). Article ADS CAS PubMed PubMed Central Google
Scholar * Yu, S., Cao, H., Shen, B. & Feng, J. Tumor-derived exosomes in cancer progression and treatment failure. _Oncotarget_ 6, 37151–37168 (2015). Article PubMed PubMed Central
Google Scholar * Urabe, F. _et al._ Urinary extracellular vesicles: A rising star in bladder cancer management. _Transl. Androl. Urol._ 10, 1878–1889. https://doi.org/10.21037/tau-20-1039
(2021). Article PubMed PubMed Central Google Scholar * The Cancer Genome Atlas Program—NCI. https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (2022). *
_GTEx Portal_. https://gtexportal.org/home/ (2022). * Murakami, T. _et al._ Bladder cancer detection by urinary extracellular vesicle mRNA analysis. _Oncotarget_ 9, 32810–32821.
https://doi.org/10.18632/oncotarget.25998 (2018). Article PubMed PubMed Central Google Scholar * Hajdinjak, T. UroVysion FISH test for detecting urothelial cancers: Meta-analysis of
diagnostic accuracy and comparison with urinary cytology testing. _Urol. Oncol.: Semin. Orig. Investig._ 26, 646–651. https://doi.org/10.1016/j.urolonc.2007.06.002 (2008). Article CAS
Google Scholar * Matsumoto, H. _et al._ Clinical practice guidelines for bladder cancer 2019 update by the japanese urological association: Summary of the revision. _Int. J. Urol._ 27,
702–709. https://doi.org/10.1111/iju.14281 (2020). Article PubMed Google Scholar * Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: The 7th edition of the AJCC
cancer staging manual and the future of TNM. _Ann Surg Oncol_ 17, 1471–1474. https://doi.org/10.1245/s10434-010-0985-4 (2010). Article PubMed Google Scholar * Eble, J., Sauter, G.,
Epstein, J. I. & Sesterhenn, I. A. Classification of tumours: Pathology and genetics of tumours of the urinary system and male genital organs. _World Health Org. Classif. Tumours_ 2004,
255–257 (2004). Google Scholar * Flaig, T. W. _et al._ NCCN guidelines® insights: Bladder cancer, version 2.2022: Featured updates to the NCCN guidelines. _J. Natl. Comprehens. Cancer
Netw._ 20, 866–878. https://doi.org/10.6004/jnccn.2022.0041 (2022). Article Google Scholar * Papanicolaou, G. N. & Marshall, V. F. Urine sediment smears as a diagnostic procedure in
cancers of the urinary tract. _Science_ 101, 519–520. https://doi.org/10.1126/science.101.2629.519 (1945). Article ADS CAS PubMed Google Scholar * Barkan, G. A. _et al._ The paris
system for reporting urinary cytology: The quest to develop a standardized terminology. _Acta Cytol._ 60, 185–197. https://doi.org/10.1159/000446270 (2016). Article PubMed Google Scholar
* Murakami, T. _et al._ Development of glomerulus-, tubule-, and collecting duct-specific mRNA assay in human urinary exosomes and microvesicles. _PLoS ONE_ 9, e109074.
https://doi.org/10.1371/journal.pone.0109074 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Team, R. C. _R: A Language and Environment for Statistical Computing_.
https://www.r-project.org/ (2022). * Robin, X. _et al._ _pROC: Display and Analyze ROC Curves_. https://cran.r-project.org/web/packages/pROC/pROC.pdf (2021). * Wickham, H. _et al._ _ggplot2:
Create Elegant Data Visualisations Using the Grammar of Graphics_. https://search.r-project.org/CRAN/refmans/ggplot2/html/ggplot2-package.html (2022). * Breen, V. _et al._ A holistic
comparative analysis of diagnostic tests for urothelial carcinoma: A study of Cxbladder Detect, UroVysion® FISH, NMP22® and cytology based on imputation of multiple datasets. _BMC Med. Res.
Methodol._ 15, 145. https://doi.org/10.1186/s12874-015-0036-8 (2015). Article CAS Google Scholar * Wang, Z. _et al._ Overexpression of KRT17 promotes proliferation and invasion of
non-small cell lung cancer and indicates poor prognosis. _Cancer Manage. Res._ 11, 7485–7497. https://doi.org/10.2147/CMAR.S218926 (2019). Article CAS Google Scholar * Ujiie, D. _et al._
KRT17 as a prognostic biomarker for stage II colorectal cancer. _Carcinogenesis_ 41, 591–599. https://doi.org/10.1093/carcin/bgz192 (2020). Article CAS PubMed Google Scholar * Li, C. _et
al._ A pan-cancer analysis of the oncogenic role of Keratin 17 (KRT17) in human tumors. _Transl. Cancer Res._ 10, 4489–4501. https://doi.org/10.21037/tcr-21-2118 (2021). Article ADS CAS
PubMed PubMed Central Google Scholar * Wu, J. _et al._ Low expression of keratin17 is related to poor prognosis in bladder cancer. _OncoTargets Therapy_ 14, 577–587.
https://doi.org/10.2147/OTT.S287891 (2021). Article PubMed PubMed Central Google Scholar * Babu, S. _et al._ Keratin 17 is a sensitive and specific biomarker of urothelial neoplasia.
_Modern Pathol._ 32, 717–724. https://doi.org/10.1038/s41379-018-0177-5 (2019). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank for Dr. Hiroki Chiba (SGH), Dr.
Masaya Miyazaki (SGH), Dr. Kiyoto Nagamori (SGH), Dr. Hiroshi Tanaka (SGH), Dr. Satoshi Nagamori (HCC), Dr. Takuya Moriguchi (HCC), Dr. Chihiro Kamijo (HCC), Dr. Naoto Miyajima (TKH), Dr.
Toshiki Koyama (TKH), Dr. Kanta Hori (TKH), Dr. Kazushi Hirakawa (SKH) for collecting clinical data and samples and providing those to this study. We also thank for Ms. Yuka Tanaka (HUH) for
preparing and shipping the clinical samples. We thank for Discovery Life Sciences team to support this study by recruiting patients and organizing their clinical specimen and clinical data.
We thank for Showa Denko Material America co-workers, especially Ms. Mieko Ogura and Dr. Cindy Yamamoto for supplying the assay reagents and having thoughtful discussions and suggestions.
We also thank for Dr. Melanie Oakes, University of California Irvine and Dr. Masato Mitsuhashi, Nanosomix, for providing thoughtful suggestions/advice. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Research & Development, Showa Denko Materials (America), Inc., Irvine, CA, USA Taku Murakami * Departments of Kidney Transplant Surgery and Urology, Sapporo City General
Hospital, Sapporo, Japan Keita Minami & Hiroshi Harada * Department of Urology, Hokkaido Cancer Center, Sapporo, Japan Toru Harabayashi, Satoru Maruyama & Norikata Takada *
Department of Urology, Teine Keijinkai Hospital, Sapporo, Japan Akira Kashiwagi * Department of Urology, Sapporo Keiyukai Hospital, Sapporo, Japan Yasuyuki Sato * Department of Urology,
Hokkaido University Hospital, N15 W7 Kita-ku, Sapporo, 060-8638, Japan Haruka Miyata, Ryuji Matsumoto, Hiroshi Kikuchi, Takashige Abe, Sachiyo Murai, Nobuo Shinohara & Takahiro Osawa *
Clinical Research and Medical Innovation Center, Hokkaido University Hospital, Sapporo, Japan Yoichi M. Ito Authors * Taku Murakami View author publications You can also search for this
author inPubMed Google Scholar * Keita Minami View author publications You can also search for this author inPubMed Google Scholar * Toru Harabayashi View author publications You can also
search for this author inPubMed Google Scholar * Satoru Maruyama View author publications You can also search for this author inPubMed Google Scholar * Norikata Takada View author
publications You can also search for this author inPubMed Google Scholar * Akira Kashiwagi View author publications You can also search for this author inPubMed Google Scholar * Haruka
Miyata View author publications You can also search for this author inPubMed Google Scholar * Yasuyuki Sato View author publications You can also search for this author inPubMed Google
Scholar * Ryuji Matsumoto View author publications You can also search for this author inPubMed Google Scholar * Hiroshi Kikuchi View author publications You can also search for this author
inPubMed Google Scholar * Takashige Abe View author publications You can also search for this author inPubMed Google Scholar * Yoichi M. Ito View author publications You can also search for
this author inPubMed Google Scholar * Sachiyo Murai View author publications You can also search for this author inPubMed Google Scholar * Nobuo Shinohara View author publications You can
also search for this author inPubMed Google Scholar * Hiroshi Harada View author publications You can also search for this author inPubMed Google Scholar * Takahiro Osawa View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.M., T.O., and H.H. conceptualized and designed the study. T.O., S.M., K.M., T.H., A.K., and Y.S.
prepared and obtained IRB approval. T.O., H.H., T.H., N.T., K.M., A.K., H.M., Y.S., R.M., H.K., T.A., S.M., and N.S. recruited patients and collected clinical data and urine samples. TM
performed biomarker assays and analyzed study data. Y.I. advised and supervised statistical analyses. T.M. and T.O. discussed the data and wrote the manuscript. All the authors reviewed the
manuscript. CORRESPONDING AUTHOR Correspondence to Takahiro Osawa. ETHICS DECLARATIONS COMPETING INTERESTS The authors of this manuscript have the following conflicts of interest to
disclose. TM is an employee of Showa Denko Materials America, formerly known as Hitachi Chemical Co. America. The present study was funded by the Japan Society for the Promotion of Science
(KAKENHI, Grant Number 22K09439) and Showa Denko Materials America. Parts of this study were presented in American Urological Association annual meetings in 2019 and 2020. Parts of this
study were presented in American Urological Association annual meetings in 2019 and 2020. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1. SUPPLEMENTARY TABLE 2. SUPPLEMENTARY TABLE 3. SUPPLEMENTARY TABLE 4.
SUPPLEMENTARY TABLE 5. SUPPLEMENTARY FIGURE 1. SUPPLEMENTARY FIGURE 2. SUPPLEMENTARY FIGURE 3. SUPPLEMENTARY FIGURE 4. SUPPLEMENTARY FIGURE 5. RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material
in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Murakami, T., Minami, K., Harabayashi, T. _et
al._ Cross-sectional and longitudinal analyses of urinary extracellular vesicle mRNA markers in urothelial bladder cancer patients. _Sci Rep_ 14, 6801 (2024).
https://doi.org/10.1038/s41598-024-55251-x Download citation * Received: 22 October 2023 * Accepted: 21 February 2024 * Published: 21 March 2024 * DOI:
https://doi.org/10.1038/s41598-024-55251-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Non-muscle invasive bladder cancer * Cancer recurrence
* Biomarker * Extracellular vesicles * Exosome * mRNA
