Preparation and physicochemical characterization of hyaluronic acid-lysine nanogels containing serratiopeptidase to control biofilm formation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Remarkable resistance of bacterial biofilms to high doses of antimicrobials and antibiotics is one of their main challenges. Encapsulation of proteolytic enzymes is one of the
suggested strategies to tackle this problem. In this regard, the antibacterial and anti-biofilm activity of biocompatible hyaluronic acid- Lysine nanogels containing serratiopeptidase
(SRP-loaded HA-Lys nanogel) was assessed against _P. aeruginosa_ and _S. aureus_ strains_._ SRP-loaded HA-Lys nanogel was prepared using dropping method and optimized by Box-Behnken
experimental design. These formulations were studied for physical characterization, release profile, stability, bioactivity, and anti-biofilm effects. The particle size, polydispersity index
(PDI), and surface charge were measured by _Z_etasizer Nano ZS. The average particle size and zeta potential of the optimum sample were 156 nm and -14.1 mV, respectively. SRP release showed
an initial burst followed by sustained release and the highest release was around 77%. Enzyme biological activity data revealed the higher efficiency of free SRP compared to SRP-loaded
HA-Lys nanogel. The time-kill assay showed that both forms of SRP-loaded HA-Lys nanogel and blank HA-Lys nanogel showed significant antimicrobial activity against examined bacteria in
comparison to the free enzyme. The obtained results demonstrated improved anti-biofilm efficacy and down regulation of tested biofilm genes for both SRP-loaded HA-Lys nanogel 100% and blank
HA-Lys nanogel 100% compared to SRP 100%. SIMILAR CONTENT BEING VIEWED BY OTHERS ENHANCED ANTIBACTERIAL ACTIVITY OF POROUS CHITOSAN-BASED HYDROGELS CROSSLINKED WITH GELATIN AND METAL IONS
Article Open access 29 March 2024 HYBRID NANOCARRIERS WITH DIFFERENT DENSITIES OF SILVER NANOPARTICLES FORMATION FEATURES AND ANTIMICROBIAL PROPERTIES Article Open access 25 February 2025
PREPARATION AND KINETIC STUDIES OF A NEW ANTIBACTERIAL SODIUM ALGINATE GELATIN HYDROGEL COMPOSITE Article Open access 25 November 2024 INTRODUCTION Multicellular structures made up of
densely packed, extremely hydrated populations of microorganisms embedded in a matrix of self-synthesized proteinaceous or polymeric materials are known as bacterial biofilms1,2. Biofilms
can display biotic or abiotic connections to surfaces. They possess remarkable abilities such as strong resistance to both the innate and adaptive immune systems, along with the capacity to
tolerate high amounts of antibiotics and antimicrobial agents. As a result of such traits, bacterial biofilms can cause chronic infections, introducing them as a crucial health and financial
challenge3. It has been discovered that secreted and surface exposed proteins are essential for the development, stability, and control of biofilms1. Proteases were therefore proposed as a
possible anti-biofilm agents, a theory that was subsequently confirmed by research. Studies using pure proteases from various organisms have shown efficacy against biofilms, with
metalloproteases specifically proving to be highly influential in combating biofilm formation and growth4. Furthermore, the removal of biofilms has also been accomplished using commercial
proteases5. Serratiopeptidase (SRP), a commercially available bacterial metalloprotease, has shown effectiveness in treating a range of biofilm-associated medical disorders6. SRP not only
can modify the virulent phenotype of bacteria in biofilms but also it is effective against mature biofilms7, Furtheremore, it enhances the bactericidal effect of antibiotics against
biofilms8. SRP is a proteolytic enzyme with immense applications in therapeutic areas, which have been validated by several in vitro_, _in vivo, and clinical studies and through historical
evidence. Its multifaceted properties, including anti-inflammatory, anti-biofilm, analgesic, anti-edemic, and fibrinolytic activities, are responsible for these applications9. However,
anti-inflammatory enzymes might be preserved against proteolytic degradation and activity loss by different methods including loading them into novel delivery carriers. As a result, due to
SRPs multi potential effects, various drug delivery systems were developed in the last decade, and a large number of clinical and pre-clinical studies were performed on encapsulated SRP.
Nanocarriers are currently the first choice in drug delivery systems due to their various benefits10. In addition, increased drug stability is one of the most important properties of
nanocarriers for the delivery of proteins and polypeptides. Ionic complexes are one of the safest nanocarriers in clinical use due to their chemical reactions. It has been shown that
Hyaluronic acid (HA) and l-lysine (Lys) can self-assemble to form these ionic complexes due to their negative and positive charges, respectively11. HA is a glycosaminoglycan (GAG) which is a
natural biopolymer and extracellular matrix component composed of d-glucuronic acid and N-acetylglucosamine repeated groups12. The antibiofilm activity of HA has been studied in number of
investigations. Drago et al. have studied the anti-adhesive and anti-biofilm activity of HA towards bacterial species commonly isolated from respiratory infections. In this study,
antibiofilm activity was investigated by spectrophotometry after the biofilm was incubated with HA and stained by crystal violet6,13. Besides, Lys is an essential and water-soluble amino
acid that was first derived from casein hydrolysis. This amino acid presents in high concentrations in organisms, and its commercial form exists as Lys monohydrochloride14. In this study, we
showed the effectiveness of SRP loaded-HA-Lys nanogel on the biofilm removal. This work aims to create a special kind of gel using HA and Lys which contains SRP as a potent anti-biofilm
agent. The present study focused on evaluating the size, stability, and release mechanism of the HA gels containing SRP, as well as their capability to maintain the enzyme activity. The
anti-bacterial and anti-biofilm effects of SRP-loaded HA-Lys nanogel were assessed against _P. aeruginosa_ and _S.aureus_ strains. Moreover, the impact of SRP-loaded HA-Lys nanogel on the
expression level of certain biofilm genes was also examined. METHODS AND MATERIALS MATERIALS SRP was purchased from Advanced Enzyme Technologies Ltd. India; HA and Lys were from
Sigma-Aldrich, UK. Chloroform was obtained from Merck Company, Germany and Spectra/Por dialysis membrane (MWCO 100KDa) from Sigma-Aldrich, U.S.A. All materials and organic solvents used were
of analytical grade. _S. aureus_ ATCC 6538 and _P. aeruginosa_ ATCC 15,442 were obtained from the microbial bank of Pasteur Institute of Iran. This study was performed in the nanotechnology
department of Pasteur Institute of Iran. PREPARATION OF BLANK HA-LYS NANOGELS AND SRP LOADED HA-LYS NANOGELS SRP-loaded HA-Lys nanogels were prepared by the dropping method. In this method,
the enzyme was added dropwise to the Lys solution and the resulting solution was then added to the HA solution by the dropping method. All steps were done whyle stirring. For blank HA-Lys
nanogels, SRP was not added to Lys solusion. OPTIMIZATION OF SRP-LOADED HA-LYS NANOGEL BY EXPERIMENTAL DESIGN In order to determine the process’s effective components, this study employed
the d-Optimal Design technique, and 19 trials were carried out with Design Expert software (version 7.0.10, State-Ease, Inc., Minneapolis, MN). Three factors of polymer ratio (the ratio of
HA to Lys), drug concentration, and stirrer speed are selected as test variables and nanoparticle size (Z-average), polydispersity index (PDI), encapsulation efficiency (EE%), and
nanoparticle charge (Zeta Potential) are considered as test responses. Range of variables were selected based on information obtained from previous studies and initial screening tests. The
quantitative variables are shown in Table 1. Table 2 presents the proposed test matrices. PHYSICOCHEMICAL CHARACTERIZATION OF SRP-LOADED HA-LYS NANOGEL DETERMINATION OF PARTICLE SIZE AND
ZETA POTENTIAL _Z_etasizer Nano ZS (Malvern Instrument Ltd. Malvern, UK) was used to evaluate the mean particle size(Z-average), polydispersity index (PDI), and zeta potential of
nanoparticles at room temperature (RT). DETERMINATION OF SRP ENTRAPMENT EFFICIENCY The centrifugation method was used to test the SRP entrapment efficiency (EE) of nanogels indirectly.
Specifically, 1 ml of SRP-loaded HA-Lys nanogel solution was centrifuged at 14,000g for 45 min at 4 °C (Eppendorf 580R centrifuge, Germany). The free SRP concentration in the aqueous phase
was evaluated using the Bradford method. The EE% of SRP was calculated using the following equation : $$EE \left(\%\right)= \left[\frac{A-B}{A}\right]\times 100$$ where A is the initial SRP
concentration used to prepare the nanogel and B is the un-entrapped SRP concentration measured in the supernatant. PARTICLE SHAPE AND MORPHOLOGY With the use of field emission scanning
electron microscopy (FE-SEM, NOVA NANOSEM 450 FEI model), the morphology of the blank HA-Lys nanogel and SRP-loaded HA-Lys nanogel was determined. The samples were coated with a layer of
gold (100 Å) for 3 min under an argon atmosphere at a pressure of 0.2 atm. IN VITRO RELEASE STUDY The optimized SRP-loaded HA-Lys nanogel was subjected to release assay with a dialysis
membrane (MWCO 100 KDa). In summary, 2 ml of the chosen formulation were placed in a dialysis tube and left to float in a constantly agitated releasing medium that contained PBS (pH 7.2) at
37 °C. Then, 1 ml of the sample was taken at 0.5, 1, 2, 4, 6, 24, 48, and 72 h, and the Bradford method was used to measure the concentration of released SRP. The same volume of fresh PBS
was added to the release medium and the enzymes cumulative release was determined and plotted against the time. Ultimately, the total amount of medication discharg was calculated. Based on
zero-order, first-order, Korsmeyer Peppas, and Higuchi models, the SRP release profile was produced. By using linear regression, the best-fitting model for entrapped SRP was identified.
FOURIER-TRANSFORM INFRARED SPECTROSCOPY (FT-IR) To analyze the interaction between SRP and excipients, FTIR spectra of SRP, HA, Lys, SRP-loaded HA-Lys nanogel, and blank HA-Lys nanogel were
examined in KBr discussing a PerkinElmer FTIR spectrophotometer (spectrum Two, USA). FTIR measurements were carefully performed at ambient temperature in the scanning range of 4000 to 400
cm-1 at a constant resolution of 4 cm-1. STABILITY STUDY The stability of SRP-loaded HA-Lys nanogel was evaluated based on EE% and particle size measurements at 4 °C and 25 °C (RT) at
different time intervals for three months. SRP BIOLOGICAL ACTIVITY AND KINETICS The modified Lowry method was used to measure the enzymatic activity of SRP. This method identifies the phenol
group in the tyrosine root and has a sensitivity of 0–2 μg/ml of protein. Non-specific measurement of protease activity may be used as a standard method to determine the activity of SRP. In
this experiment, casein acts as a substrate for the protease. When the protease digests the casein, the amino acid tyrosine is released along with other amino acids and peptide fragments.
Next, the Folin-Ciocalteu reagent (FCR) reacts with the released tyrosine followed by the absorbance being read at 660 nm by a spectrophotometer.A higher level of protease activity is
indicated by the production of more chromophores when casein hydrolysis by the protease releases more tyrosine. The protease activity of the samples can be calculated in terms of an enzyme
equivalent to tyrosine released from casein hydrolysis per minute using the tyrosine standard curve15. TIME-KILL ASSAY Time-kill test helps to understand how well antimicrobial drugs work
against different types of germs. This test measures the efficacy of antimicrobial agents against various germs, depending on the quantity and duration of usage. The time-kill evaluation was
done for all treatments against _S. aureus_, and _P. aeruginosa_. For this, the mixture of bacteria and treatments including SRP 100%, SRP-loaded HA-Lys nanogel 50%, blank HA-Lys nanogel
100%, and SRP-loaded HA-Lys nanogel 100% were left to sit for 2, 4, 6, 24, 48, and 72 h at 37 °C. Then, the optical density (OD660) was measured at particular time intervals using a
microplate reader (Epoch, Japan). The bacteria growth pattern without any treatment was used as the positive control. IN VITRO ANTIBIOFILM ACTIVITY (MICROTITER PLATE TEST) Quantification of
in vitro anti-biofilm activity of free SRP and SRP-loaded HA-Lys nanogel was performed on two strains of _S. aureus_ and _P. aeruginosa_. Firstly, biofilm formation was performed by
culturing _S. aureus_, and _P. aeruginosa_ for 24 h in LB and Thioglycolate medium, respectively. The culture is diluted 1:100 in fresh suitable culture medium and incubated in a 96-well
plate for 4–24 h at 37 °C. Biofilm formation will be assessed using a microscopic method. Then selected standard bacteria were treated with sub-MIC (1/2 MIC) values of free SRP and
SRP-loaded HA-Lys nanogel, and the plates were washed with distilled water. After drying, the attached cells were stained with 1% crystal violet and washed with distilled water three times.
The attached dye in the wells was solubilized with 30% v/v glacial acetic acid, and optical density (OD) was recorded at 550 nm using the ELISA reader. REAL TIME Real-time PCR (Applied
Biosystems, Carlsbard, CA, USA) was used to assess the effect of treatments on gene expression. The expression level of _ndvB_ and _icaA_ genes were evaluated for _P. aeruginosa_ and _S.
aureus_, respectively The bacteria were exposed to sub-MIC concentration of SRP 100%, SRP-loaded HA-Lys nanogel 50%, blank HA-Lys nanogel 100%, and SRP-loaded HA-Lys nanogel 100% for 24 h.
Tubes containing cells were treated with a cold solution called RNX TM–PLUS. Then we mixed the tubes and left them at RT for 5 min before adding chloroform. The cells were kept on ice for 5
min and then centrifuged (4000 g, 4 °C, 15 min). The materials were transferred to RNase-free small tubes that contained the same amount of isopropanol. The mixture was centrifuged at 4 °C
for 5 min. The RNA pellet was mixed with ethanol and then treated with Diethyl pyrocarbonate (DEPC) in water. RNA was removed from all parts of the cell using a special kit (Qiagen, USA).
cDNA was synthesized from RNA by mixing reaction buffer, RNA, Enzyme-Mix, and water in specialized tubes. The mixture was kept in a warm place for 10 min at 25 °C and then for 60 min at 47
°C. The reaction was stopped by heating it at 85 °C for 5 min. Then, the mixture was put on ice until it was needed. We used a PCR kit from Bioneer, Korea to do PCR. 10 pmol of each primer
was utilized, then the mix was heated at 95 °C for 5 min. Following that, a cycle of heating at 95 °C for 1 min, 60 °C for 1 min, and 72 °C for 30 s was repeated for 34 cycles. Finally, we
heated the mix at 72 °C for 5 min. The PCR results were analyzed by comparing the CT of target genes (_ndvB_, and _icaA_), and 16S rRNA gene as control. The cells treated with nanoparticles
were examined for gene activity by contrasting their mRNA levels with untreated cells. The sequence of primers for the target genes is shown in Table 3. STATISTICAL ANALYSIS Statistical
analysis of d-optimal design was performed using Design-Expert 7.0.10 software (Stat-Ease Inc., U.S.A). Responses were analyzed using the ANOVA test. The _p-value_ of <0.05 was considered
as significant. ETHICS APPROVAL There are no “human subjects” in this study RESULTS OPTIMIZING THE PROCESS OF NANOPARTICLE MANUFACTURING The results obtained by a BOX-Behnken experimental
design investigating the interactions between three independent variables namely polymer ratio (A), drug concentration (B), and stirrer speed (C) at three levels are shown in Table 1 and 19
formulations of SRP-loaded HA-Lys nanogel were obtained as shown in Table2. EFFECT OF FORMULATION VARIABLES ON PARTICLE SIZE As can be seen in Table 2, SRP-loaded HA-Lys nanogel particles
have a size range of 134–266 nm. Table 3 lists the results of the variance analysis of particle size. Interestingly, particle size did not significantly differ from the best-fitting model
(_p-value_ > 0.05). Just the combination of drug concentration and stirrer speed (B AND C) has a significant effect on particle size. The _p-value_ for B and C was less than 0.05 (Table
4). The resulting equation in terms of coded value was as follows: $${\text{Particle}}\,{\text{Size }} = { 183}.{24} - {1}.{1}*{\text{A}} - {1}0.0{4}*{\text{B}} - {11}.{93}*{\text{C}} +
{15}.{26}*{\text{AB}} + {14}.{51}*{\text{AC}} + {26}.{34}*{\text{BC}}$$ (1) There is a converse relationship between polymer ratios, drug concentration, and stirrer speed with vesicle size.
While keeping other factors constant, the increament in polymer ratio leads to a decrease in the average vesicle size. Also, there is a reduction in the vesicle size after increasing the
drug concentration. Moreover, the particle size reduces with increasing the stirring time. At higher stirring speeds, formation of the smaller size emulsion droplets lead to a significant
decrease in particle size. EFFECT OF FORMULATION VARIABLES ON ENTRAPMENT EFFICIENCY The entrapment efficiency of the prepared SRP-loaded HA-Lys nanogel was found to vary between 66 and
95%.The effects of independent variables on the EE are shown in Table 4. The models for entrapment efficiency were considered to be significant since their p values were less than < 0.05.
Drug concentration (B) has a significant effect on entrapment efficiency (_p-value_ < 0.05). However, A and B have no significant effect on EE% (_p-value_ > 0.05). The interaction
terms of the EE are shown in the below equation: $${\text{EE}} = { 82}.{58} - {2}.{8}0*{\text{A}} + {6}.0{8}*{\text{B}} - {2}.{32}*{\text{C}}$$ (2) The data (see Table 1) revealed that drug
entrapment efficiency was dependent on the polymer concentration16. It can be justified for ionic interaction between the drug and polymer component. EFFECT OF FORMULATION VARIABLES ON ZETA
POTENTIAL Zeta potential is considered as the main impact factor for product stability. Based on Table 2, the zeta potential of prepared SRP-loaded HA-Lys nanogel ranges from − 2.9 to − 35.4
mV. The analysis of variance for zeta potential is listed in Table 4. The models for zeta potential were considered to be not significant since their p values were more than > 0.05. None
of the independent variables has a significant effect on zeta potential (_p-value_ > 0.05). The interaction term of the zeta potential have been shown in the below equation:
$${\text{Zeta}}\,{\text{Potential}} = - {12}.{16} - 0.{3}*{\text{A}} - {1}.{3}*{\text{B}} + {5}.{43}*{\text{C}}$$ (3) There is an indirect relationship between polymer ratio and drug
concentration on zeta potential value. While the relationship between stirrer speed and zeta potential was direct. According to Eq. (3), zeta potential turned to be more negative while the
amount of HA was increased. EFFECT OF FORMULATION VARIABLES ON POLYDISPERSITY The PDI of all nanoparticles was obtained by Box–Behnken, ranging from 0.266 to 0.558 (Table 2). The _p-values_
of PDI for all independent variables in Table 4 were more than 0.05. Accordingly, the HA/Lys ratio, drug concentration, and stirring speed have no significant effect on PDI (_p-value_ >
0.05). According to the regression, the polymer ratio and the amount of drug have a positive effect on PDI, while the stirring speed has a negative influence on PDI. The interaction terms of
the PDI have been shown in the below equation: $$PDI = 0.45 + 0.04*A + 0.012*B - 7.4E - 003*C + 0.012*AB + 4.5E - 003*AC + 0.021*BC - 0.041*A^{2} - 0.042*B^{2} - 0.031*C^{2}$$ DATA
OPTIMIZATION Suitable nanocarriers were designed after optimization of particle size, EE%, zeta potential, and PDI. The best desirability index (desirability = 0.920) was obtained when the
polymer ratio was 0.013 w/w%, the drug concentration was 0.018 g/mol, and the speed of stirring was 700 rpm (Table 5). A multi-criteria index was applied to optimize formulations. Narrow
particle size, minimum polydispersity, the zeta potential of about − 30 mV, and the maximum percentage of entrapment efficacy were predicted in optimal conditions. The obtained desirability
index was 0.92, which confirms the validity of optimization. The anticipated nanocarriers should have the following characteristics compromizing particle size, zeta potential, polydispersity
index, and entrapment efficacy calculated as 159.147 nm, − 18.590 mV, 0.283, and 93.779, respectively. According to Table 6, the validity of the central composite design was clear because
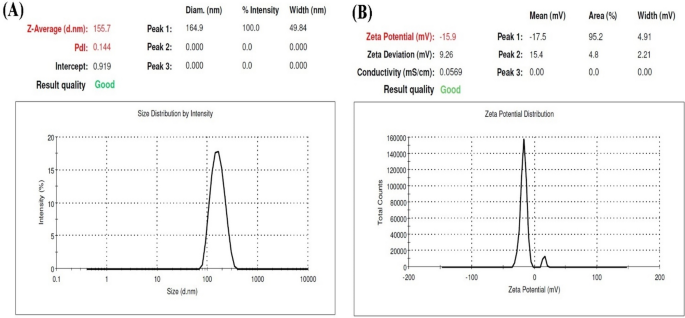
there was no significant difference between observed and predicted data for SRP-loaded HA-Lys nanogel. CHARACTERIZATION OF NANOPARTICLES PARTICLE SIZE AND SURFACE CHARGE The size and
dispersion of the particles and their morphology were evaluated by zeta sizer. As shown in Fig. 1, the average particle size and zeta potential of the optimum sample were 156 nm and − 14.1
mV, respectively. A negative value of zeta potential could be as a result of the presence of carboxyl groups on the nanoparticle surfaces, indicating good stability of formulations. The
stability of nanogels is affected by zeta potential, so the higher values of zeta potential results in enhanced stability as the weak electrostatic repulsive force between nanoparticles
inhibits aggregation. Moreover, a narrow particle size distribution (0.144) indicates a relatively homogenous dispersion. SCANNING ELECTRON MICROSCOPY (SEM) The optimized SRP-loaded HA-Lys
nanogel formulation was distinctly globular with smooth surfaces, according to SEM pictures (Fig. 2). The average size of the optimized formulation is less than 100 nm. ASSESSMENT OF NANOGEL
STRUCTURE BY FTIR Figure 3 shows blank HA-Lys nanogel's FTIR spectrum. Similar bands of raw minerals were displayed in the spectrum. The established HA structure included a large
stretching band of hydroxyl groups, two bands corresponding to carbonyl stretching bands of carboxylic acid amide, and ether bands. Nevertheless, blank HA-Lys nanogel was not allocated the
carboxylic acid of HA's carbonyl stretching band. However, Lys showed bands relating to N–H in-plane bending, C–N stretching of amine, two bands resulting from symmetric and asymmetric
CH2 stretching, a carbonyl stretching band of carboxylic acid, and bands corresponding to amine groups. The quaternary ammonium salt was identified as the source of a band at 2685 cm−1 in
the FTIR spectra of blank HA-Lys nanogel (Fig. 3). All ammonium salts exhibited a broad band between 2200 and 3300 cm−1, as has been covered in other articles, because of a mixture of NR4 +
group bands. Thus, these findings support the ionic interaction between the amine group of Lys and the carboxyl group of HA that was postulated for self-assembled blank HA-Lys nanogel17. The
published FTIR spectra in references were compared with the FTIR spectra of SRP in the SRP-loaded HA-Lys nanogel (Fig. 3). In light of all of the information provided, the FTIR spectrum of
the SRP-loaded HA-Lys nanogels (Fig. 3) revealed no appreciable shift in the enzyme peaks following the entrapment of SRP in the nanoparticles, indicating that there is no incompatibility,
either chemically or physically, between SRP and blank HA-Lys nanogels. RELEASE PROFILE The release of SRP through the optimized structure was evaluated at physiological pH (7.4) for 72 h.
As shown in Fig. 4, initially the burst release of free SRP was observed in first 8 h and were then released monotonously for the remaining 12, 20, and 24 h. The drug release of free SRP was
approximately 75% during the first 8 h, and then approximately 100% of the drug was released within 48 h. However, the release of SRP from HA nanogels was about 55% after 8 h then the
release was monotonous for the remaining 12, 20, and 24 h (Fig. 4). Finally, the release of the SRP reached nearly 77% after 72 h. STABILITY STUDIES The physical stability of SRP-loaded
HA-Lys nanogel was investigated by characterization of vesicle size, PDI, and EE% after storage at 4 °C and 25 °C for 90 days. As can be seen in Fig. 5, an increase in the percentage of size
and PDI and reduction in EE% was shown during storage time which could be due to the aggregation of nanoparticles and the leakage of the drug. Mean vesicle size, PDI, and EE% of nanogels
showed fewer changes after 90 days at 4 °C in contrast to 25 °C. This can be explained by the lower stability of blank HA-Lys nanogel at higher temperatures. ENZYME BIOLOGICAL ACTIVITY
Enzyme biological activity assay was carried out by protease activity, as described in method section, and the amount of SRP activity was calculated based on the calibration curve (Fig. 6).
There was a linear relationship between the concentration of enzyme and enzyme activity. This showed that the enzyme activity assay for the determination of enzyme concentrations during
formulation development was reliable and accurate. The most important factor in this study is the retention of the biological activity of the SRP after loading in blank HA-Lys nanogel.
Enzymatic activity was calculated according to the following formula (Eq. 4), and the activity of free enzyme and enzyme loaded in the nanogels was compared18.
$${\text{The}}\,{\text{amount}}\,{\text{of}}\,{\text{enzyme}}\,{\text{activity}} = \left( {{\text{T}} \times {\text{Vc}}} \right) \times \left( {{\text{D}} \times {\text{Ve}}} \right)/\left(
{{\text{B}} \times {\text{Vt}}} \right)$$ (4) where T is reaction time, Vc is calorimetric volume, D is dilution factor, Ve is volume of enzyme used, B is enzyme substrate concentration and
Vt is total volume $${\text{Enzyme}}\,{\text{activity}}\,{\text{of}}\,{\text{free}}\,{\text{SRP}} = \left( {{93}0.{7} \times {2}/0.{1} \times {1}0 \times {1}} \right) \times {2} =
{3722}.{8}$$ $${\text{Enzyme}}\,{\text{activity}}\,{\text{of}}\,{\text{nanogels}} = \left( {{463} \times {2}/0.{1} \times {1}0 \times {1}} \right) \times {2} = {1852}$$ The results obtained
from Eq. 4 show that the activity of SRP in blank HA-Lys nanogel was less than that of the free enzyme. TIME-KILL ASSAY The time-killing assay was done using different samples to assess
their effect against _P. aeruginosa_ and _S. aureus_ was tracked for 2 days (Fig. 7). The largest and most rapid decline against both examined bacteria was seen using SRP-loaded HA-Lys
nanogel 100%.This results might be due to association of HA to the bacterial cell membrane as an antimicrobial agent, making SRP to get into the cell more efficient. According to the Fig.
7A, B, applying the SRP-loaded HA-Lys nanogel 100% leaded to the lowest growth of both examined bacteria, which means it is the most efficient on bacteria’s growth compared to other groups.
Furthermore, it is evident that SRP 100% had minimal impact on the bacterial growth of tested strains. ANTIBIOFILM ACTIVITY ASSESSMENT Figures 8A, B show the obtained results of
microbiological studies. The biofilm formation rate of examined bacteria was calculated based on the following formula19: $${\text{Biofilm}}\,{\text{formation}}\,{\text{rate}} = \left(
{{\text{mean}}\,{\text{optical}}\,{\text{absorption}}\,{\text{of}}\,{\text{control}}/{\text{mean}}\,{\text{optical}}\,{\text{absorption}}\,{\text{of}}\,{\text{test}}} \right) \times {1}00$$
All samples showed considerable microbial activity against _P. aeruginosa_ and _S. aureus_ compared to the control groups (_p-value_ < 0.001). The results show that the concentration of
free SRP and blank HA-Lys nanogel has a converse relationship with bacterial biofilm formation. Increasing the concentration of enzymes and blank nanogels results in a decrease in the rate
of biofilm of bacteria. Interestingly, the rate of biofilm formation of nanogels with SRP/blank HA-Lys nanogel 50% was about 30%, while treating bacteria with blank HA-Lys nanogel 100% was
results less than 20%. REAL-TIME PCR To gain more insights into the effectiveness of HA-Lys nanogel on the formation of _P. aeruginosa_ and _S. aureous_ biofilms, the expression level of
specific genes was studied by real time PCR (Fig. 9A, B). SRP-loaded HA-Lys nanogel 100% treatment results in the highest reduction of genes expression and blank HA-Lys nanogel 100% was
places in the second place. In all species tested, the PCR test revealed lower expression of the genes _ndvB_ and _icaA_, which are associated with biofilm formation, following treatment
with SRP100%, SRP/blank HA-Lys nanogel 50%, blank HA-Lys nanogel 100%, and SRP-loaded HA-Lys nanogel 100% in comparison to untreated samples (_p-value_ < 0.001). These findings showed
that HA-Lys nanogel can help SRP to get into bacterial cells and stop biofilms to be formed. The blank HA-Lys nanogel 100%, and SRP-loaded HA-Lys nanogel 100% might directly interact with
certain transcription factors, leading to reduced _ndvB_ and _icaA_ expression. DISCUSSION There were opposite changes between size and scattered intensity attributed to the formation of
complexes with strong aggregates and even precipitations. These results were consistent with other polyelectrolyte complexes consisting of polyelectrolytes with hydrophobic backbones20.
According to the Sheetu Wadhwa study, since chitosan (CS) is cationic in nature : and HA is onionic it expected that a possibly close binding may retard the release of the loaded drug at the
site of action and prolong the residence time of the carrier. These polysaccharides are expected to interact with each other, possibly through hydrogen bonding, ionic interactions, and
other intermolecular forces. Therefore, different ratios of internal polymer including CS:HA 1:0.05–1:0.15 was further optimized concerning particle size, zeta potential, and entrapment
efficiency21. Also, there was an increase in the numerical value of zeta potential after increasing the amount of drug concentration, while other independent variables were kept constant. An
increase in HA leads to highly negative zeta potential because HA is an acidic polysaccharide with a negative charge. According to Fahmy et al. the incorporation of negatively charged HA
causes extensive adsorption on the elastosomal22. However, the results showed that zeta potential increases linearly with enhancing stirring speed. This might be due to the agglomeration of
individual particles that tends to reduce surface area and change the zeta potential value associated with that surface23. Increased PDI by raising the ratio of HA/Lys and drug concentration
showed that an increase in these two factors may decrease nanogel stability24,25. But PDI decreased with increasing stirring speed. Moreover, the viscosity of the solution was reduced by
elevating the stirring speed because it always exerts a constant amount of shear stress on the emulsifying mixture. As a result, the PDI of nanogels reduces due to the decrementof solution
viscosity23. The average size of the optimized formulation is less than 100 nm. The particle diameter obtained by the DLS method is larger than SEM, which is attributed to the hydration
effect of the SRP-loaded HA-Lys nanogel in aqueous medium26. The release profiles of SRP from HA nanogels were biphasic release processes27,28. The initial phase involves a relatively rapid
release of the drug and is then followed by a slower release phase. The rapid drug release in the initial phase may be due to the desorption of the drug from the outer surface of the
nanoparticles and slower drug release is mainly related to the diffusion of the drug through the nano structure29,30,31. A wide size distribution and heterogeneous particle sizes are
observed as a result of temperature increment. The loaded protein content is another influencing factor that should be considered. The best sample, as can be observed, lost SRP in lesser
extent when stored at 4 °C in comparison to 25 °C. Aggregation of the vesicles may induce a rise in their size as storage time increases. According to thermodynamic theory, surface energy is
a size-dependent quantity. Smaller nanoparticles have a greater surface energy and tend to combine to reduce surface energy. The stability study showed that the size changes at 25 °C were
significantly higher, as well as PDI and EE. Overall, nanoparticles seem to be more stable at 4 °C. Reduction in the activity of SRP after loading in nanogels can be attributed to
electrostatic attachment of SRP with HA or the steric hindrance, size distribution of nanogels, and diffusion effect32,33 of SRP in nanogels. Despite the decreasing activity of SRP-loaded
nanogels, according to previous reports, enzyme-loaded nanogels had better stability than free enzyme exposing environmental conditions. The results show that the loading process increases
protease resistance against environmental fluctuations and improves enzymatic activity in the pharmaceutical industry and medicine because SRP loaded in HA nanogels can accumulate on the
surface of bacterial cell walls34. Generally, blank HA-Lys nanogel showed significant microbial activity against _P. aeruginosa_ and _S. aureus_ compared to free enzyme. A possible
explanation is because of the high antimicrobial properties of HA35,36. The antibacterial effect of HA itself have been reported before. For example Wenzhen et al. used HA as antibacterial
agent to modify surface of microneedles37. Another reason may be due to poor penetration of free SRP in the biofilm compared with SRP-loaded HA-Lys nanogels. Also, the investigation of the
samples’ effect on bacterial viability indicated that the reduction of bacterial viability in SRP-loaded HA-Lys nanogel was not significantly greater than blank HA-Lys nanogel. One of the
primary mechanisms of SRPs therapeutic action is most likely its anti-biofilm activity. The anti-biofilm properties of SRP has been documented in an increasing number of research38.
According to a prior study, SRP considerably boosted the efficiency of antibiotics against bacteria that produce biofilms8. In a comparable way, SRP was reported by Panagariya et al39. to
augment the activity of other antibiotics, including ampicillin, cephalexin, diclacillin, minocycline, and cefotiam. In another investigation, Mecikogu et al. showed that treatment using SRP
dramatically increased the action of antibiotics against bacteria that produce biofilms in animal models. According to Papa et al7. research, down regulation of surface proteins expression
in pathogens that help biofilm formationis probably the way how the SRPs anti-biofilm function is mediated. Similarly, some research has shown that SRPs activity is increased when it is
incorporated into nanostructures. For example, Kumar et al40. discovered that the anti-inflammatory activity of SRP was increased by immobilizing the enzyme to magnetic nanoparticles
utilizing glutaraldehyde as a chemical linker. In a different investigation, Kaur et al41. showed that arthritic inflammation was considerably reduced when SRP was conjugated to albumin
nanoparticles via glutaraldehyde linkages. CONCLUSION The present study focused on the preparation, optimization, and antibiofilm activity evaluation of SRP-loaded HA-Lys nanogels.
SRP-loaded HA-Lys nanogel was prepared via the dropping method. The results of the Box-Behnken experimental design showed that polymer ratio, drug concentration, and stirrer speed had a
converse effect on the particle size of blank HA-Lys nanogel. Nonetheless, the entrapment efficiency of nanogels is highly dependent on the quantity of enzyme. For the ideal sample, the
average particle size was 156 nm, and the zeta potential was -14.1 mV. Moreover, the release of SRP reached about 77% after 72 h. Also, the physical characterization of blank HA-Lys nanogel
indicated that nanogels had higher stability and lower changes in EE after 90 days at 4 °C compared to 25 °C.Anti-biofilm activity assessment showed the noticeable anti-biofilm effect of
SRP-loaded HA-Lys nanogel against _P. aeruginosa_ and _S.aureus_. Therefore, this nanocarrier can be considered as a suitable candidate for managing infections caused by biofilm forming
pathogens. DATA AVAILABILITY The datasets generated during and/or analyzed during the current study are available from the corresponding author upon a reasonable request. ABBREVIATIONS * HA:
Hyaluronic acid * Lys: Lysine * SRP-loaded HA-Lys nanogel: Nanogels containing Serratiopeptidase * Blank HA-Lys nanogel: HA-Lys nanogel * BBD: Box-Behnken experimental design * SRP:
Serratiopeptidase * _S. aureus_ : _Staphylococcus aureus_ * _P. aeruginosa_ : _Pseudomonas aeruginosa_ * PDI: Polydispersity index * EE%: Encapsulation efficiency percentage * GAG:
Glycosaminoglycan * FT-IR: Fourier-transform infrared spectroscopy REFERENCES * Mukherji, R., Patil, A. & Prabhune, A. Role of extracellular proteases in biofilm disruption of gram
positive bacteria with special emphasis on _Staphylococcus aureus_ biofilms. Enzyme Eng. 4 (2015). * Gupta, P. V. & Nagarsenker, M. S. _Antimicrobial and Antibiofilm Activity of
Enzybiotic Against Staphylococcus aureus_ 364–372 (Formatex Research Center, 2015). Google Scholar * Hogan, S. _et al._ Potential use of targeted enzymatic agents in the treatment of
_Staphylococcus aureus_ biofilm-related infections. _J. Hosp. Infect._ 96, 177–182 (2017). Article CAS PubMed Google Scholar * Saggu, S. K., Jha, G. & Mishra, P. C. Enzymatic
degradation of biofilm by metalloprotease from Microbacterium sp. SKS10. _Front. Bioeng. Biotechnol._ 7, 192 (2019). Article PubMed PubMed Central Google Scholar * Elchinger, P. H. _et
al._ Effect of proteases against biofilms of _Staphylococcus aureus_ and _Staphylococcus epidermidis_. _Lett. Appl. Microbiol._ 59, 507–513 (2014). Article CAS PubMed Google Scholar *
Klodzinska, S. N. _et al._ Hyaluronic acid-based nanogels improve in vivo compatibility of the anti-biofilm peptide DJK-5. _Nanomed. Nanotechnol. Biol. Med._ 20, 102022.
https://doi.org/10.1016/j.nano.2019.102022 (2019). Article CAS Google Scholar * Papa, R. _et al._ A new anti-infective strategy to reduce the spreading of antibiotic resistance by the
action on adhesion-mediated virulence factors in _Staphylococcus aureus_. _Microbial Pathog._ 63, 44–53 (2013). Article CAS Google Scholar * Passariello, C., Lucchese, A., Pera, F. &
Gigola, P. Clinical, microbiological and inflammatory evidence of the efficacy of combination therapy including serratiopeptidase in the treatment of periimplantitis. _Eur. J. Inflamm._ 10,
463–472 (2012). Article CAS Google Scholar * Jadhav, S. B., Shah, N., Rathi, A., Rathi, V. & Rathi, A. J. B. R. Serratiopeptidase: Insights into the therapeutic applications.
_Biotechnol. Rep._ 28, e00544 (2020). Article Google Scholar * Mehrarya, M. _et al._ Niosomal formulation for Antibacterial applications. _J. Drug Target._ 30, 1–44 (2022). Article Google
Scholar * Lankalapalli, S. & Kolapalli, V. R. M. Polyelectrolyte complexes: A review of their applicability in drug delivery technology. _Indian J. Pharmaceut. Sci._ 71, 481 (2009).
Article CAS Google Scholar * Oyarzun-Ampuero, F. A., Goycoolea, F. M., Torres, D. & Alonso, M. A new drug nanocarrier consisting of polyarginine and hyaluronic acid. _Eur. J.
Pharmaceut. Biopharmaceut._ 79, 54–57 (2011). Article CAS Google Scholar * Drago, L. _et al._ Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for
respiratory tract infections. _Apmis_ 122, 1013–1019 (2014). Article CAS PubMed Google Scholar * Anastassiadis, S. L-Lysine fermentation. _Recent Patents Biotechnol._ 1(1), 11–24 (2007).
Article MathSciNet CAS Google Scholar * Cupp-Enyard, C. J. J. Sigma’s non-specific protease activity assay-casein as a substrate. _JoVE_ 19, e899 (2008). Google Scholar * Saha, P.,
Goyal, A. & Rath, G. Formulation and evaluation of chitosan-based ampicillin trihydrate nanoparticles. _Pharm. Res. Trop. J. Pharmaceut. Res._ https://doi.org/10.4314/tjpr.v9i5.61061
(2010). Article Google Scholar * Carneiro, J. _et al._ Development and characterization of hyaluronic acid-lysine nanoparticles with potential as innovative dermal filling. _Braz. J.
Pharmaceut. Sci._ 52, 645–651 (2016). Article CAS Google Scholar * Harris, T. & Keshwani, M. Measurement of enzyme activity. _Methods Enzymol._ 463, 57–71 (2009). Article CAS PubMed
Google Scholar * Nikolić, M., Vasić, S., Đurđević, J., Stefanović, O. & Čomić, L. J. K. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic
extract. _Kragujevac J. Sci._ 36, 129–136 (2014). Article Google Scholar * Zheng, C. _et al._ Long-term kinetics of DNA interacting with polycations. _Polymer_ 55, 2464–2471.
https://doi.org/10.1016/j.polymer.2014.03.038 (2014). Article CAS Google Scholar * Wadhwa, S., Paliwal, R., Paliwal, S. R. & Vyas, S. P. Hyaluronic acid modified chitosan
nanoparticles for effective management of glaucoma: Development, characterization, and evaluation. _J. Drug Target._ 18, 292–302. https://doi.org/10.3109/10611860903450023 (2010). Article
CAS PubMed Google Scholar * Fahmy, A. M., Hassan, M., El-Setouhy, D. A., Tayel, S. A. & Al-Mahallawi, A. M. Statistical optimization of hyaluronic acid enriched ultradeformable
elastosomes for ocular delivery of voriconazole via Box-Behnken design: In vitro characterization and in vivo evaluation. _Drug Deliv._ 28, 77–86.
https://doi.org/10.1080/10717544.2020.1858997 (2021). Article CAS PubMed Google Scholar * Hussain, Z. & Sahudin, S. Preparation, characterisation and colloidal stability of
chitosan-tripolyphosphate nanoparticles: Optimisation of formulation and process parameters. _Int. J. Pharm. Pharmaceut. Sci._ 8, 297–308 (2016). CAS Google Scholar * Folchman-Wagner, Z.,
Zaro, J. & Shen, W. C. Characterization of polyelectrolyte complex formation between anionic and cationic poly(amino acids) and their potential applications in pH-dependent drug
delivery. _Molecules_ https://doi.org/10.3390/molecules22071089 (2017). Article PubMed PubMed Central Google Scholar * Supachawaroj, N., Damrongrungruang, T. & Limsitthichaikoon, S.
Formulation development and evaluation of lidocaine hydrochloride loaded in chitosan-pectin-hyaluronic acid polyelectrolyte complex for dry socket treatment. _Saudi Pharmaceut. J. SPJ Off.
Publ. Saudi Pharmaceut. Soc._ 29, 1070–1081. https://doi.org/10.1016/j.jsps.2021.07.007 (2021). Article CAS Google Scholar * Mirzaie, A. _et al._ Preparation and optimization of
ciprofloxacin encapsulated niosomes: A new approach for enhanced antibacterial activity, biofilm inhibition and reduced antibiotic resistance in ciprofloxacin-resistant
methicillin-resistance _Staphylococcus aureus_. _Bioorgan. Chem._ 103, 104231. https://doi.org/10.1016/j.bioorg.2020.104231 (2020). Article CAS Google Scholar * Nguyen, T. B. & Lee,
B. T. A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. _Tissue Eng. Part A_ 20, 1993–2004.
https://doi.org/10.1089/ten.TEA.2013.0352 (2014). Article CAS PubMed PubMed Central Google Scholar * Al-Qadi, S., Alatorre-Meda, M., Martin-Pastor, M., Taboada, P. & Remunan-Lopez,
C. The role of hyaluronic acid inclusion on the energetics of encapsulation and release of a protein molecule from chitosan-based nanoparticles. _Colloids Surf. B Biointerfaces_ 141,
223–232. https://doi.org/10.1016/j.colsurfb.2016.01.029 (2016). Article CAS PubMed Google Scholar * Kumari, N. & Pathak, K. Dual controlled release, in situ gelling periodontal sol
of metronidazole benzoate and serratiopeptidase: Statistical optimization and mechanistic evaluation. _Curr. Drug Deliv._ 9, 74–84. https://doi.org/10.2174/156720112798375998 (2012). Article
CAS PubMed Google Scholar * Lim, S. T., Martin, G. P., Berry, D. J. & Brown, M. B. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel
microspheres of hyaluronic acid and chitosan. _J. Control. Release Off. J. Control. Release Soc._ 66, 281–292. https://doi.org/10.1016/s0168-3659(99)00285-0 (2000). Article CAS Google
Scholar * Wang, J. _et al._ Hyaluronic acid-modified liposomal honokiol nanocarrier: enhance anti-metastasis and antitumor efficacy against breast cancer. _Carbohydr. Polym._ 235, 115981
(2020). Article CAS PubMed Google Scholar * Kumar, S., Jana, A. K., Dhamija, I., Singla, Y. & Maiti, M. Preparation, characterization and targeted delivery of serratiopeptidase
immobilized on amino-functionalized magnetic nanoparticles. _Eur. J. Pharmaceut. Biopharmaceut._ 85, 413–426 (2013). Article CAS Google Scholar * Zhu, H., Pan, J., Hu, B., Yu, H.-L. &
Xu, J.-H. Immobilization of glycolate oxidase from _Medicago falcata_ on magnetic nanoparticles for application in biosynthesis of glyoxylic acid. _J. Mol. Catal. B Enzym._ 61, 174–179
(2009). Article CAS Google Scholar * Filby, B. W., Weldrick, P. J. & Paunov, V. N. Overcoming beta-lactamase-based antimicrobial resistance by nanocarrier-loaded clavulanic acid and
antibiotic cotreatments. _ACS Appl. Bio Mater._ 5, 3826–3840. https://doi.org/10.1021/acsabm.2c00369 (2022). Article CAS PubMed Google Scholar * Pirnazar, P. _et al._ Bacteriostatic
effects of hyaluronic acid. _J. Periodontol._ 70, 370–374 (1999). Article CAS PubMed Google Scholar * Romanò, C. _et al._ Hyaluronic acid and its composites as a local
antimicrobial/antiadhesive barrier. _J. Bone Joint Infect._ 2, 63–72 (2017). Article Google Scholar * Du, W., Li, X., Zhang, M., Ling, G. & Zhang, P. Investigation of the antibacterial
properties of hyaluronic acid microneedles based on chitosan and MoS 2. _J. Mater. Chem. B_ 11, 7169–7181 (2023). Article CAS PubMed Google Scholar * Rouhani, M. _et al._ Improved
anti-biofilm activity and long-lasting effects of novel serratiopeptidase immobilized on cellulose nanofibers. _Appl. Microbiol. Biotechnol._ 107, 6487–6496 (2023). Article CAS PubMed
Google Scholar * Panagariya, A. & Sharma, A. A preliminary trial of serratiopeptidase in patients with carpal tunnel syndrome. _J. Assoc. Phys. India_ 47, 1170–1172 (1999). CAS Google
Scholar * Kumar, S. _et al._ Preparation, characterization and targeted delivery of serratiopeptidase immobilized on amino-functionalized magnetic nanoparticles. _Eur. J. Pharmaceut.
Biopharmaceut._ 85, 413–426 (2013). Article CAS Google Scholar * Kaur, H. & Singh, A. J. Design, development and characterization of serratiopeptidase loaded albumin nanoparticles.
_J. Appl. Pharmaceut. Sci._ 5, 103–109 (2015). Article Google Scholar * Yaghoobi, M. M., Khaleghi, M., Rezanejad, H. & Parsia, P. Antibiofilm activity of _Dracocephalum polychaetum_
extract on methicillin-resistant _Staphylococcus aureus_. _Avicenna J. Clin. Microbiol. Infect._ 5(1), 61772–61772 (2018). Article Google Scholar * Mah, T.-F. Biofilm-specific antibiotic
resistance. _Future Microbiol._ 7(9), 1061–1072 (2012). Article CAS PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmaceutical
Nanotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran Hanieh Mahdiani, Haleh Bakhshandeh & Rassoul Dinarvand * Nanotechnology Research Centre, Faculty
of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran Hanieh Mahdiani & Rassoul Dinarvand * Nanobiotechnology Department, New Technologies Research Group, Pasteur Institute of
Iran, Tehran, Iran Hanieh Mahdiani, Faegheh Yazdani, Mahsa Khoramipour, Vahideh Valizadeh & Haleh Bakhshandeh * QC Department, Osve Pharmaceutical Co, Tehran, Iran Haleh Bakhshandeh
Authors * Hanieh Mahdiani View author publications You can also search for this author inPubMed Google Scholar * Faegheh Yazdani View author publications You can also search for this author
inPubMed Google Scholar * Mahsa Khoramipour View author publications You can also search for this author inPubMed Google Scholar * Vahideh Valizadeh View author publications You can also
search for this author inPubMed Google Scholar * Haleh Bakhshandeh View author publications You can also search for this author inPubMed Google Scholar * Rassoul Dinarvand View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.B., R.D. developed the idea and designed the experiments. H.M., M.K., and F.Y. conducted the
experiments. H.M., and V.V., analyzed the data. H.M. wrote the first draft. All authors confirmed the final manuscript before the submission and agreed to the published version of the
manuscript. CORRESPONDING AUTHORS Correspondence to Vahideh Valizadeh or Haleh Bakhshandeh. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mahdiani, H., Yazdani, F.,
Khoramipour, M. _et al._ Preparation and Physicochemical Characterization of Hyaluronic Acid-Lysine Nanogels Containing Serratiopeptidase to Control Biofilm Formation. _Sci Rep_ 14, 6111
(2024). https://doi.org/10.1038/s41598-024-56732-9 Download citation * Received: 29 December 2023 * Accepted: 11 March 2024 * Published: 13 March 2024 * DOI:
https://doi.org/10.1038/s41598-024-56732-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Therapeutic enzyme * Serratiopeptidase (SRP) *
Hyaluronic acid (HA) * Lysine * Nanogel * Bacterial biofilm
