Murine vaginal secretory responses to a male volatile chemical messenger
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:

ABSTRACT Many species use chemical messengers to communicate a remarkable range of information. Mice appear to make particular use of chemical messengers, including effects on estrous
cycling and initiation, pregnancy, aggression, stress and of course attraction. Behavioral studies have helped identify several candidate messengers, or pheromones, that mediate attraction
in mice. One question is whether attractive chemical messengers induced a physical vaginal secretory response. The preparation hypothesis posits that increased vaginal secretion would
lubricate and protect the vagina in response to the prospect of imminent coitus, but this has been difficult to assess experimentally, particularly in mice. We developed a rapid, sensitive,
minimally invasive method of quantifying vaginal moisture in mice and used this model to test vaginal secretory responses to male bedding. We report that female mice experience an increase
in vaginal moisture after exposure to male, but not female, bedding. This response is induced by either physical or airborne exposure to male urine, to preputial gland extract, and to the
preputial gland-derived pheromone alpha/beta farnesenes. This vaginal response is diurnally regulated, seen only during their active phase. The response is sensitive to the estrous phase,
with a clear response during estrus but not during metestrus. We conclude that mice may serve as a model for aspects of vaginal function and that this assay will be readily applicable to
other small animals. The identification of a pheromone-mediated vaginal secretory response offers a window into the regulation of the vaginal environment and the neurobiology of sexual
responses in mice. SIMILAR CONTENT BEING VIEWED BY OTHERS OLFACTORY SIGNALS AND FERTILITY IN OLIVE BABOONS Article Open access 19 April 2021 PHEROMONES THAT CORRELATE WITH REPRODUCTIVE
SUCCESS IN COMPETITIVE CONDITIONS Article Open access 09 November 2021 HYPOTHALAMIC DOPAMINE NEURONS MOTIVATE MATING THROUGH PERSISTENT CAMP SIGNALLING Article 25 August 2021 INTRODUCTION
Many species use chemical messengers to communicate a wide range of information1. These messengers are generally thought to act through the olfactory system that is broadly divided into two
separate systems. Many of these messengers – sometimes also referred to as pheromones – are thought to act via the vomeronasal organ, a sensory epithelium that responds to non-volatile
messengers, and whose neurons project to the accessory olfactory bulb and from there to the rest of the brain2. However volatile messengers likely act via the main olfactory bulb via inputs
from olfactory neurons that line the sinus3. These messengers can derive from different sources: though many of these messengers are present in urine, released in part by preputial and
clitoral glands of male and female mice, there is also evidence for release of messengers through saliva, sweat, and even tears4,5. Taken as a whole, the range of signals communicated by
such chemical messengers and their consequent behavioral responses is remarkable. Some species such as mice appear to make particular use of these chemical messengers and several such
responses have already been characterized. For example, the “Whitten Effect” sees synchronized estrus in female mice after exposure to specific chemical messengers in male urine6,7. Other
effects have been described in mice, including the termination of pregnancy in response to the odor of an unfamiliar male, the so-called “Bruce effect”8, or the alteration of the duration of
estrous cycles in isolated group-housed females, the so-called “Lee-Boot effect”9. Typically these studies have made use of laboratory mice. Chemical messengers are of considerable interest
to the farming industry both as livestock ‘biostimulants’3,10 and to facilitate pest control (i.e. rodent attractants for traps)11. The role of chemical messengers in mediating female
attractive responses to males has been the subject of many studies. Several chemicals and proteins have been reported to serve as attractants for female mice, including the major urinary
protein darcin12, 3,4-dehydro-exo-brevicomin and 2-sec-butyl-4,5- dihydrothiazole (SBT)13, a mixture of alpha and beta farnesenes14 as well as hexadecanol and hexadecyl acetate15. Volatile
messengers such as alpha/beta farnesenes and hexadecanol/hexadecyl acetate are produced by the male preputial gland of the mouse14,15 and the rat16. Vaginal responses to attractive stimuli
have seen little study, despite the medical importance of vaginal health and function. The vaginal environment is tightly regulated, while serving as a unique interface with the external
world. Much remains unknown about how this vaginal environment is regulated. Our poor understanding of the neurobiology of vaginal secretion is also reflected in the prevalence of and
limited treatments for vaginal dryness, a condition that affects millions17. Frequently painful, vaginal dryness affects quality of life, is accompanied by risk of infection, and, as a side
effect of commonly prescribed drugs, is a contributor to patient non-compliance18,19,20. The preparation hypothesis posits that animals experience an autonomic/involuntary increase in
vaginal moisture when confronted with the prospect of imminent coitus in order to protect the reproductive apparatus (discussed in21). However, this has been difficult to assess
experimentally. Studies of chemoattractants have relied on behavioral preference tests that quantify time spent investigating or loitering by a murine biochemical marker (e.g22). Vaginal
secretion is under autonomic control (e.g23) and so offers a rapidly quantifiable physiological response, but has seen limited study, particularly in small animals such as mice, due to the
challenge of reliably quantifying small volumes of vaginal secretions. The aim of this study was to develop a simple method for the quantification of vaginal moisture in female laboratory
mice and to apply this method to test vaginal secretory responses to attractive male stimuli. To accomplish this we adapted a long-established method from studies of lacrimation in mice24 to
quantify vaginal secretion. We describe this rapid, minimally invasive and readily repeatable assay including a method for the manufacture of a colorimetric thread. When initial studies
indicated that exposure to male bedding increased vaginal moisture, we examined the nature and origin of the chemical messenger, testing physical and airborne exposure to male urine, male
glandular secretion, and the volatile chemical messengers alpha/beta farnesenes that have previously been implicated in female attractive responses to males25. We additionally investigated
the impact of the active/rest phase and estrous phases on this vaginal moisture response. METHODS STUDY ANIMALS Mice were group-housed in standard ventilated caging (cage floor dimensions:
33.5 × 18 cm, depth 14 cm), with corn cob-based bedding (Bed-o’combs laboratory animal bedding, The Andersons, Maumee, OH). Mice were group-housed 3–4 mice per cage and were fed Inotiv
Teklad 2918 irradiated rodent diet _ad libitum_. Because mice are nocturnal, we tested their responses during their active phase, by maintaining them on a reverse light cycle, except to test
for diurnal contributions as described below. With one exception, C57BL/6 strain mice were tested. That exception was the study of diurnal regulation, for which CD1 strain mice were used.
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). CD1 strain mice for studies of diurnal variation were bred in a colony in the same facility and kindly provided by Dr.
Ken Mackie (Indiana University, Bloomington IN). Mice were 3–6 months of age. All animal care and experimental procedures used in this study were approved by the Institutional Animal Care
and Use Committee of Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Laboratory Animals. Experiments complied with ARRIVE
guidelines. Sex and number of mice used for each experiment: Only adult female mice were tested for vaginal responses, but bedding and urine samples were obtained from both males and females
and preputial glands were obtained from males. For study of response to bedding the same group of females were exposed to male or female bedding with a 2 week ‘washout’ period. male
bedding: 12 mice, female bedding: 11 mice. For responses to olfactory stimuli numbers were as follows. Urine (contact): _n_ = 19, Urine scent: _n_ = 27, Preputial gland scent: _n_ = 16,
alpha/beta farnesenes scent: _n_ = 13. For tests of active vs. rest phase, 25 and 22 females, respectively. For test of estrous phase, 43 mice. Female mice were generally tested in two but
no more than three experiments. This means that in the second or third experience had previous experience with male scents. With the exception of one cohort of mice housed in standard light
cycle, mice were housed in the same room but the males and females were sexually naïve, meaning that they had not had an opportunity to physically interact with one another. As noted above,
the C57BL/6 mice were purchased from Jackson laboratories, so the cage-mates were related but it is unknown whether the mice in different cages were related. A given cage of CD1 strain
females were siblings, but females from different cages in the diurnal variation study were from different dams. MEASUREMENT OF VAGINAL MOISTURE The use of colorimetric threads is an
established method for the measurement of small volumes of exocrine secretions such as tears in small animals including mice24,26. Fluids readily travel along the threads,
wetting/discoloring them for later quantification; more fluid results in a longer discolored portion of the thread and can therefore be taken as a measure of fluid levels, though no claim is
made that a given distance traveled corresponds to a specific volume of fluid. Until recently a commercially produced version of these threads (ZoneQuick) was available (e.g26). These
threads were impregnated with phenol red, pH sensitive colorimetric dye that shifts between yellow at pH 6.8 to fuchsia at pH 8.2. The manufacturer ceased production of these threads in
2020, but the method is not difficult to replicate in-house and is described here. After testing a variety of threads, we found that Coats & Clark 60:40 (cotton over a polyester core)
35wt had the best combination of stiffness and color absorption. This thread is readily available from a variety of sewing supply vendors (e.g. www.joann.com). As a first step, thread is
submerged in 100mL PBS (pH 7.4) for 10 min. After this, the thread is removed and allowed to air-dry for 5–6 h. The rationale for this is to give the thread a consistent embedded pH of 7.4
so that even a small sample with a lower pH will still discolor the thread. To prepare a phenol red/HEPES-buffered saline solution, we combine equal volumes of phenol red solution (1% in
alcohol, Cat# UN1987, Sigma-Aldrich, St. Louis MO) and HBS (adjusted to pH 6.5 with NaOH) in a beaker. We generally prepare 10mL at a time. Using forceps, dip the strings into the beaker for
2 s. The phenol red strings are placed between two paper towels to absorb excess dye mix. A fresh paper towel is placed over the threads with a flat weight above to pin the thread between
the paper towel sheets. Threads are allowed to dry for two days. Threads are initially red, but soon change to a golden yellow color as they dry. Store the thread in a sealable plastic bag
to minimize contact with air. These threads can be stored for several months. Threads are cut as needed on the day of experiments. To allow for consistent placement in the vaginal cavity, a
thread is inserted into a glass capillary (A-M Systems, 0.5 mm inner diameter, Cat#: 626000, fire-polished to prevent injury), leaving 3 mm of thread outside the opening of the capillary.
The capillary is then placed into the vaginal cavity of an unanesthetized mouse for 10 s. We have previously adapted these threads for the measurement of salivation27 and Fig. 1 in that
publication shows a sample thread positioned in a cannula as well as a sample discolored thread positioned next to a ruler for measurement. See Supplementary Fig. S1 for a volume/thread
length response curve. We pipetted up a given volume of phosphate buffered saline with 5% kolliphor as a wetting agent, then expressed this volume at the tip and promptly touched the end of
the thread to the tip of the pipette to allow for uptake of liquid by the thread. Based on uptake of 0.8, 1.0, 1.2 and 1.4 uL, a linear regression analysis yielded a slope of 23.9 ± 4.1 mm
of distance along thread per microliter of saline. We did not include volumes below 0.8uL since these come with a risk of pipetting error as well as adhesion of liquid to the outside of the
pipette tip that may result in an exaggerated response. On a practical note, it is important to measure and record the distance traveled within ~ 1 min since the sample gradually returns to
a golden color as it dries. Moreover, a liquid sample with a lower pH will discolor the thread at the leading edge (because of the embedded pH of the thread) so it is sometimes necessary to
track the ‘leading edge’ of the discoloration. On a related note, we find that frequent prior handling of the mice is important for these experiments, partly to reduce the stress of
handling, but also because this greatly reduces the likelihood that mice will urinate during the procedure and so foul the sample. The colorimetric threads are also an approximate pH sensor
and the acidic urine28 overwhelms the embedded pH and so does not discolor the threads; this combined with the difference in volume make it easy to determine when a sample is due to
urination. Mice that see a urine response are excluded from a given experiment. PREPARATION OF OLFACTORY STIMULI BEDDING Bedding for a given experiment was obtained in coordination with the
weekly animal facility cage-change. Bedding (from 6 days of habitation) was obtained from a cage of 3–4 male (or in one experiment (Fig. 1B) female) mice. Bedding was used within 1 h. For a
bedding exposure, males were relocated to a clean cage and females were moved into the soiled male cage and allowed to roam for 1 h, after which the vaginal moisture measurement was obtained
and females were moved back into their home cage. A given soiled male cage was used only once (for one cage of females). URINE For the urine(contact) testing condition, urine is obtained
from male mice by scruffing the animal and collecting, with a kim-wipe, freely secreted urine. Urine from 4 males is sufficient for 3–4 females (i.e. one cage). Because not all males urinate
‘on demand’ the males originate from 1 to 2 cages. The kim-wipes are immediately mixed in with bedding of a fresh cage. Females are placed into the cage and allowed to explore and as a rule
they soon dig up the soiled tissue. After 1 h, after which the vaginal moisture measurement was obtained and females were moved back into their home cage. A cage prepared in this manner was
used only once (for one cage of females). For urine-smell only condition, the urine is collected as above, but now the kim-wipes from two males are placed on a separator positioned on the
wire caging, in a plastic weigh boat, but below the cage filter top, so preventing physical contact with the mice while sealing in the scent. OBTAINING AND PREPARING PREPUTIAL GLAND
SECRETION Preputial glands were dissected and physically pressed using a spatula to release gland contents into a small plastic dish. Glands from two males (C57BL/6, age 3–4 months) were
taken as a single sample and the sample was used for 1 experiment. A total of 8 males were used for this experiment. Samples were used within 5 min of dissection then exposed as for
urine-smell only condition. PREPARATION OF FARNESENE AS AN ODORANT Implicated as a female attractant, farnesene is found in male urine at 5ppm and has been demonstrated to have attractant
properties at 10ppm25. Farnesene occurs naturally as isomers several of which have attractant properties25. For these experiments, farnesene was purchased from Sigma-Aldrich as a mixture of
isomers (trans-β, cis-α, trans-α; cat: w383902). Farnesene was diluted to 10 ppm (1:100 in ethanol, then 1:1000 in water, accounting for the density of the original stock) in a volume of 1mL
and absorbed into paper towel and suspended in a plastic dish as above. METHOD FOR DETERMINING ESTROUS PHASE To determine the estrous cycle phase of a given mouse we use the method
described by Byers et al.29. We obtain a vaginal smear by inserting 10ul of sterile saline into the proximal vaginal canal using a 20uL pipette. The liquid is then collected back into the
pipette and is placed onto a slide and covered with a cover slip. The slide is then imaged within 3 h using a camera-equipped phase-contrast microscope. The picture is saved for scoring.
Evaluation of the images is done via a two-person consensus approach: each person scores the images; in the case of disagreement, each makes a case for a given phase and the scorers arrive
at a consensus. Occasionally scorers are uncertain about the phase because it exhibits characteristics of both. This occurs most frequently for estrus vs. metestrus. In that case the sample
is scored accordingly (e.g. estrus/metestrus) and the vaginal response data is excluded from analyses. These ambiguous estrous typings represented ~ 10–15% of the total. GENERAL METHOD OF
EXPOSURE TO ODORANT (BEDDING, URINE(CONTACT/SCENT), ALPHA/BETA FARNESENES) For odorant exposure experiments, several cages containing a group of C57BL/6 female mice (3–4/cage) are brought
into an adjoining room separated by a closed door. Mice were tested in late-morning, starting at 10AM to reduce the likelihood of unusual scents. All baseline vaginal moisture readings are
obtained for each mouse as described under Measurement of Vaginal Moisture above. This takes less than a minute per mouse so is accomplished in under half an hour. After this, the first cage
of female mice are transferred into a scent-containing cage and allowed to explore. Subsequent groups of mice are transferred at ~ 4–5 min intervals. After an hour of exploration, the first
group of mice are tested, with vaginal moisture readings taken for each female a second time to allow comparison to her own pre-exposure baseline. Subsequent cages were tested as they
reached 1 h. Experimenters (both the experimenter obtaining a sample and the individual quantifying the distance that the sample traveled along the thread) were aware of the experiment that
was being conducted (i.e. they were not blinded). In principle, a skilled observer could discern clues about the current state of estrus of a given mouse. DIURNAL EXPERIMENTS To test for the
impact of diurnal rhythm, we tested responses to male urine (odor) in female mice maintained either standard or reverse light cycles. These responses were taken to represent rest and active
phases, respectively. Mice were tested in late-morning, starting at 10AM. C57BL/6 mice have been reported to be melatonin-deficient and to therefore not exhibit a standard profile of
diurnal/circadian responses30. We therefore instead tested CD1 strain mice. VAGINAL RESPONSES TO URINE BY ESTROUS PHASE To test for the role of estrous phase in vaginal responses, we tested
the effect of exposure to urine (contact) as above in mice, then determined the estrus phase immediately after the second (odor-response) vaginal moisture test. We compared mice in estrus or
metestrus. Estrous phase was not routinely measured for the remaining experiments. STATISTICS Analyses were done using Graphpad Prism. With one exception, experiments were analyzed using a
two-tailed paired t-test comparing an experimental condition to the same-animal baseline. Our analyses assumed that the data were normally distributed. A normality test of a sample baseline
vaginal moisture data set (Smell-only, Fig. 2B, _n_ = 27) using a D’Agostino & Pearson omnibus normality test indicated that the data had a normal distribution (K2: 0.016, P value:
0.992). The coefficient of variance in the same data set was 47.2%. In Fig. 3, vaginal moisture data are recorded by phase of estrus, and so a 1-way ANOVA was used, with a Bonferroni
post-hoc test with a single pooled variance. REGARDING INDEPENDENCE OF SUBJECTS Female mice are housed as a group, generally 3–4 to a cage. This is in keeping with their nature as a social
species; social isolation is considered a stressful condition. A given cage of female mice are exposed to a scent as group for an hour. During that time, they are free to explore/investigate
their environment as they see fit. They are then tested individually. This experimental arrangement closely mirrors pharmacological treatments where group-housed mice are treated then
returned to their home cage until the time of testing. Separating mice during this treatment would add an additional variable (stress). RESULTS VAGINAL MOISTURE IN MICE BY ESTROUS PHASE
Using the colorimetric method to quantify vaginal moisture in C57BL/6 mice described in Methods, we examined basal levels of vaginal moisture by estrous phase. We found that levels were
significantly lower during metestrus relative to estrus and diestrus (Fig. 3 way ANOVA with; F(3,44) = 4.62 _p_ = 0.006 for differences among means; Bonferroni _post hoc_ test: *, diestrus
vs. metestrus, _p_ = 0.034; ** estrus vs. metestrus, _p_ = 0.007. Sample sizes: proestrus, _n_ = 8; estrus, _n_ = 17, metestrus: _n_ = 13, diestrus _n_ = 10). EXPOSURE TO MALE BUT NOT FEMALE
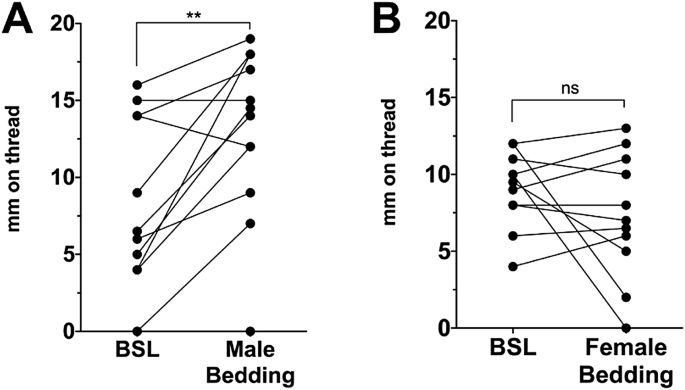
BEDDING INCREASES BASELINE VAGINAL MOISTURE We next tested whether exposure to C57BL/6 males would elicit an increase in vaginal moisture in C57BL/6 female mice, finding that it did (Fig.
1A paired t-test vs. baseline, _p_ = 0.003, _n_ = 12, t = 3.78 df = 11). Females did not similarly respond to the bedding of female mice (Fig. 1B paired t-test vs. baseline, _p_ = 0.023, _n_
= 11, t = 1.27 df = 10). THE CHEMICAL MESSENGER IS CARRIED IN MALE URINE AND IS VOLATILE To determine whether the effect was urine-specific, we tested whether urine itself could elicit a
similar response, finding that C57BL/6 females responded to physical contact with C57BL/6 male urine with an increase in vaginal moisture (Fig. 2A, paired t-test vs. baseline, _p_ = 0.0044,
_n_ = 19, t = 3.3 df = 18). To test whether the effect is due to a volatile compound we repeated the test of urine but now prevented physical access to the urine. We found that this method
of exposure mimicked that for urine itself (Fig. 2B paired t-test vs. baseline, _p_ = 0.0011, _n_ = 27, t = 3.66 df = 26), indicating that this response is mediated by a volatile messenger
present in urine. THE VOLATILE MESSENGER IS LIKELY SECRETED BY THE PREPUTIAL GLAND To test the preputial gland as a potential source of this chemical messenger we exposed C57BL/6 female mice
to the scent of preputial gland extract from two C57BL/6 males, finding that this also induced a vaginal secretory response (Fig. 2C, paired t-test vs. baseline, _p_ < 0.0001, _n_ = 16,
t = 7.3 df = 15). THE PREPUTIAL-DERIVED FARNESENES INDUCE A VAGINAL SECRETORY RESPONSE We found that exposure to alpha/beta farnesenes at 10ppm reliably induced a strong vaginal secretory
response (Fig. 2D; paired t-test vs. baseline, _p_ = 0.0027, _n_ = 13, t = 3.8 df = 12). Taken together our results indicate that the response to bedding may be mediated by the preputial
gland-derived alpha/beta farnesenes. THE VAGINAL RESPONSE IS ONLY SEEN DURING THE ACTIVE PHASE We tested for a diurnal role in this vaginal response. C57BL/6 mice have been reported to be
melatonin-deficient and to therefore not exhibit a standard profile of diurnal/circadian responses30. We therefore instead tested CD1 strain mice as above. We found that CD1 strain mice
maintained on a reverse light cycle -- and so tested during their active phase -- experienced the same increase in vaginal moisture in response to male bedding (Fig. 4A, paired t-test vs.
baseline, _p_ = 0.0025, _n_ = 25, t = 3.38 df = 24). However, a separate cohort of female mice maintained on standard light cycle (and so tested during their rest/sleep phase) did not
respond to male urine (Fig. 4B, paired t-test vs. baseline, _p_ = 0.98, _n_ = 22, t = 0.022 df = 21). This indicates that the vaginal response is under diurnal control and that the female
vaginal response is limited to the active phase. BEDDING RESPONSES ARE SENSITIVE TO THE PHASE OF ESTROUS We tested whether mice were differentially responsive depending on whether they are
in estrus or metestrus. We found that females in their estrus phase had a significant response while those in metestrus did not (Fig. 5 paired t-test vs. baseline. Estrus phase: _p_ <
0.0001, _n_ = 25, t = 7.96 df = 24; metestrus phase: _p_ = 0.24, _n_ = 18. t = 1.21, df = 17). DISCUSSION Studies of vaginal function have been limited in part for want of a method to
reliably quantify vaginal moisture in animal models. Mice are a widely used animal model that include a wealth of transgenic mouse lines. We adapted a method for quantifying tears using a
colorimetric thread to the measurement of vaginal moisture in mice. Our chief finding is the identification of a vaginal secretory response to male, but not female bedding. In dissecting the
source of this effect, we determined that it can be induced by a specific volatile chemical messenger from the male preputial gland. The preputial-derived alpha/beta farnesenes25,
previously implicated as a female attractant, successfully elicit this response. In addition, these responses are diurnally regulated, seen only during the mouse’s active phase and are more
pronounced during the estrus but not metestrus. These findings point to a pheromonally mediated vaginal secretory response in mice that is under complex regulation. This model will allow for
a more detailed examination of the neurobiology of vaginal secretion in the context of sexual attraction. The assay described here has the advantages of being sensitive, measuring
submicroliter volumes (Fig. S1), while still being rapid, simple and minimally invasive. It offers a complement to behavioral measures of female responses to males such as lordosis,
investigation time, or vocalizations. At 1 h, it is also more rapidly discernible than some other chemical-messenger induced physiological effects such as those on the estrous cycle6. We
were able to observe both increases (due to pheromonal stimulation) and decreases (due to estrous phase), indicating that the method is sensitive enough to see both increases and decreases
without the need for parasympathetic stimulation by agents such as pilocarpine. We hypothesized that female mice would have a stronger secretory response during their sexually receptive
phase (pro-estrus and estrus) than during their non-receptive phase (metestrus and diestrus)31. Testing estrus and metestrus phases, we did see a sensitivity of the vaginal moisture response
to the phase of estrus, with a strong response during estrus but none during metestrus. Our finding that mice exhibited a vaginal response only during their active phase has implications
for studies that use laboratory mice. Most research groups maintain mice on a so-called ‘standard’ light cycle and so tests them only during their sleep phase. If we had done this work in
standard light cycle, we would have erroneously concluded that there was nothing to learn. One major question related to the source and identity of this chemical messenger. Male bedding
contains numerous compounds derived from urine, sweat, saliva, and feces all of which have been explored as sources of chemical messengers. Several chemicals and proteins have been reported
to serve as attractants for female mice. This includes the major urinary protein darcin12, 3,4-dehydro-exo-brevicomin and 2-sec-butyl-4,5-dihydrothiazole (SBT)13, alpha/beta farnesenes14,25
as well as hexadecanol and hexadecyl acetate15. Once the preputial gland was implicated as a source, this narrowed down the list to farnesenes and hexadecanol/hexadecyl acetate. We found
that farnesenes mimicked the effect but our findings do not rule out the possibility that other compounds will similarly induce such a response. Future studies will investigate whether
hexadecanol and/or hexadecyl acetate induce a similar vaginal response. Farnesenes themselves will also merit further study. Their production appears to be sexually dimorphic, produced in
the preputial gland of males14, with higher levels in dominant vs. subordinate males32. Farnesenes also appear to have sexually distinct effects, stimulating attraction in females25, but
aggression in males33. An interesting question is whether farnesenes explicitly contribute to mating or reproductive success. While it seems likely, to our knowledge studies that
specifically quantify number of matings or reproductive outcomes have not been reported. There are important advantages to preparing the vaginal environment for coitus both in terms of
lubrication, regulating pH and maintaining an antibacterial milieu. It therefore seems likely that this pheromonally mediated vaginal secretory response will be seen in other species. What
is not clear is whether farnesenes will play a similar role across species. Our findings do have relevance to one current debate on the role of vaginal lubrication. The so-called
“preparation hypothesis” posits that vaginal lubrication is an automatic response to coital cues, with the purpose of protecting the genital tissues (discussed in21). Based on the
preparation hypothesis, female mice would invariably respond to male urine cues used here with vaginal lubrication. But we find that the response is less likely during their sleep cycle or
during metestrus. It is possible that the relationship is more complex, factoring in the likelihood of intercourse occurring, which in turn may be the result of a ‘conversation’ of
pheromones and behaviors. How relevant are these findings to other species, including humans? Some species such as mice appear to make particular use of chemical messengers and several such
responses have already been characterized in mice. For example, the “Whitten Effect” sees synchronized estrus in female mice after exposure to specific chemical messengers in male urine6,7.
Other effects have been described in mice, including the acceleration of sexual development in females in response to a protein-associated messenger from males, the so-called “vandenBergh
effect”34,35, the termination of pregnancy in response to the odor of an unfamiliar male, the so-called “Bruce effect”8, or the alteration of the duration of estrous cycles in isolated
group-housed females, the so-called “Lee-Boot effect”9. Substantial evidence supports a social communicative role for oxytocin, though this may not be mediated by the olfactory system36,37.
But a series of studies have examined the effects of androstadienone, a compound found in male sweat, on human female physiological/behavioral parameters such as sweat38 and salivary
cortisol39, as well as sexual arousal40. Some of the research has come in for criticism of methodology but taken as a whole the limited research that has been done strongly suggests that
some olfactory pheromone-responsive system is present in humans. This has given rise to the lamentation that the field of human pheromone research has lost decades41. This question has
remained largely academic since most pheromone-like responses described in animal models do not have clear parallels in humans; i.e. there is no reason to think that an analogue of the Bruce
Effect is terminating human pregnancies. But altered vaginal secretion in response to pheromonal cues is another matter. Human females experience increased vaginal moisture with sexual
arousal. The ability to quantify vaginal responses in animal models may make this question more experimentally tractable. But unless there is a messenger in human male urine that has been
overlooked, any hypothetical human male pheromone would presumably be derived from another exocrine gland. As a final note, it is worth pointing out that moving forward it will be important
to validate laboratory findings in the wild or at least semi-natural contexts. Doing so would be an important step to validate the ecological relevance of the findings and also to ascertain
the generalizability of the findings across strains or even species. In summary, we find that a method for quantifying vaginal moisture in mice offers a new window into the regulation of the
vaginal environment. Using this method, we have identified a novel secretory response to a volatile chemical messenger, specifically the preputial gland-derived alpha/beta farnesenes. This
model may find application for the study of the neurobiology of vaginal secretion including factors that contribute to vaginal dryness such as aging, changing health, or polypharmacy. DATA
AVAILABILITY Data is provided within the manuscript or supplementary information files. REFERENCES * Petrulis, A. Chemosignals and hormones in the neural control of mammalian sexual
behavior. _Front. Neuroendocrinol._ 34, 255–267. https://doi.org/10.1016/j.yfrne.2013.07.007 (2013). Article CAS PubMed Google Scholar * Halpern, M. The organization and function of the
vomeronasal system. _Annu. Rev. Neurosci._ 10, 325–362. https://doi.org/10.1146/annurev.ne.10.030187.001545 (1987). Article CAS PubMed Google Scholar * Dorries, K. M., Adkins-Regan, E.
& Halpern, B. P. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. _Brain Behav. Evol._ 49, 53–62.
https://doi.org/10.1159/000112981 (1997). Article CAS PubMed Google Scholar * Kimoto, H., Haga, S., Sato, K. & Touhara, K. Sex-specific peptides from exocrine glands stimulate mouse
vomeronasal sensory neurons. _Nature_. 437, 898–901. https://doi.org/10.1038/nature04033 (2005). Article ADS CAS PubMed Google Scholar * Barabas, A. J., Aryal, U. K. & Gaskill, B.
N. Protein profiles from used nesting material, saliva, and urine correspond with social behavior in group housed male mice, Mus musculus. _J. Proteom._ 266, 104685.
https://doi.org/10.1016/j.jprot.2022.104685 (2022). Article CAS Google Scholar * Whitten, W. K. Modification of the oestrous cycle of the mouse by external stimuli associated with the
male. _J. Endocrinol._ 13, 5 (1956). Article Google Scholar * Jemiolo, B., Harvey, S. & Novotny, M. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary
constituents. _Proc. Natl. Acad. Sci. U S A_. 83, 4576–4579. https://doi.org/10.1073/pnas.83.12.4576 (1986). Article ADS CAS PubMed PubMed Central Google Scholar * Bruce, H. M. An
exteroceptive block to pregnancy in the mouse. _Nature_. 184, 105. https://doi.org/10.1038/184105a0 (1959). Article ADS CAS PubMed Google Scholar * Van Der Lee, S. & Boot, L. M.
Spontaneous pseudopregnancy in mice. II. _Acta Physiol. Pharmacol. Neerl_. 5, 213–215 (1956). PubMed Google Scholar * Landaeta-Hernandez, A. J., Ungerfeld, R. & Chenoweth, P. J.
Biostimulation and pheromones in livestock: a review. _Anim. Reprod. Sci._ 248, 107154. https://doi.org/10.1016/j.anireprosci.2022.107154 (2023). Article CAS PubMed Google Scholar *
Klassen, D., Lennox, M. D., Dumont, M. J., Chouinard, G. & Tavares, J. R. Dispensers for pheromonal pest control. _J. Environ. Manage._ 325, 116590.
https://doi.org/10.1016/j.jenvman.2022.116590 (2023). Article CAS PubMed Google Scholar * Roberts, S. A. et al. Darcin: a male pheromone that stimulates female memory and sexual
attraction to an individual male’s odour. _BMC Biol._ 8 https://doi.org/10.1186/1741-7007-8-75 (2010). * Novotny, M., Harvey, S., Jemiolo, B. & Alberts, J. Synthetic pheromones that
promote inter-male aggression in mice. _Proc. Natl. Acad. Sci. U S A_. 82, 2059–2061. https://doi.org/10.1073/pnas.82.7.2059 (1985). Article ADS CAS PubMed PubMed Central Google Scholar
* Ma, W., Miao, Z. & Novotny, M. V. Induction of estrus in grouped female mice (Mus domesticus) by synthetic analogues of preputial gland constituents. _Chem. Senses_. 24, 289–293.
https://doi.org/10.1093/chemse/24.3.289 (1999). Article CAS PubMed Google Scholar * Liu, Q. et al. Two Preputial Gland-secreted pheromones evoke sexually dimorphic neural pathways in the
mouse vomeronasal system. _Front. Cell. Neurosci._ 13, 455. https://doi.org/10.3389/fncel.2019.00455 (2019). Article CAS PubMed PubMed Central Google Scholar * Gawienowski, A. M.,
Orsulak, P. J., Stacewicz-Sapuntzakis, M. & Joseph, B. M. Presence of sex pheromone in preputial glands of male rats. _J. Endocrinol._ 67, 283–288. https://doi.org/10.1677/joe.0.0670283
(1975). Article CAS PubMed Google Scholar * The, N. G. S. M. P. S. E. P. The 2020 genitourinary syndrome of menopause position statement of the North American Menopause Society.
_Menopause_. 27, 976–992. https://doi.org/10.1097/GME.0000000000001609 (2020). Article Google Scholar * Reisman, Y. Sexual consequences of Post-SSRI syndrome. _Sex. Med. Rev._ 5, 429–433.
https://doi.org/10.1016/j.sxmr.2017.05.002 (2017). Article PubMed Google Scholar * Lua, L. L., Pathak, P. & Dandolu, V. Comparing anticholinergic persistence and adherence profiles in
overactive bladder patients based on gender, obesity, and major anticholinergic agents. _Neurourol. Urodyn._ 36, 2123–2131. https://doi.org/10.1002/nau.23256 (2017). Article CAS PubMed
Google Scholar * Shea, A. K., Meschino, D. & Wolfman, W. The effect of serotonin reuptake inhibitors on the vaginal epithelium in postmenopausal women. _Climacteric_. 22, 507–510.
https://doi.org/10.1080/13697137.2019.1604655 (2019). Article CAS PubMed Google Scholar * Dawson, S. J., Suschinsky, K. D. & Lalumiere, M. L. Habituation of sexual responses in men
and women: a test of the preparation hypothesis of women’s genital responses. _J. Sex. Med._ 10, 990–1000. https://doi.org/10.1111/jsm.12032 (2013). Article PubMed Google Scholar * Ramm,
S. A., Cheetham, S. A. & Hurst, J. L. Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. _Proc. Biol. Sci._ 275, 1727–1735.
https://doi.org/10.1098/rspb.2008.0302 (2008). Article PubMed PubMed Central Google Scholar * Ottesen, B. et al. Vasoactive intestinal polypeptide (VIP) provokes vaginal lubrication in
normal women. _Peptides_. 8, 797–800. https://doi.org/10.1016/0196-9781(87)90061-1 (1987). Article CAS PubMed Google Scholar * Hamano, H. et al. A new method for measuring tears. _CLAO
J._ 9, 281–289 (1983). CAS PubMed Google Scholar * Jemiolo, B., Xie, T. M. & Novotny, M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals.
_Physiol. Behav._ 50, 1119–1122. https://doi.org/10.1016/0031-9384(91)90570-e (1991). Article CAS PubMed Google Scholar * Thayer, A. et al. THC regulates tearing via cannabinoid CB1
receptors. _Invest. Ophthalmol. Vis. Sci._ 61, 48. https://doi.org/10.1167/iovs.61.10.48 (2020). Article CAS PubMed PubMed Central Google Scholar * Andreis, K. et al. Cannabinoid CB1
receptors regulate salivation. _Sci. Rep._ 12, 14182. https://doi.org/10.1038/s41598-022-17987-2 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Boswald, L. F., Matzek,
D., Kienzle, E. & Popper, B. Influence of strain and Diet on urinary pH in Laboratory mice. _Anim. (Basel)_. 11 https://doi.org/10.3390/ani11030702 (2021). * Byers, S. L., Wiles, M. V.,
Dunn, S. L. & Taft, R. A. Mouse estrous cycle identification tool and images. _PLoS ONE_. 7, e35538. https://doi.org/10.1371/journal.pone.0035538 (2012). Article ADS CAS PubMed
PubMed Central Google Scholar * Stehle, J. H., von Gall, C. & Korf, H. W. Organisation of the circadian system in melatonin-proficient C3H and melatonin-deficient C57BL mice: a
comparative investigation. _Cell. Tissue Res._ 309, 173–182. https://doi.org/10.1007/s00441-002-0583-2 (2002). Article CAS PubMed Google Scholar * Chari, T., Griswold, S., Andrews, N. A.
& Fagiolini, M. The stage of the Estrus cycle is critical for interpretation of female mouse Social Interaction Behavior. _Front. Behav. Neurosci._ 14, 113.
https://doi.org/10.3389/fnbeh.2020.00113 (2020). Article PubMed PubMed Central Google Scholar * Harvey, S., Jemiolo, B. & Novotny, M. Pattern of volatile compounds in dominant and
subordinate male mouse urine. _J. Chem. Ecol._ 15, 2061–2072. https://doi.org/10.1007/BF01207438 (1989). Article CAS PubMed Google Scholar * Novotny, M., Harvey, S. & Jemiolo, B.
Chemistry of male dominance in the house mouse, Mus domesticus. _Experientia_. 46, 109–113. https://doi.org/10.1007/BF01955433 (1990). Article CAS PubMed Google Scholar * Vandenbergh, J.
G., Whitsett, J. M. & Lombardi, J. R. Partial isolation of a pheromone accelerating puberty in female mice. _J. Reprod. Fertil._ 43, 515–523. https://doi.org/10.1530/jrf.0.0430515
(1975). Article CAS PubMed Google Scholar * Vandenbergh, J. G. Male odor accelerates female sexual maturation in mice. _Endocrinology_. 84, 658–660. https://doi.org/10.1210/endo-84-3-658
(1969). Article CAS PubMed Google Scholar * Gordon, I., Martin, C., Feldman, R. & Leckman, J. F. Oxytocin and social motivation. _Dev. Cogn. Neurosci._ 1, 471–493.
https://doi.org/10.1016/j.dcn.2011.07.007 (2011). Article PubMed PubMed Central Google Scholar * Feldman, R., Gordon, I. & Zagoory-Sharon, O. Maternal and paternal plasma, salivary,
and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. _Dev. Sci._ 14, 752–761. https://doi.org/10.1111/j.1467-7687.2010.01021.x
(2011). Article PubMed Google Scholar * Jacob, S., Hayreh, D. J. & McClintock, M. K. Context-dependent effects of steroid chemosignals on human physiology and mood. _Physiol. Behav._
74, 15–27. https://doi.org/10.1016/s0031-9384(01)00537-6 (2001). Article CAS PubMed Google Scholar * Wyart, C. et al. Smelling a single component of male sweat alters levels of cortisol
in women. _J. Neurosci._ 27, 1261–1265. https://doi.org/10.1523/JNEUROSCI.4430-06.2007 (2007). Article CAS PubMed PubMed Central Google Scholar * Spencer, N. A. et al. Social
chemosignals from breastfeeding women increase sexual motivation. _Horm. Behav._ 46, 362–370. https://doi.org/10.1016/j.yhbeh.2004.06.002 (2004). Article CAS PubMed Google Scholar *
Wyatt, T. D. The search for human pheromones: the lost decades and the necessity of returning to first principles. _Proc. Biol. Sci._ 282, 20142994. https://doi.org/10.1098/rspb.2014.2994
(2015). Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank our colleagues Drs. Milos Novotny, Jeffrey Alberts and Helena Soini for
stimulating discussions on the subject of murine chemical messengers. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Gill Institute for Neuroscience, Department of Psychological and Brain
Sciences, Indiana University, Bloomington, 1101 E 10th St, IN 47405, USA Natalia Murataeva, Sam Mattox, Kyle Yust, Wenwen Du & Alex Straiker Authors * Natalia Murataeva View author
publications You can also search for this author inPubMed Google Scholar * Sam Mattox View author publications You can also search for this author inPubMed Google Scholar * Kyle Yust View
author publications You can also search for this author inPubMed Google Scholar * Wenwen Du View author publications You can also search for this author inPubMed Google Scholar * Alex
Straiker View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.S. wrote the main manuscript text. N.M., S.M. and K.Y. conducted experiments and
analyzed data. W.D. provided materials. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Alex Straiker. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ELECTRONIC SUPPLEMENTARY MATERIAL Below is the link to the electronic supplementary material. SUPPLEMENTARY MATERIAL 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not
have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Murataeva, N., Mattox, S., Yust, K. _et al._ Murine vaginal secretory
responses to a male volatile chemical messenger. _Sci Rep_ 14, 27707 (2024). https://doi.org/10.1038/s41598-024-77983-6 Download citation * Received: 10 May 2024 * Accepted: 28 October 2024
* Published: 12 November 2024 * DOI: https://doi.org/10.1038/s41598-024-77983-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Vagina * Vaginal *
Method * Secretion * Exocrine * Pheromone * Chemical messenger
